Abstract
Background and objectives: Short daily hemodialysis (SDHD) is an alternative to thrice-weekly HD because of its putative physiologic benefits. The purpose of this study was to investigate the effect of SDHD on the pharmacokinetics and pharmacodynamics of vancomycin.
Design, setting, participants, & measurements: Six noninfected adults who had anuria and were treated with SDHD were studied and received four dialysis sessions over 4 days. After completion of the first SDHD, each patient received vancomycin 15 mg/kg by intravenous infusion. Blood samples were collected over the ensuing 3 days during each subsequent inter- and intradialytic period. Pharmacokinetic parameters were determined. Serum concentration-time profiles were simulated for four vancomycin regimens with maintenance doses administered after every other SDHD. Area under the serum-concentration time curve (AUC) from 0 to 48 hours, 48 to 96 hours, and 96 to 144 hours were calculated, and Monte Carlo simulations were performed to determine the probability of target attainment at an AUC/minimum inhibitory concentration (MIC) ratio ≥800 for each 48-hour AUC at MICs ranging from 0.5 to 2.0 μg/ml.
Results: Median (range) systemic clearance was 7.2 ml/min (5.3 to 10.0 ml/min), and dialytic clearance was 104 ml/min (94 to 106 ml/min). The steady-state volume of distribution was 55.4 L (34.8 to 77.2 L). At MICs ≤1 μg/ml, probability of target attainment was >90% for each 48-hour AUC when vancomycin was administered as a 20-mg/kg loading dose followed by 10 mg/kg after every other SDHD.
Conclusions: Vancomycin pharmacokinetic parameters in SDHD are consistent with data from thrice-weekly HD. A loading dose of 20 mg/kg followed by 10 mg/kg after every other SDHD provides adequate exposure for pathogens with MICs ≤1 μg/ml.
According to the US Renal Data System, infection continues to exact a heavy toll among patients with ESRD. Hospitalization rates for infections among hemodialysis (HD) patients increased 37.5% from 1993 to 2005 (1). The mortality rate attributable to septicemia was 19.8% in HD patients, second only to the mortality rate for cardiac arrest (1). Quotidian HD regimens are gaining in popularity. These modalities provide patients with greater scheduling flexibility, because they can perform the HD in their home with a caregiver rather than at an HD center. More important, studies have shown that short daily HD (SDHD) and nocturnal HD improve quality of life and attenuate the medical complications that are associated with ESRD (2–11). Despite these reported improvements in quality of life and medical complications provided by SDHD, infection will likely remain a persistent medical issue for dialysis patients. As a result, the appropriate provision of antibiotic therapy to patients who receive SDHD will remain paramount.
Staphylococcus aureus and coagulase-negative staphylococci have been implicated in >60% of infections that occur in patients who undergo long-term HD (8). The prevalence of methicillin resistance in S. aureus isolated from blood cultures from outpatients who undergo HD has been reported to be >40% (12). In health care–associated infections, the prevalence of methicillin-resistant S. aureus is >55% (13). Coagulase-negative staphylococci are less virulent than S. aureus, but the rate of methicillin resistance is substantially higher (approximately 90%) (14). As a result of the high rates of staphylococcal infections and the prevalence of methicillin resistance in these organisms, vancomycin is frequently used for empiric and directed therapy of serious Gram-positive infections in patients who receive HD; however, vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) have been recovered from patients who were undergoing dialysis (15,16). In addition, there have been reports of recovery of inducible, heterogeneous VISA strains from patients whose therapy failed with standard doses of vancomycin (17–19); therefore, the importance of the judicious use of vancomycin in this patient population cannot be overemphasized.
