Abstract
Background and objectives: Sarcoidosis is a multisystem disorder of unknown etiology. The outcome of renal transplantation on patients with sarcoidosis is not well known. A few case reports have described recurrence of sarcoidosis after transplant. Here, we report for the first time results and outcome of renal transplantation in a series of patients with sarcoidosis.
Design, setting, participants, & measurements: Eighteen patients with sarcoidosis who underwent renal transplantation were identified retrospectively in eight French renal transplantation departments. Patient medical charts, demographics, and the outcome of renal transplantation were reviewed.
Results: Initial renal disease was related to sarcoidosis in 10 patients. At the end of the follow-up (median, 42 months), patient and death-censored graft survival were 94.4% and the mean GFR was 60 ml/min per 1.73 m2. Five patients (27%) experienced recurrence of sarcoidosis including extra-renal involvement in two patients and renal involvement in three patients. Median GFR was lower in the group of patients with renal recurrence compared with that of the entire cohort: 31 ml/min per 1.73 m2. Recurrence occurred shortly after transplantation (median period, 13 months). Risk factors for recurrence included primary renal disease related to sarcoidosis and a shorter delay between the last episode of sarcoidosis and renal transplantation.
Conclusions: Our results indicate that renal transplantation may be carried out safely in transplant candidates with sarcoidosis. Recurrence is not rare and is likely to affect graft outcome. These results fully justify a specific clinical and histologic monitoring mainly during the early posttransplant period.
Sarcoidosis is a multisystem disorder of unknown etiology that generally occurs in young adults between the ages of 20 and 39 years (1). It is characterized by the presence of noncaseating granulomas in various organs, usually involving the respiratory tract (2). The highest annual incidence reported for sarcoidosis was 40 cases per 100,000 people in northern Europe (3).
Although sarcoidosis is benign in >50% of cases, it can also be severe, involving extrapulmonary sites, such as the heart, kidneys, central nervous system, liver, larynx, or eyes (4). Renal involvement in sarcoidosis is rare, but it is probably underestimated (5). The current prevalence of renal failure ranges from 0.7 to 4.3% (6–8). Renal disease is mainly related to disturbed calcium metabolism including hypercalciuria (40% of patients), hypercalcemia (11% of patients), and renal lithiasis (10% of patients). Granulomatous tubulointerstitial nephritis, a less common cause of renal impairment, is present in 7 to 27% of all patients in postmortem studies (9) and is associated with both acute and chronic renal failure. Corticosteroids remain the cornerstone of renal therapy, with a good success rate, but prolonged therapy is often necessary to preserve renal function and to delay the onset of ESRD (10–13).
End-stage organ disease that is secondary to sarcoidosis is uncommon (2,14). Sarcoidosis as the initial disease accounts for only a minority of organ transplantations. Thus, only 3% of lung transplants and <1% of heart and liver transplants involve sarcoidosis as the primary disease (2). Neither the incidence of graft rejection nor patient or graft survival appears to be different from the results observed in an overall population of similar organ recipients (15–19). By contrast, sarcoidosis recurrence does not appear to be uncommon, particularly after lung transplantation, with a recurrence rate close to 50% (16,20). Although there are case reports for patients that have undergone liver and heart transplantation, the sarcoidosis recurrence rate remains undetermined (21–25).
There is even less information on sarcoidosis recurrence in the field of renal transplantation. Only a few case reports describe renal sarcoidosis recurrence (26–30), and there is no available information on patient and graft outcome. Here, we describe the first series of 18 patients with sarcoidosis who underwent renal transplantation, including patient and graft outcomes, incidence, and potential risk factors of recurrence.
Patients and Methods
This multicenter retrospective study was conducted in eight French renal transplantation departments (Henri Mondor Hospital, AP-HP, Créteil; Necker-Enfants Malades Hospital, AP-HP, Paris; Rangueil Hospital, Toulouse; Strasbourg Hospital, Strasbourg; Pellegrin Hospital, Bordeaux; Hospices Civils de Lyon, Lyon; Bretonneau Hospital, Tours; Bichat Hospital, AP-HP, Paris).
Patient medical charts and demographics were retrospectively reviewed; information recorded included age, gender, history of sarcoidosis before transplantation, initial nephropathy, date of transplantation, donor source, panel reactive antibody levels, and postoperative immunosuppressive treatment. We examined the outcome of renal transplantation in these patients, including patient and graft survival, occurrence of posttransplant sarcoidosis recurrence, acute rejection episodes, cytomegalovirus infection or reactivation, causes of graft loss, and patient death. The GFR was estimated using the Modification of Diet in Renal Disease (MDRD) formula (31). Protocol biopsies were not performed routinely; they were performed according to the protocols of each transplant center and were available for six patients (patients #4, #8, #11, #12, #13, and #14).
Quantitative data are presented as means (SD) or medians (range) in cases of asymmetric distribution and were compared using the nonparametric Mann-Whitney test. Qualitative data are presented as percentages. Categorical data were compared using the χ2 test or the Fisher exact test when appropriate. A value of P < 0.05 was considered significant.
Results
Between 1992 and 2007, 18 sarcoidosis patients (12 men and 6 women; man/woman ratio = 2:1) underwent renal transplantation and were included in this multicenter retrospective study. All patients met the criteria for sarcoidosis, as defined by the American Thoracic Society, the European Respiratory Society, and the World Association of Sarcoidosis and Other Granulomatous Disorders in 1999 (32).
