Abstract
Background and objectives: Conversion from central venous catheters to a graft or a fistula is associated with lower mortality risk in long-term hemodialysis (HD) patients; however, a similar association with hospitalization risk remains to be elucidated.
Design, setting, participants, & measurements: We conducted a prospective observational study all maintenance in-center HD patients who were treated in Fresenius Medical Care, North America legacy facilities; were alive on January 1, 2007; and had baseline laboratory data from December 2006. Access conversion (particularly from a catheter to a fistula or a graft) during the 4-month period from January 1 through April 30, 2007, was linked using Cox models to hospitalization risk during the succeeding 1-year follow-up period (until April 30, 2008).
Results: The cohort (N = 79,545) on January 1, 2007 had 43% fistulas, 29% catheters, and 27% grafts. By April 30, 2007, 70,852 patients were still on HD, and among 19,792 catheters initially, only 10.3% (2045 patients) converted to either a graft or a fistula. With catheters as reference, patients who converted to grafts/fistulas had similar adjusted hazard ratios (0.69) as patients on fistulas (0.71), while patients with fistulas/grafts who converted to catheters did worse (1.22), all P < 0.0001.
Conclusions: Catheters remain associated with the greatest hospitalization risk. Conversion from a catheter to either graft or fistula had significantly lower hospitalization risk relative to keeping the catheter. Prospective studies are needed to determine whether programs that reduce catheters will decrease hospitalization risk in HD patients.
In long-term hemodialysis (HD) patients, an arteriovenous fistula is preferred over arteriovenous grafts and central venous catheters (1–3). Within the campaign to increase fistulas, our group and others have expressed concern over the alarming rates of catheter use, the access type with the worst associated outcomes (4–8). Data derived from the Hemodialysis (HEMO) Study indicated substantial reduction in death risk associated with conversion from catheters to fistulas or grafts in prevalent HD patients who had survived for 1 year (9). Recently, a report from the Dialysis Outcomes and Practice Patterns Study (DOPPS) also showed an association of improved survival with conversion from initial catheter accesses to either fistulas or grafts for incident HD patients, with 30% lower adjusted mortality risk compared with patients who were maintained on catheter access (10). Of note, the survival advantage that was associated with catheter removal was similar, whether patients converted to fistulas or grafts.
We recently reported a similar reduction of death risk associated with conversion from catheters to grafts or fistulas in a cohort of 79,545 prevalent HD patients whose access types were tracked during a 4-month baseline period, with mortality monitored for the remainder of the year (11). As a follow-up to this study, we used the same large prevalent cohort of HD patients to test the hypothesis that conversion from a catheter to either a fistula or a graft during a 4-month period may be associated with lower hospitalization risk in the succeeding year, compared with patients with continuous catheter use. To frame this hypothesis in context, we initially determined the association between baseline access type and hospitalization risk.
Materials and Methods
Study Population
All permanent HD patients who were alive as of January 1, 2007; were treated in Fresenius Medical Care North America (FMCNA) facilities, excluding recently acquired Renal Care Group; and had at least one laboratory result for the month of December 2006 for albumin, hemoglobin, phosphorus, or urea were included in the cohort. Age, gender, race, diabetes status, and vintage (defined as time on dialysis) were recorded as of January 1, 2007. The equilibrated Kt/V (eKt/V) was calculated from the single-pool Kt/V using the Tattersall equation; single-pool Kt/V in turn was derived from variable-volume double-urea sample urea kinetic modeling, consistent with current national guidelines (12,13).
Definitions
Vascular accesses were defined as fistulas (anastomosis of native vein and artery), grafts (surgically implanted conduit of predominantly synthetic material between the vein and the artery), or catheters (partly external device that provides access predominantly to the central veins or right atrium). The access type recorded in the FMCNA Knowledge Center data warehouse as of January 1, 2007, was defined as the initial study access (not necessarily a prevalent patient's first ever access type). For patients who survived the baseline period from January 1 through April 30, 2007, the last access in place was specifically recorded for determination of whether there was a change. Patients who started the year with a fistula or a graft and had a catheter on May 1, 2007, were classified as having converted to catheter use. When a catheter remained in place despite the presence of a maturing fistula or graft, the patient was considered to incur the risk of a catheter and was classified as remaining on the catheter (if this assumption was incorrect [i.e., the patient was already using a fistula but the catheter was not yet terminated in the system], then the resulting bias would be toward the null). Furthermore, categorization of exposure under catheter for patients with dual accesses makes clinical sense, because reports have indicated disappointing primary fistula maturation rates of only 40 to 45% (14–18).