Several recommendations have been published regarding vancomycin dosing for patients who receive high-flux HD (14,20–23). A common approach is to administer a 1-g loading dose (LD) after dialysis or during the last hour of a dialysis session followed by administration of maintenance doses (MDs) of 0.5 to 1.0 g after dialysis or during the last hour of a dialysis session (21,22). The authors of these studies reported that 96 to 97% of predialysis vancomycin concentrations were between 5 and 20 μg/ml; however, 30 to 40% of those concentrations were <10 μg/ml (21,22). This finding is important because studies have suggested that trough concentrations <10 μg/ml may predict treatment failure and the potential for emergence of VISA or VRSA (24,25). On the basis of these data, a recently published consensus review on the therapeutic monitoring of vancomycin recommended that the minimum trough concentration always be >10 μg/ml to avoid the development of resistance (26). From a pharmacodynamic standpoint, the area under the serum-concentration time curve (AUC)/minimum inhibitory concentration (MIC) ratio has been shown to predict vancomycin efficacy, and an AUC0 to 24/MIC ratio ≥400 has been advocated as the target for vancomycin efficacy (27,28). Unfortunately there is no consensus on the appropriate AUC/MIC target in patients who have chronic kidney disease and receive vancomycin.
A limited number of studies have evaluated the pharmacokinetics of vancomycin in SDHD patients. Clinicians rely on pharmacokinetic data obtained from the 4-hour, thrice-weekly HD patient population and frequent monitoring of serum vancomycin concentrations. Because it is not known whether this approach is appropriate for patients who receive SDHD, the purpose of this study was to characterize the pharmacokinetics and pharmacodynamics of vancomycin in otherwise healthy patients who had ESRD and were receiving SDHD. Moreover, this study was performed to evaluate several potential vancomycin dosing regimens.
Materials and Methods
Participants and Study Protocol
Six noninfected patients with anuria from the outpatient SDHD cohort at Indiana University Hospital-Clarian Health Partners (Indianapolis, IN) were enrolled in the study. Individuals were considered for enrollment when they were at least 18 years of age, they received SDHD six times per week, they had no other acute intercurrent illness, and their postdialysis weight was within 30% of their ideal body weight. Patients were excluded when they had a history of vancomycin allergy or had received vancomycin within 3 weeks of study initiation. The study was approved by the Indiana University-Purdue University-Indianapolis Institutional Review Board. All patients gave written informed consent before participating in the study.
After completion of a scheduled SDHD session (SDHD-1), each patient received vancomycin 15 mg/kg as a 1-hour intravenous infusion through the postdialyzer port of the patient's HD access. Venous blood samples were subsequently collected at time 0 (end of SDHD-1 and start of vancomycin infusion), 0.5, 1 (end of vancomycin infusion), 1.5, 2, 3, and 5 hours. The next day, patients had blood samples collected just before (0 hour), at the midpoint of (1 hour) and at the completion of the second dialysis (SDHD-2; 2 hours), and 0.5, 1, 2, and 4 hours after SDHD-2 ended. The patients also had predialysis, 1-hour, and 2-hour (post) samples of SDHD-3 and -4 the subsequent 2 days. All blood samples were collected into nonheparinized evacuated blood collection test tubes, allowed to clot for at least 30 minutes, and centrifuged, and serum was stored at −70°C until assay.
All patients underwent dialysis with a new cellulose triacetate high-flux (Exeltra 150 Baxter Healthcare) dialyzer with a surface area of 1.5 m2 and an ultrafiltration coefficient of 31.5 ml/h per mmHg. Each SDHD session was 2.0 to 2.5 hours long, and the HD operating characteristics were the same as used those for the patients' regularly scheduled treatments.
Analytical Methods
Serum concentrations of vancomycin were determined by the Enzyme Multiplied Immunoassay Technique (EMIT; Syva Co., Dade Behring Inc., Cupertino, CA) at the Indiana University Hospital clinical laboratory. The EMIT assay has intra- and interassay coefficients of variation of <5%. Urea nitrogen and creatinine concentrations were determined by colorimetric methods (29). This assay had intra- and interassay coefficients of variation of <5% for both urea nitrogen and creatinine.
Pharmacokinetic Analysis
On the basis of visual examination of the vancomycin serum concentration-time curves and previous data from our laboratory, a two-compartment structural pharmacokinetic model was chosen. Differential equations describing the model were fit to each patient's vancomycin serum concentration-time data:
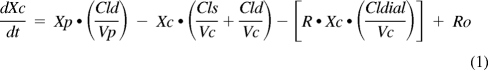 |
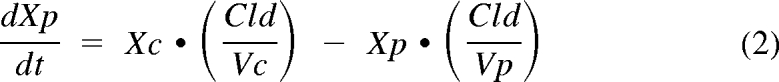 |
 |
where Xc and Xp are the amounts of vancomycin in the central and peripheral compartments, Vc and Vp are the apparent volumes of distribution of the central and peripheral compartments, Cld is the distribution clearance between the central and peripheral compartments, Cls is systemic clearance, R has a value of 0 during the interdialytic period and 1 during dialysis, Cldial is dialytic clearance, Ro is the vancomycin infusion rate, and Yc is the ADAPT output equation describing the estimated vancomycin concentrations. The inter- and intradialytic periods were fitted simultaneously. The models were fitted to the data using ADAPT 5 computer software package (30) using maximum a posteriori estimation. The variance model assumed that the SD of the residuals were linear with increasing vancomycin concentrations:
where, yint, is the y-intercept of the residual plot and m, is the slope of the line. Drug volumes of distribution were assumed to be constant during the entire study period. This assumption is based on the premise that, although patient plasma volumes will change with each inter- and intradialytic period, this will have minimal impact on the estimation of vancomycin apparent volume of distribution because it is considerably larger than plasma volume. Apparent volume of distribution at steady state (Vss) was calculated as the sum of Vc and Vp, and interdialytic elimination rate constant and half-life were calculated by standard equations.
Rebound was calculated as follow:
where Cpostdialysis is the vancomycin concentration after HD was completed and Cend is the maximum vancomycin concentration after rebound was completed. Single-pool Kt/Vurea, eqilibrated KT/Vurea, and standard KT/Vurea and urea reduction ratios (URRs) were calculated using standard methods (31).
Pharmacokinetic Simulations and Pharmacodynamic Analysis
Using the pharmacokinetic parameters derived for each patient, serum concentration-time profiles were simulated using ADAPT 5 for four vancomycin dosing regimens: 15-mg/kg LD followed by 7.5-mg/kg MD; 15-mg/kg LD followed by 15-mg/kg MD; 20-mg/kg LD followed by 7.5-mg/kg MD; and 20-mg/kg LD followed by 10-mg/kg MD. All MDs were administered after every other SDHD session. The postdialysis trough concentrations were determined at 48, 96, and 144 hours, and area under the serum concentration-time curves were calculated for each regimen from 0 to 48 hours, 48 to 96 hours, and 96 to 144 hours using the linear trapezoidal rule.
Pharmacodynamic exposures, as measured by the AUC/MIC ratio, were calculated by performing 10,000-patient Monte Carlo simulations (Crystal Ball 2000.2.2 software; Decisioneering, Inc., Denver, CO) for each regimen using the AUC0 to 48, AUC48 to 96, and AUC96 to 144. For the simulations, the AUC was assumed to follow log-Gaussian distribution. The pharmacodynamic target was a 48-hour AUC/MIC ratio ≥800 because an AUC0 to 24/MIC ratio ≥400 has been advocated as a target to ensure clinical effectiveness for vancomycin (26,28). Because the vancomycin doses were administered after every other SDHD session, the 24-hour pharmacodynamic target was doubled from 400 to 800. The probability of target attainment (PTA) for the 48-hour AUC/MIC ratio was calculated using MIC values of 0.50, 0.75, 1.00, 1.50, and 2.00 μg/ml. For completeness, the intermediate MICs of 0.75 and 1.50 μg/ml were included in the analysis because these MICs can be obtained by using the Etest (AB Biodisk, Solna, Sweden) method, a commonly used clinical method of determining MICs. This technique has been used previously for determining vancomycin MICs (32,33). The dosing regimen was considered optimal when the PTA was ≥90% for every 48-hour dosing interval.
Statistical Analysis
Regression analysis was used to assess the relationship between URR, Kt/V, and vancomycin dialytic clearance. Statistical analyses were performed using GraphPad 5.0 for Windows (La Jolla, CA). Overall differences were considered statistically significant at P < 0.05.
Results
Six patients (five men and one woman) completed the study. The median (range) age and weight were 53.5 years (28 to 59 years) and 91 kg (59 to 110 kg), respectively. No adverse effects related to vancomycin were reported.