Patients' first hospitalization (i.e., separately for all causes, vascular access related, and sepsis/bacteremia) was evaluated for the subsequent year from May 1, 2007, through April 30, 2008, excluding the baseline period. The FMCNA policy requires that all patients and/or caregivers be asked at the beginning of every treatment whether they had visited the hospital since the last treatment was performed; if so, then this information should be recorded. Unscheduled absences are routinely tracked by patient care staff who contact patients (or caregivers) at home and/or the physician to determine the reason(s) for missing one or more treatments. In many cases, they contact the local hospital directly to determine whether a patient was admitted during absence. Hospital admissions from all causes were recorded. In addition, hospitalization primarily for vascular access–related issues (International Classification of Diseases, Ninth Revision codes 996.1, 996.60, 996.62, 996.70, 996.73, 996.74, and 997.2) and, separately, hospitalization primarily for sepsis or bacteremia (International Classification of Diseases, Ninth Revision codes 38, 38.0, 38.1, 38.10, 38.11, 38.19, 38.2, 38.3, 38.40, 38.41, 38.42, 38.43, 38.44, 38.49, 38.8, 38.9, and 790.7) were also tracked. Patients who received a kidney transplant or transferred out of FMCNA facilities contributed exposure time at risk until the transplant or transfer, respectively.
Statistical Analysis
Baseline characteristics and hospitalization information were summarized as mean ± SD or as percentages of the total. Categorical variables were compared using χ2 tests and continuous variables with two-sample t tests, with patients who were using fistulas or grafts (each) separately compared with patients who were using catheters as the reference group.
Preliminary Analysis: Vascular Access Type and Hospitalization Risk
HD catheters have historically been associated with greater hospitalization risk relative to fistulas and grafts (19–22). To determine whether this is true in this contemporary cohort, the simple association between first access of record on January 1, 2007, and hospitalization risk was determined for the whole year, up to December 31, 2007. We constructed standard Cox models (i.e., “intention-to-treat”) in three forms: (1) Unadjusted; (2) case-mix adjusted for age, gender, race, presence of diabetes, and vintage (transformed to square root); and (3) case-mix and laboratory adjusted (i.e., albumin, hemoglobin, phosphorus, and eKt/V). Laboratory variables that were used in the models were selected on the basis of known contribution to hospitalization risk as well as their current use as quality-of-care indicators (23–25). To determine whether facility (i.e., representing a combination of environmental geography/location, local practice, and socioeconomic or source population factors) could alter the results, we used a sensitivity analysis that adjusted for “center effect,” using a mixed-effects Cox model with adjustment for facility as a random effect (26). Finally, we also constructed time-dependent models (i.e., “as-treated”), whereby the vascular access on the last day of the previous month was recorded and the mean of all available laboratory values were collated monthly (when performed more than once during the month).
Catheter Conversion Analysis
Patients' access type during the first 4 months was classified into 5 categories: (1) Fistula (unchanged), (2) graft (unchanged), (3) catheter (unchanged), (4) catheter converted to graft or fistula, and (5) other (all other changes in access type, predominantly involving a temporary or permanent switch to a catheter). Few patients had multiple access changes during the baseline period and were included within this “other” category. Case-mix factors that were associated with greater likelihood of converting from catheters to fistula/graft during the 4-month period were determined using logistic regression models. Case-mix factors that were associated with dysfunction or failure of extant fistulas/grafts and resulted in catheter use were also determined.
Cox models were similarly constructed as (1) unadjusted, (2) case-mix adjusted, and (3) case-mix and laboratory adjusted. Patients who remained on catheters were used as the reference group. Two additional sensitivity analyses were performed. First, a shorter follow-up time of only 8 months was used, to be directly comparable to our previous mortality analyses (11); second, alternative models that excluded patients who were hospitalized during the 4-month baseline conversion period were constructed, eliminating the potential influence of immediate previous hospitalization to future hospitalization risk. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
The study cohort included 79,545 prevalent patients at baseline: 34,335 (43%) with fistulas, 23,433 (29%) with catheters, and 21,777 (27%) with grafts. Among patients with catheters, 32% had a concomitant maturing fistula, whereas 14% had grafts. Mean age was 62 ± 15 years, 54% were male, 51% were white, 53% had diabetes, and mean vintage was 3.6 ± 3.9 years. Compared with the cohort mean, patients with fistulas were younger (60 ± 15 years), were predominantly male (65%), had less diabetes (approximately 50%), and had higher albumin and phosphorus. Table 1 summarizes the study patients' baseline clinical and laboratory characteristics. The catheter group had the lowest albumin, hemoglobin, and eKt/V and shortest vintage (2.5 ± 3.4 years). More black patients (49%) and patients with longer vintage (4.6 ± 3.9 years) were using grafts.