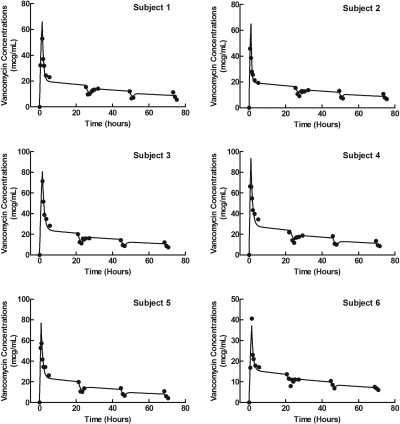
The vancomycin pharmacokinetic parameters for each patient are shown in Table 1. A two-compartment, open-model best fit the pharmacokinetic data. Individual vancomycin serum concentration-time curves, both observed and modeled data, in the six patients are illustrated in Figure 1. As evidenced by Figure 1, there was good agreement between the fitted and observed data. This finding is further supported by the median (range) r2 values for the observed versus predicted concentrations of 0.96 (0.87 to 0.99). Individual and median estimates of vancomycin dialytic clearance, percentage of urea and vancomycin removed, and vancomycin rebound characteristics are shown in Table 2. There was no statistically significant relationship between vancomycin dialytic clearance and either URR or Kt/V.
Table 1.
Individual and median vancomycin pharmacokinetic parameters and body weights
| Patient | Cls (ml/min) | Cld (ml/min) | Vc (L) | Vss (L) | t1/2β(hours) | Body Weight (kg) |
|---|---|---|---|---|---|---|
| 1 | 7.4 | 11.1 | 9.3 | 56.4 | 91.1 | 81 |
| 2 | 10.0 | 23.5 | 11.0 | 77.2 | 90.7 | 101 |
| 3 | 7.9 | 9.8 | 12.2 | 63.5 | 95.4 | 110 |
| 4 | 7.1 | 9.8 | 11.7 | 52.3 | 87.3 | 104 |
| 5 | 5.3 | 7.1 | 8.4 | 34.8 | 78.1 | 59 |
| 6 | 6.9 | 12.9 | 14.8 | 54.4 | 92.4 | 59 |
| Median | 7.2 | 10.4 | 11.3 | 55.4 | 90.9 | 91 |
Cls, systemic clearance; Cld, distribution clearance between the central and peripheral compartments; Vc, apparent volume of distribution in the central compartment; Vss, apparent volume of distribution at steady-state; and t1/2β, terminal elimination half-life (i.e., interdialytic half-life).
Figure 1.
Individual observed and modeled vancomycin serum concentration-time curves in six patients. Observed data are indicated by ● and modeled data by the solid lines.
Table 2.
Individual and median vancomycin dialytic clearance, vancomycin and urea removal estimates, and rebound characteristics
| Patient | Blood Flow Rate (ml/min) | HD Duration (hours)a | Volume of Dialysate (L) | URR | Delivered spKt/V | eKt/V | Weekly Std Kt/V | Vancomycin Cldial (ml/min) | Vancomycin Tmax,rebound (hours)b | Vancomycin Rebound (%)b |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 500 | 2.50 | 20 | 0.46 | 0.54 | 0.49 | 2.38 | 103 | 2.7 | 28.6 |
| 2 | 500 | 2.00 | 25 | 0.45 | 0.79 | 0.53 | 2.50 | 101 | 1.6 | 17.1 |
| 3 | 500 | 2.25 | 20 | 0.54 | 0.84 | 0.52 | 2.49 | 106 | 3.6 | 35.4 |
| 4 | 500 | 2.50 | 20 | 0.39 | 0.63 | 0.47 | 2.29 | 104 | 3.2 | 26.2 |
| 5 | 500 | 2.50 | 20 | 0.42 | 0.61 | 0.52 | 2.50 | 104 | 3.2 | 36.3 |
| 6 | 500 | 2.50 | 20 | 0.42 | 0.67 | 0.57 | 2.66 | 94 | 2.7 | 11.6 |
| Median | 500 | 2.50 | 20 | 0.44 | 0.62 | 0.52 | 2.49 | 104 | 3.0 | 27.3 |
spKT/Vurea, single-pool Kt/Vurea; eKt/V, equilibrated Kt/Vurea; Cldial, vancomycin dialytic clearance.
Average dialysis duration during the study.
Based on modeled concentrations.
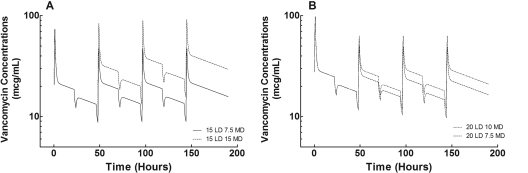
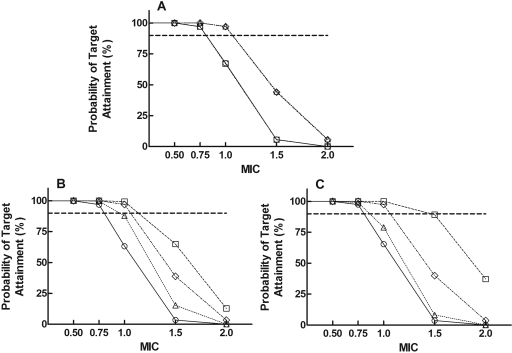
The simulated serum concentration-time curves for the four vancomycin dosing regimens are illustrated in Figure 2, and the Cmin and AUCs for the first three vancomycin doses are shown in Table 3. The mean Cmin at 48 hours, immediately after SDHD, was <10 μg/ml after the LD of 15 mg/kg, but the mean Cmin at 48 hours was >10 μg/ml after the 20-mg/kg LD. Of the four dosing regimens, a 20-mg/kg LD followed by 10 mg/kg after every other dialysis session was the only regimen to achieve Cmin values >10 μg/ml for each period. Finally, the results of the pharmacodynamic analysis are shown in Figure 3. For pathogens with a vancomycin MIC <1 μg/ml, the PTA was >90% for each period for all for dosing regimens; however, when the vancomycin MIC was 1 μg/ml, the only regimen that achieved a PTA >90% for each period was the 20-mg/kg LD followed by 10 mg/kg after every other SDHD session. None of the dosing regimens achieved a PTA >90% when the vancomycin MIC was >1 μg/ml. This finding is significant because staphylococci are reported to be susceptible to vancomycin when the MIC is ≤2 μg/ml.
Figure 2.
Simulated vancomycin mean concentration-time profiles for four dosing regimens in the six patients. The profiles are shown for the intradialytic and interdialytic periods for 190 hours after the administration of the three drug doses and during six dialysis sessions. The times for the dialysis sessions were 22 to 24, 46 to 48, 70 to 72, 94 to 96, 118 to 120, and 142 to 144 hours. The vancomycin doses were administered at 0, 48, and 96 hours. For a patient who is following traditional SDHD, the next scheduled HD session would be at 190 hours. (A) Dosing with a 15-mg/kg LD. (B) Dosing with a 20-mg/kg LD.
Table 3.
AUC and Cmin after simulations of the first, second, and third doses of vancomycin
| Regimen (mg/kg) | First |
Second |
Third |
|||
|---|---|---|---|---|---|---|
| Cmin48(μg/ml) | AUC0 to 48(μg/h per ml) | Cmin96(μg/ml) | AUC48 to 96(μg/h per ml) | Cmin144(μg/ml) | AUC96 to 144(μg/h per ml) | |
| 15 LD, 7.5 MD | 8.7 ± 1.4 | 892 ± 182 | 8.9 ± 1.5 | 866 ± 164 | 9.0 ± 1.6 | 873 ± 163 |
| 15 LD, 15 MD | 8.7 ± 1.4 | 892 ± 182 | 13.2 ± 2.2 | 1310 ± 253 | 15.6 ± 2.7 | 1530 ± 289 |
| 20 LD, 7.5 MD | 11.7 ± 1.9 | 1190 ± 243 | 10.4 ± 1.8 | 1006 ± 188 | 9.7 ± 1.7 | 945 ± 175 |
| 20 LD, 10 MD | 11.7 ± 1.9 | 1190 ± 243 | 11.8 ± 2.0 | 1160 ± 218 | 11.9 ± 2.1 | 1160 ± 217 |
Data are means ± SD.
Figure 3.