Table 1.
Baseline patient characteristics by access type on January 1, 2007, and subsequent hospitalization during a 1-year period
| Parameter | All Patients | Catheter | Graft | Fistula |
|---|---|---|---|---|
| Patient characteristics | ||||
| no. of patients (%) | 79,545 | 23,433 (29.5) | 21,777 (27.4) | 34,335 (43.2) |
| age (years; mean ± SD) | 61.6 ± 15.0 | 62.3 ± 15.4 | 63.8 ± 14.1a | 60.4 ± 15.1a |
| male (%)c | 54.2 | 47.7 | 44.3 | 65.0 |
| race (%)c | ||||
| white | 50.8 | 54.9 | 42.4 | 53.4 |
| black | 40.5 | 38.3 | 48.9 | 36.7 |
| other | 8.7 | 6.8 | 8.7 | 9.9 |
| diabetesc | 53.0 | 55.8 | 55.3 | 49.7 |
| vintage (years; mean ± SD) | 3.6 ± 3.9 | 2.5 ± 3.4 | 4.6 ± 3.9a | 3.6 ± 3.5a |
| eKt/V (mean ± SD) | 1.48 ± 0.33 | 1.42 ± 0.37 | 1.51 ± 0.31a | 1.49 ± 0.32a |
| albumin (g/L; mean ± SD) | 3.82 ± 0.42 | 3.68 ± 0.47 | 3.85 ± 0.37a | 3.90 ± 0.38a |
| hemoglobin (g/dl; mean ± SD) | 12.12 ± 1.40 | 11.98 ± 1.55 | 12.15 ± 1.33a | 12.19 ± 1.33a |
| phosphorus (mg/dl; mean ± SD) | 5.47 ± 1.63 | 5.44 ± 1.71 | 5.40 ± 1.57b | 5.54 ± 1.61a |
| All hospitalizations | ||||
| exposure days (n [%]) | 24,756,616 | 6,414,312 (26) | 6,968,933 (28) | 11,373,371 (46) |
| event counts (n [%]) | 118,218 | 42,141 (36) | 33,912 (29) | 42,165 (36) |
| hospital days/patient-year (mean) | 13.0 | 18.8 | 13.0 | 9.8 |
| LOS (days; mean) | 7.5 | 7.8 | 7.3 | 7.25 |
LOS, length of stay.
P < 0.001,
P = 0.03 versus central venous catheter.
P < 0.001 by χ2 (within group).
Fifty-nine percent of patients were hospitalized within the year, averaging 1.5 episodes per patient. Patients with fistulas composed 46% of exposure days at risk for hospitalization (in part because of higher prevalence as well as greater survival over time) but proportionately fewer hospitalization events (36%) and hospital days (35%), also shown in Table 1. Conversely, catheters composed the fewest exposure days (26%, nearly half that for fistulas) yet accounted for 36% of hospital events (same as fistulas) and a greater proportion of hospital days (37%). Grafts had a proportional share of hospitalization (as expected from exposure days).
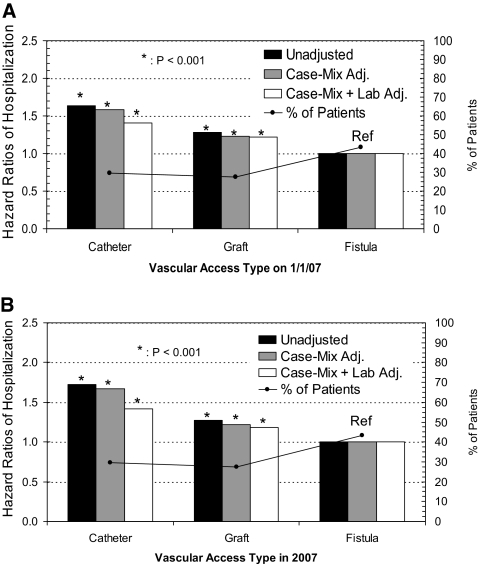
Preliminary analyses revealed that hospitalization risk from all causes was highest for patients who were using catheters (Figure 1A). Compared with patients with fistulas, there was 41% greater hazard risk (HR) for baseline catheter use and 22% for baseline graft use in the conventional case-mix– and laboratory-adjusted models (P < 0.001). The corresponding time-varying models in Figure 1B show nearly identical results at 42 and 18% greater HR for catheters and grafts, respectively (both P < 0.001). A sensitivity analysis that adjusted for “center effect” using a mixed-effects Cox model (facility entered as a random effect) resulted in minimal changes that did not substantially alter the results (data not shown). Of note, a slight increase in HR was associated with catheters and a slight decrease for grafts in time-varying models, indicating that the as-treated analysis likely detected a greater proximal risk for hospitalization associated with catheters-in-use (i.e., access type updated each month) than the intention-to-treat model (standard Cox models that assign exposure by initial access type). The reverse effect was true for grafts, whereby the HR was lower in the time-dependent models.