PTA for AUC/MIC ratio ≥800 for each 48-hour AUC: AUC0 to 48 (A), AUC48 to 96 (B), and AUC96 to 144 (C). MICs ranged from 0.5 to 2.0 μg/ml. The four vancomycin dosing regimens were 15-mg/kg LD followed by 7.5-mg/kg MD (○); 15-mg/kg LD followed by 15-mg/kg MDs (□); 20-mg/kg LD followed by 7.5-mg/kg MDs (△); and 20-mg/kg LD followed by 10-mg/kg MDs (♢).
Discussion
SDHD is an alternative renal replacement therapy to conventional thrice-weekly HD; however, the impact on drug dosing has been less well studied than conventional thrice-weekly HD. Dosing of vancomycin in conventional high-flux dialysis typically involves the administration of a vancomycin LD (e.g., 1000 mg, 15 mg/kg) followed by an MD or a replacement dose (e.g., 500 mg, 7.5 mg/kg) during or after each dialysis session. Numerous articles have been published regarding the pharmacokinetics of vancomycin and dosing in this patient population, but less emphasis has been given to the importance of achieving appropriate pharmacodynamic targets. Current guidelines suggest maintaining vancomycin serum trough concentrations between 15 and 20 μg/ml in patients who receive HD therapy, although this is not supported by clinical trial evidence (26).
In this study, we determined the pharmacokinetics of vancomycin and its removal by SDHD in otherwise healthy patients with chronic kidney disease. We performed pharmacokinetic and pharmacodynamic simulations to evaluate vancomycin dosing strategies to develop a rational dosing method. This study incorporated pharmacodynamic targets rather than simply measuring vancomycin serum concentrations. We simulated four potential vancomycin dosing regimens for patients who were undergoing SDHD, assuming an HD treatment scheme of 6 days of 2 hours of HD followed by 1 day off. Thus, the total dialysis time during the week was 12 hours. Serum concentrations were simulated over a 1-week period, as illustrated in Figure 3 and Table 3.
Like β-lactam antibiotics, vancomycin exhibits time-dependent bactericidal activity against susceptible bacteria; however, the bactericidal pattern of the agent is not entirely predictive of the pharmacodynamic parameter associated with efficacy. The presence and duration of the postantibiotic effect is also important factor. In vitro, animal, and limited human studies have demonstrated that the AUC/MIC ratio is the pharmacodynamic parameter that best predicts vancomycin efficacy against staphylococci (26). In patients with lower respiratory tract infections caused by S. aureus, clinical and bacteriologic success with vancomycin therapy were higher in patients with 24-hour AUC/MIC ratios ≥400 compared with patients with 24-hour AUC/MIC ratios <400 (28). On the basis of all available data, the consensus review on therapeutic monitoring of vancomycin supports the 24-hour AUC/MIC ratio ≥400 as the pharmacodynamic target for vancomycin effectiveness (26). In this study, serum concentration-time profiles were simulated for administration of vancomycin doses every 48 hours; therefore, the pharmacodynamic target chosen for our analyses was a 48-hour AUC/MIC ratio ≥800. In the simulation portion of our study, we evaluated the 48-hour AUC/MIC ratio for the first three 48-hour periods, each of these immediately following the vancomycin doses. For maximization of clinical efficacy and minimize the potential for resistance, the goal of any vancomycin dosing regimen should be to achieve the pharmacodynamic target with the LD and all subsequent MDs of the drug.
In the first 48-hour period, the PTA at an AUC/MIC ≥800 was 97 to 100% for the 15- and 20-mg/kg LDs when the vancomycin MIC was <1 μg/ml; however, at an MIC of 1 μg/ml, the PTA was 97% for the 20-mg/kg LD and only 67% for the 15-mg/kg LD (Figure 3). Because the susceptibility of a pathogen is not known at the initiation of therapy, a 20-mg/kg LD of vancomycin should be administered empirically to maximize the pharmacodynamics of the drug. These data are similar to the results of a previous study that demonstrated more rapid achievement of desirable serum vancomycin concentrations with a 20-mg/kg LD compared with a 1-g LD (34). After a 20-mg/kg LD, MDs of 10 mg/kg administered after every other SDHD session provide adequate vancomycin exposures for the periods of 48 to 96 hours and 96 to 144 hours. For this regimen, the PTA was 97% for both periods at an MIC of 1 μg/ml; however, when the MIC is <1 μg/ml, the PTA is almost 100% for MDs of 7.5 mg/kg (after the 20-mg/kg LD) administered after every other SDHD session. It is likely that culture and susceptibility results would be known before administration of the MD, so the 7.5- or 10.0-mg/kg dose could be chosen on the basis of the MIC for the pathogen.