Figure 1.
Vascular access type as of January 1, 2007, and associated 1-year hospitalization risk from all causes (N = 79,545), using standard Cox models (A) and time-dependent Cox models (B). The black line tracks the distribution of patients by vascular access category. Case mix includes age, gender, race, diabetes, and vintage, whereas lab includes eKt/V, albumin, hemoglobin, and phosphorus.
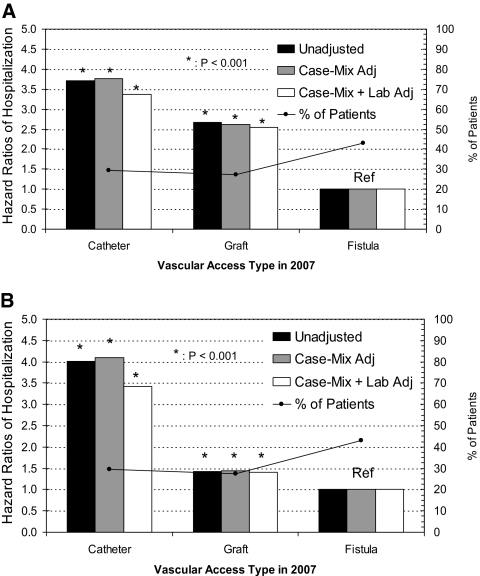
Results from time-dependent models for vascular access–related hospitalization and sepsis- or bacteremia-associated hospitalizations each are presented in Figure 2, A and B, respectively. The associated HR for catheter use remained consistently higher than that associated with use of grafts in comparison with fistulas (reference group). For vascular access–related hospitalization (including access dysfunction and mechanical complications), even though grafts had HR of 2.5 relative to fistula (P < 0.001), catheters maintained HR an order of magnitude higher at 3.4 (P < 0.001). In contrast, for sepsis- or bacteremia-related hospitalizations, the associated HR was 3.4 for catheters and only 1.4 for grafts (both P < 0.001 compared with fistula), confirming greater infection risk associated with catheter use.
Figure 2.
Time-dependent Cox models depicting vascular access type as of January 1, 2007 (N = 79,545), and associated 1-year hospitalization risk from vascular access–related issues (A) and sepsis/bacteremia (B). The black line tracks the distribution of patients by vascular access category. Case mix includes age, gender, race, diabetes, and vintage, whereas lab includes eKt/V, albumin, hemoglobin, and phosphorus.