The pharmacodynamics of vancomycin were woefully inadequate for all four dosing regimens when the MIC was >1 μg/ml. This finding is very important because the vancomycin susceptibility breakpoint, which is established by the Clinical and Laboratory Standards Institute, is an MIC ≤2 μg/ml (26). Any staphylococcal isolate with an MIC of 1.5 to 2.0 μg/ml would be reported as susceptible to vancomycin by the clinical microbiology laboratory; however, on the basis of our pharmacodynamic analysis, suboptimal clinical outcomes would be expected if vancomycin were used to treat infections that were caused by organisms with MICs >1 μg/ml. In a case-control study, outcomes were evaluated for patients who were undergoing HD and received vancomycin for the treatment of bacteremia caused by methicillin-resistant S. aureus (35). Patients were divided into two groups on the basis of the vancomycin MIC, and vancomycin was given every 5 to 7 days when the serum concentration was 5 to 15 μg/ml (35). Hospital length of stay, total hospitalization costs, and mortality were significantly higher in patients with vancomycin MICs of 2 μg/ml compared with those with vancomycin MICs ≤0.5 μg/ml (35). Similarly, poor clinical outcomes with vancomycin have been reported in other patient populations when the MICs were 1.5 to 2.0 μg/ml (33,36–39); therefore, alternative therapy should be used when treating staphylococcal infections with vancomycin MICs >1 μg/ml.
Conclusions
The pharmacokinetics of vancomycin are similar in patients who receive SDHD. An LD of 20 mg/kg followed by 10 mg/kg after every other SDHD session provides adequate exposure for pathogens with MICs ≤1 μg/ml. Given the small number of patients enrolled in this study and the use of simulations to test these regimens, future studies are needed to apply the data and recommendations from this study to a larger population of infected patients. Alternative therapy is recommended when treating staphylococcal infections with vancomycin MICs >1 μg/ml. As recommended in the consensus vancomycin therapeutic monitoring guidelines, serum concentration monitoring may be useful to assist clinicians with vancomycin dosing in these patients.
Disclosures
None.
Acknowledgments
This study was supported in part by grants from the National Kidney Foundation of Indiana (Indianapolis, IN) and the Indiana Clinical and Translational Sciences Institute, Indiana Clinical Research Center, and Young Investigator awards (NIH UL RR025761).
This study was presented in part at the annual meeting of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.US Renal Data System: USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 2.Pierratos A: Daily (quotidian) nocturnal home hemodialysis: Nine years later. Hemodial Int 8: 45–50, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Vos PF, Zilch O, Kooistra MP: Clinical outcome of daily dialysis. Am J Kidney Dis 37: S99–S102, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Pierratos A: Daily hemodialysis: An update. Curr Opin Nephrol Hypertens 11: 165–171, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Nissenson AR: Daily hemodialysis: Challenges and opportunities in the delivery and financing of end-stage renal disease patient care. Adv Ren Replace Ther 8: 286–292, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S: Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: A prospective, controlled study. J Am Soc Nephrol 16: 2778–2788, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Pierratos A, McFarlane P, Chan CT: Quotidian dialysis: Update 2005. Curr Opin Nephrol Hypertens 14: 119–124, 2005 [DOI] [PubMed] [Google Scholar]
- 8.D'Agata EM: Antimicrobial-resistant, Gram-positive bacteria among patients undergoing chronic hemodialysis. Clin Infect Dis 35: 1212–1218, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Martins Castro MC, Luders C, Elias RM, Abensur H, Romao Junior JE: High-efficiency short daily haemodialysis: Morbidity and mortality rate in a long-term study. Nephrol Dial Transplant 21: 2232–2238, 2006 [DOI] [PubMed] [Google Scholar]
- 10.McFarlane PA: More of the same: Improving outcomes through intensive hemodialysis. Semin Dial 22: 598–602, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Punal Rioboo J, Sanchez-Iriso E, Ruano-Ravina A, Varela Lema ML, Sanchez-Guisande D, Gonzalez-Rodriguez L, Herrero JA, Barril G, Maduell F, Hernandez J, Otero A, Bajo MA, Sanchez R: Short daily versus conventional hemodialysis quality of life: A cross-sectional multicentric study in Spain. Blood Purif 28: 159–164, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Klevens RM, Edwards JR, Andrus ML, Peterson KD, Dudeck MA, Horan TC: Dialysis surveillance report: National Healthcare Safety Network (NHSN)—Data summary for 2006. Semin Dial 21: 24–28, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK: NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections—Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 29: 996–1011, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Pai AB, Pai MP: Optimizing antimicrobial therapy for gram-positive bloodstream infections in patients on hemodialysis. Adv Chronic Kidney Dis 13: 259–270, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Fridkin SK: Vancomycin-intermediate and -resistant Staphylococcus aureus: What the infectious disease specialist needs to know. Clin Infect Dis 32: 108–115, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep 51: 565–567, 2002 [PubMed] [Google Scholar]