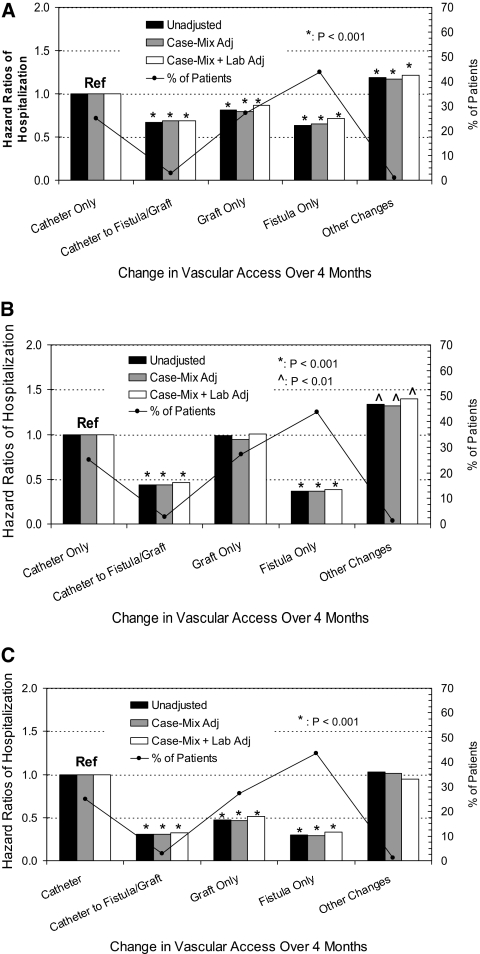
From 70,852 (89%) patients who were alive on May 1, 2007, we determined the association of a change in vascular access from catheter to graft or fistula (during the previous 4 months) to hospitalization risk during the subsequent year. Overall, 96% of patients maintained the same baseline vascular access. Among patients with a catheter and either a fistula or a graft as of January 1, 19% with fistulas had converted (i.e., catheter removed) to solely fistulas and 14% with grafts had converted to solely grafts by April 30. A total of 10.3% (2045) patients who initially had catheters converted to using fistulas (n = 1484) or grafts (n = 561) within 4 months. Case-mix factors that were associated with catheter conversion are shown in Table 2. Compared with patients who remained on catheters, patients who underwent dialysis solely by fistula and patients who were converted from catheters to a permanent access (fistula or graft) had lower hospitalization risk from all causes (HR 0.71 and 0.69, respectively; both P < 0.001; Figure 3A). Patients who underwent dialysis solely with grafts had HR of 0.87 (P < 0.001). Separating conversions to fistulas versus grafts, case-mix– plus laboratory-adjusted hospitalization HR was 0.67 versus 0.75, respectively (both P < 0.001 relative to staying on catheters). Patients with all other changes, mostly single conversions from graft/fistula to catheter but with a few having multiple access turnover, the hospitalization risk was increased to HR of 1.22 (P < 0.001).
Table 2.
Demographic characteristics associated with conversion from baseline catheters to graft/fistula and conversion from baseline fistula/graft to catheter use at the end of the 4-month conversion period
| Characteristic | Catheter to Fistula/Graft |
Fistula/Graft to Catheter |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age (years) | ||||
| <45 | 1.00 | — | 1.00 | — |
| 45 to 55 | 1.01 | 0.86 to 1.18 | 0.90 | 0.66 to 1.25 |
| 56 to 65 | 0.98 | 0.84 to 1.14 | 1.10 | 0.82 to 1.47 |
| 66 to 75 | 0.89 | 0.77 to 1.03 | 1.27 | 0.96 to 1.69 |
| >75 | 0.75a | 0.64 to 0.88 | 1.61b | 1.21 to 2.15 |
| Gender | ||||
| female | 1.00 | — | 1.00 | — |
| male | 1.48a | 1.35 to 1.62 | 0.75a | 0.64 to 0.88 |
| Race | ||||
| other | 1.00 | — | 1.00 | — |
| white | 0.99 | 0.82 to 1.19 | 1.36 | 0.99 to 1.88 |
| black | 1.02 | 0.92 to 1.10 | 1.33 | 0.96 to 1.84 |
| Diabetes | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 1.01 | 0.92 to 1.10 | 1.25b | 1.06 to 1.48 |
| Vintage | ||||
| ≤90 days | 1.00 | — | 1.00 | — |
| 91 to 365 days | 1.19c | 1.04 to 1.36 | 0.79 | 0.45 to 1.37 |
| >1 to 2 years | 0.71a | 0.61 to 0.84 | 0.71 | 0.41 to 1.22 |
| >2 years | 0.57a | 0.50 to 0.66 | 0.69 | 0.41 to 1.17 |
Reference group has odds ratio (OR) of 1.00, with factors that show OR >1.00 indicating greater likelihood for conversion. CI, confidence interval.
P < 0.001.
P < 0.01.
P = 0.01.
Figure 3.
Changes in access type (or maintaining the same access type during 4 months between January 1 and April 30, 2007) in all surviving patients (N = 70,852) and subsequent 1-year hospitalization risks from all causes (A); vascular access–related issues (B); and sepsis/bacteremia (C). The black line tracks the distribution of patients by vascular access category. Case mix includes age, gender, race, diabetes, and vintage, whereas lab includes eKt/V, albumin, hemoglobin, and phosphorus.