- 17.Liu C, Chambers HF: Staphylococcus aureus with heterogeneous resistance to vancomycin: Epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 47: 3040–3045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore MR, Perdreau-Remington F, Chambers HF: Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 47: 1262–1266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK: Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50: 3039–3047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Launay-Vacher V, Izzedine H, Mercadal L, Deray G: Clinical review: Use of vancomycin in haemodialysis patients. Crit Care 6: 313–316, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai AB, Pai MP: Vancomycin dosing in high flux hemodialysis: A limited-sampling algorithm. Am J Health Syst Pharm 61: 1812–1816, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ariano RE, Fine A, Sitar DS, Rexrode S, Zelenitsky SA: Adequacy of a vancomycin dosing regimen in patients receiving high-flux hemodialysis. Am J Kidney Dis 46: 681–687, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pallotta KE, Manley HJ: Vancomycin use in patients requiring hemodialysis: A literature review. Semin Dial 21: 63–70, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Sakoulas G, Gold HS, Cohen RA, Venkataraman L, Moellering RC, Eliopoulos GM: Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother 57: 699–704, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Howden BP, Ward PB, Charles PG, Korman TM, Fuller A, du Cros P, Grabsch EA, Roberts SA, Robson J, Read K, Bak N, Hurley J, Johnson PD, Morris AJ, Mayall BC, Grayson ML: Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin Infect Dis 38: 521–528, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Jr, Craig WA, Billeter M, Dalovisio JR, Levine DP: Therapeutic monitoring of vancomycin in adults: Summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29: 12751279, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, Dalovisio JR, Levine DP: Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66: 82–98, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ: Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43: 925–942, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lucksiri A, Scott MK, Mueller BA, Hamburger RJ, Sowinski KM: CAHP-210 dialyzer influence on intra-dialytic vancomycin removal. Nephrol Dial Transplant 17: 1649–1654, 2002 [DOI] [PubMed] [Google Scholar]
- 30.D'Argenio DZ, Schumitzky A: ADAPT 5, User's Guide: Pharmacokinetic and Pharmacodynamic Systems Analysis Software, Los Angeles, Biomedical Simulations Resource, University of Southern California, 2009 [Google Scholar]
- 31.Leypoldt JK, Jaber BL, Zimmerman DL: Predicting treatment dose for novel therapies using urea standard Kt/V. Semin Dial 17: 142–145, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Prakash V, Lewis JS, 2nd, Jorgensen JH: Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob Agents Chemother 52: 4528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J: Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46: 193–200, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Barth RH, DeVincenzo N: Use of vancomycin in high-flux hemodialysis: Experience with 130 courses of therapy. Kidney Int 50: 929–936, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Maclayton DO, Suda KJ, Coval KA, York CB, Garey KW: Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 microg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin Ther 28: 1208–1216, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM: Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 42: 2398–2402, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moise PA, Sakoulas G, Forrest A, Schentag JJ: Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 51: 2582–2586, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A: High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch Intern Med 166: 2138–2144, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K: Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 52: 3315–3320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]