The corresponding risks for vascular access–related hospitalization are shown in Figure 3B and for hospitalizations as a result of sepsis or bacteremia in Figure 3C. Similar trends were observed in the latter two figures, summarized as follows: (1) Patients who had catheters converted to either graft or fistula had lower hospitalization risk, the magnitude of which increased progressively with vascular access–related hospitalization and even more with hospitalization from sepsis or bacteremia; (2) patients who underwent dialysis solely with grafts had similar risks for vascular access–related hospitalization as catheters but much lower risk for sepsis/bacteremia; and (3) conversion from arteriovenous access to catheters was associated with an even higher hospitalization risk than patients with a stable catheter. Specific HRs from the case mix– plus laboratory-adjusted models, including results from two sensitivity analyses, are shown in Table 3. The primary results (model 1) were shown to be robust, consistent even in the short term (within 8 months, represented by model 2) and persistent even with exclusion of patients who were hospitalized during the 4-month access conversion period, represented in model 3.
Table 3.
Case mix– and laboratory-adjusted HRs for hospitalization in patients who converted from catheters to either fistula or graft during a 4-month period, showing consistency among three Cox models with varying assumptions
| Hospitalization Event | HR | 95% CI |
|---|---|---|
| All causes | ||
| model 1 | 0.69 | 0.64 to 0.74 |
| model 2 | 0.67 | 0.63 to 0.73 |
| model 3 | 0.71 | 0.65 to 0.78 |
| Vascular access related | ||
| model 1 | 0.47 | 0.38 to 0.57 |
| model 2 | 0.43 | 0.34 to 0.55 |
| model 3 | 0.44 | 0.34 to 0.57 |
| Sepsis/bacteremia | ||
| model 1 | 0.31 | 0.22 to 0.43 |
| model 2 | 0.29 | 0.19 to 0.45 |
| model 3 | 0.27 | 0.18 to 0.43 |
Model 1: Primary analysis, 12 months of follow-up (n = 70,852); model 2: Short-term (8 months) follow-up (n = 70,852), consistent with previous report (11); model 3: Excluded patients who were hospitalized during the 4-month conversion period (n = 47,472). All models were adjusted for age, gender, race, diabetes, vintage, and baseline laboratory values: eKt/V, albumin, hemoglobin, and phosphorus. All P < 0.0001, compared with patients who continued dialysis via catheter as the reference group.
Discussion
This study confirms that catheters continue to be the access type with the greatest associated hospitalization risk, even when patients with dual accesses were included in this category, such as patients with coexisting maturing fistulas or grafts. Apart from hospitalization from all causes, catheters posed the greatest risk for both hospitalizations as a result of vascular access–related issues and sepsis or bacteremia. These results proved to be robust—consistent in both standard and time-dependent Cox models—supporting recommendations for minimizing or avoiding exposure to HD catheters, even as caregivers strive to increase the fistula use (4–8). Hospitalization risk from sepsis or bacteremia for grafts was closer to that of fistulas, with catheters exhibiting the highest risk, findings consistent with known associations between catheters and infections (27–30); however, the hospitalization risk associated with vascular access–related issues for grafts were closer to that of catheters—not surprising because grafts have been shown to have greater dysfunction rates and require more interventions relative to fistulas (31,32).
Conversion from a catheter to a graft or a fistula (observed only for 10.3% of catheter patients) was indeed associated with significantly lower subsequent hospitalization risk from all causes, including from vascular access–related issues and for sepsis or bacteremia, in this large cohort of prevalent patients. The difference in hospitalization risk was realized early (within 8 months) and persisted for up to 1 year. These results further support benefits associated with catheter removal in HD patients, beyond improved survival documented in the literature (9,10). We recently reported that patients who did not have a catheter access reported better quality of life (33). Catheters have also been associated with high C-reactive protein and inflammation even in the absence of overt infections (34), perhaps as a foreign body or because of biofilm (35,36). More important, within 6 months after conversion from catheters to fistulas in incident patients, a significant 82% reduction in C-reactive protein was observed relative to patients who remained on the catheter when fistulas failed to mature (37). Furthermore, others have shown that patients who converted from catheters to fistulas or grafts had subsequent improvement in biomarkers such as albumin, normalized protein catabolic rate, eKt/V, hemoglobin, and white blood cell count (37,38).
Thus, the impact of removing HD catheters may be greater than originally thought. It is a key actionable determinant of hospitalization and mortality in HD patients (25). Moreover, catheter-free HD may prove to be a lynchpin that leads to improvement in several aspects of dialysis care, including clinical quality goals such as adequacy of dialysis dosage, albumin, and hemoglobin (37,38). Because meeting multiple quality goals has been associated with cumulative improvement in hospitalization, mortality, and costs (23,24), programs to reduce and avoid catheter use should be a top priority in dialysis care. If we extrapolate expenses for the number of hospital days that are associated with patients who undergo dialysis on the catheter (Table 1), even at Medicare costs of approximately $1800 per hospital day (39), additional hospitalization costs could reach $28.4 million with 9.0 fewer hospital days per patient-year for fistulas or up to $18.3 million from 5.8 fewer hospital days per patient-year for grafts. Even converting only half of the catheter patients and achieving only half of the hospitalization gains in these patients may potentially save one quarter of such costs, amounting to $4 to $7 million in this cohort annually (approximately $15 to $25 million extrapolated to the entire United States); therefore, it is in the best interest of patients, nephrologists, health care providers, and payers that catheter exposure be minimized in this population. Importantly, further alignment of interests to include hospitals, access surgeons, and primary care physicians becomes necessary to remove extant barriers and close the loop. Recommendations that are designed to achieve this objective and improve care processes before dialysis and immediately upon admission to the dialysis facility have been recently reviewed (4,40).
This study has several limitations. First, it is an observational study, which is subject to biases that are inherent therein, and associations reported do not prove causation. Second, there remains residual confounding from unmeasured variables such as comorbidity (other than diabetes); medical therapy; and psychosocial, environmental, and economic factors. For example, underlying illness that may predispose to catheter use likely influenced the observed outcomes, evident when the observed hospitalization risk for patients who converted from arteriovenous accesses to catheters surpassed those for patients who underwent dialysis solely with catheters. A third limitation of the study was our inability to track outpatient vascular access procedures. These data represent potential missed complications that may have further increased morbidity differences between access types. Finally, the study did not address multiple hospitalizations, potentially underestimating the associations that we observed.
Nevertheless, a strong association exists between the type of vascular access and hospitalization risk for patients who are on maintenance HD—with fistulas having the lowest risk, catheters with the highest, and grafts in between, although the risks that are associated with grafts are much more favorable than those for catheters. Avoiding catheters altogether, particularly in predialysis patients who have chronic kidney disease and are under the care of nephrologists is ideal; however, few will argue against minimizing time at risk on catheters within the dialysis unit, when conversion of extant catheters to arteriovenous accesses, preferably fistulas, is associated not only with greater survival but also less hospitalization. Prospective studies are needed to test whether programs that reduce catheter use will decrease hospitalization risk for HD patients. In the meantime, the stakes remain high, and there is increasing evidence that implementing catheter avoidance and reduction programs will improve overall patient outcomes.
Disclosures
All authors are employees of Fresenius Medical Care, North America.
Acknowledgments
Part of this material was submitted as an abstract at the annual meeting of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA.
We thank Allison Baginski for ensuring that the quality of the figures in our manuscript meets the CJASN's requirements. We also thank the clinical staff of Fresenius Medical Services for diligently collecting information that forms the basis of studies such as these.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis 30: S150–S191, 1997 [PubMed] [Google Scholar]
- 2.III. NKF-K/DOQI clinical practice guidelines for vascular access: Update 2000. Am J Kidney Dis 37: S137–S181, 2001 [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations: 2006 updates—Vascular access. Am J Kidney Dis 48: S176–S307, 2006. 16813989 [Google Scholar]
- 4.Lacson E, Jr, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM: Balancing Fistula First with Catheters Last. Am J Kidney Dis 50: 379–395, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Neumann ME: Catheter use remains center of debate in success of Fistula First. Nephrol News Issues 22: 38: 40, 2008 [PubMed] [Google Scholar]
- 7.Sands JJ: Increasing AV fistulae and decreasing dialysis catheters: Two aspects of improving patient outcomes. Blood Purif 25: 99–102, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Robbin ML: Resolved: Fistulas are preferred to grafts as initial vascular access for dialysis—Con. J Am Soc Nephrol 19: 1632–1633, 2008 [PubMed] [Google Scholar]
- 9.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ: Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 47: 469–477, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Bradbury BD, Chen F, Furniss A, Pisoni RL, Keen M, Mapes D, Krishnan M: Conversion of vascular access type among incident hemodialysis patients: Description and association with mortality. Am J Kidney Dis 53: 804–814, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Lacson E, Jr, Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Tattersall JE, DeTakats D, Chamney P, Greenwood RN, Farrington K: The post-hemodialysis rebound: Predicting and quantifying its effect on Kt/V. Kidney Int 50: 2094–2102, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K: Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 42: 1000–1012, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Lee T, Barker J, Allon M: Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistula. J Am Soc Nephrol 18: 1936–1941, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH: The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 47: 415–421, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Metcalfe W, Khan IH, Prescott GJ, Simpson K, Macleod AM: Hospitalization in the first year of renal replacement therapy for end-stage renal disease. QJM 96: 899–909, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, Piera L, Bragg-Gresham JL, Feldman HI, Goodkin DA, Gillespie B, Wolfe RA, Held PJ, Port FK: Mortality and hospitalization in haemodialysis patients in five European countries: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 19: 108–120, 2004 [DOI] [PubMed] [Google Scholar]
- 21.O'Connor AS, Wish JB, Sehgal AR: The morbidity and cost implications of hemodialysis clinical performance measures. Hemodial Int 9: 349–361, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Nissenson AR, Dylan ML, Griffiths RI, Yu HT, Dean BB, Danese MD, Dubois RW: Clinical and economic outcomes of Staphylococcus aureus septicemia in ESRD patients receiving hemodialysis. Am J Kidney Dis 46: 301–308, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Rocco MV, Frankenfield DL, Hopson SD, McClellan WM: Relationship between clinical performance measures and outcomes among patients receiving long-term hemodialysis. Ann Intern Med 145: 512–519, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Plantinga LC, Fink NE, Jaar BG, Sadler JH, Levin NW, Coresh J, Klag MJ, Powe NR: Attainment of clinical performance targets and improvement in clinical outcomes and resource use in hemodialysis care: A prospective cohort study. BMC Health Serv Res 7: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Pankratz VS, de Andrade M, Therneau TM: Random-effects Cox proportional hazards model: General variance components methods for time-to-event data. Genet Epidemiol 28: 97–109, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Taylor GD, McKenzie M, Buchanan-Chell M, Caballo L, Chui L, Kowalewska-Grochowska K: Central venous catheters as a source of hemodialysis-related bacteremia. Infect Control Hosp Epidemiol 19: 643–646, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Hoen B, Paul-Dauphin A, Hestin D, Kessler M: EPIBACDIAL: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 9: 869–876, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Powe NR, Jaar B, Furth SL, Hermann J, Briggs W: Septicemia in dialysis patients: Incidence, risk factors, and prognosis. Kidney Int 55: 1081–1090, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Taylor G, Gravel D, Johnston L, Embil J, Holton D, Paton S: Incidence of bloodstream infection in multicenter inception cohorts of hemodialysis patients. Am J Infect Control 32: 155–160, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Huber TS, Carter JW, Carter RL, Seeger JM: Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: A systematic review. J Vasc Surg 38: 1005–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM: Superiority of autogenous arteriovenous hemodialysis access: Maintenance of function with fewer secondary interventions. Ann Vasc Surg 18: 66–73, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Lacson E, Jr, Xu J, Lin SF, Dean SG, Lazarus JM, Hakim R: Association between achievement of hemodialysis quality-of-care indicators and quality-of-life scores. Am J Kidney Dis 54: 1098–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Hung A, Pupim L, Yu C, Shintani A, Siew E, Ayus C, Hakim RM, Ikizler TA: Determinants of C-reactive protein in chronic hemodialysis patients: Relevance of dialysis catheter utilization. Hemodial Int 12: 236–243, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Passerini L, Lam K, Costerton JW, King EG: Biofilms on indwelling vascular catheters. Crit Care Med 20: 665–673, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Donlan RM, Costerton JW: Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15: 167–193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein SL, Ikizler TA, Zappitelli M, Silverstein DM, Ayus JC: Non-infected hemodialysis catheters are associated with increased inflammation compared to arteriovenous fistulas. Kidney Int 76: 1063–1069, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Wystrychowski G, Kitzler TM, Thijssen S, Usvyat L, Kotanko P, Levin NW: Impact of switch of vascular access type on key clinical and laboratory parameters in chronic haemodialysis patients. Nephrol Dial Transplant 24: 2194–2200, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Lacson E, Jr, Ikizler TA, Lazarus JM, Teng M, Hakim RM: Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 17: 363–371, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Hakim RM, Himmelfarb J: Hemodialysis access failure: A call to action—Revisited. Kidney Int 76: 1040–1048, 2009 [DOI] [PubMed] [Google Scholar]