Clinical characteristics of sarcoidosis and the various treatments before transplantation are depicted in Table 1. Two patients had sarcoidosis limited to the thoracic area (stage II on chest radiographs). The 16 other patients had extra-thoracic sarcoidosis with or without thoracic sarcoidosis. Before renal transplantation, 15 patients received steroid therapy and 2 received additional immunosuppressive therapy (patients #7 and #11). The median time between the last sarcoidosis episode and renal transplantation was 78 (8 to 900) months. Treatments had been stopped for a median time of 54 (4 to 132) months before renal transplantation in all patients except three who remained on immunosuppressive therapy at the time of transplantation (patients #7, #9, and #11): mycophenolate mofetil in one patient, azathioprine in one patient, and steroids in one patient. No patient exhibited clinical or biologic signs of active sarcoidosis at the time of transplantation.
Table 1.
Features of sarcoidosis before renal transplantation
| Patient | Age (years) | Gender | Ethnic Origin | Renal Involvement | Extra-Renal Involvement | Biopsy | Treatment |
Relapse | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | IS | Duration (months) | ||||||||
| 1 | 22 | M | Caucasian | + | − | + | − | − | + | |
| 2 | 27 | M | Caucasian | + | Lung, neuromuscular | + | + | − | NA/12 | + |
| 3 | 47 | M | Caucasian | + | Lung | + | + | − | 60/60 | + |
| 4 | 18 | F | North-African | + | Liver | + | + | − | 12/18 | + |
| 5 | 37 | M | African/Native Caribbean | + | Lung, liver | + | + | − | 2/66 | + |
| 6 | 25 | M | Caucasian | + | Hilar lymphadenopathy | + | + | − | 3 | − |
| 7 | 39 | M | North-African | + | Uveitis, lung | + | + | Mycophenolate mofetil | 40 | − |
| 8 | 48 | F | North-African | + | Lung | + | + | − | 60 | − |
| 9 | 18 | F | North-African | + | − | + | + | − | NA | NA |
| 10 | 28 | M | African/Native Caribbean | + | Lung, lymphadenopathy | + | + | − | NA | − |
| 11 | 42 | F | African/Native Caribbean | − | Lung, liver | + | + | Cyclophosphamide azathioprine | 14/8 | + |
| 12 | 45 | F | Caucasian | − | Lung, articular | + | + | − | 12 | − |
| 13 | 28 | F | North−African | − | Lung, skin | + | − | − | − | + |
| 14 | 42 | M | African/Native Caribbean | − | Lung, liver | + | + | − | 0/72 | + |
| 15 | 24 | M | African/Native Caribbean | − | Lung | − | − | − | − | − |
| 16 | 33 | M | Caucasian | − | Lung | − | − | − | − | − |
| 17 | 32 | M | Caucasian | − | Lung, liver, uveitis | + | + | − | 24 | − |
| 18 | 53 | M | Caucasian | − | Lung, liver | + | + | − | 60 | − |
CT, corticosteroids; F, female; IS, immunosuppressors; M, male; NA, not available; +, yes; −, no.
Characteristics of patients at the time of transplantation and graft outcomes are depicted in Table 2 Initial renal disease was related to renal sarcoidosis in 10 of the 18 patients and was attributed to other causes in 8 patients. All cases of renal sarcoidosis were documented by histologic analysis, which showed granulomatous interstitial nephritis in all cases. The mean age at transplantation was 43.5 (±11) years. Three patients received a second transplant (patients #11, #16, and #18) and one patient received simultaneous kidney and pancreas transplants (patient #13). All grafts except one were from deceased donors. The mean donor age was 36.5 (±15) years. The mean cold ischemia time was 16.6 (±8) hours. Delayed graft function was observed in three patients. Eleven patients received induction therapy, which included rabbit anti-thymocyte globulin in four patients (patients #7, #8, #9, and #13) and IL-2 receptor antagonists in seven patients (patients #3, #4, #5, #10, #12, #14, and #17). Maintenance immunosuppression included calcineurin inhibitors (CNI) for all patients, mycophenolate mofetil or azathioprin for 16 patients, sirolimus for 2, and corticosteroids for 16 patients (all patients except #3 and #17). In three patients (patients #2, #4, and #13), steroids were withdrawn during the first year after transplantation.
Table 2.
Patient characteristics at renal transplantation and outcome after transplantation
| No. | Age (years) | Initial Nephropathy | Donor Age (years) | PRA | Cold Ischemia Time | DGF | IS Regimen | Acute Rejection | Sarcoidosis Recurrence | Follow-up Duration (months) | End of Follow-up |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GFR (ml/min per 1.73 m2) | Proteinuria (g/day) | |||||||||||
| 1 | 50 | Sarcoidosis | 26 | 0 | 10 hours | − | CNI/SRL/CT | − | − | 86 | 85 | 0 |
| 2 | 46 | Sarcoidosis | 18 | 0 | 20 hours, 30 minutes | − | CNI/AZA/CT | + | − | 84 | 103 | 0 |
| 3 | 60 | Sarcoidosis | 46 | 0 | 10 hours, 50 minutes | − | CNI/MMF | − | − | 79 | 31 | 0.5 |
| 4 | 26 | Sarcoidosis | 38 | 0 | 24 hours | + | CNI/MMF/CT | − | − | 44 | 53 | 0 |
| 5 | 50 | Sarcoidosis | 61 | 0 | 22 hours, 35 minutes | − | CNI/MMF/CT | − | + | 21 | 90 | 3 |
| 6 | 27 | Sarcoidosis | 44 | 0 | 14 hours | − | CNI/MMF/CT | − | − | 60 | 67 | 0.5 |
| 7 | 43 | Sarcoidosis | 16 | 0 | 20 hours | − | CNI/MMF/CT | − | − | 25 | 66 | 0 |
| 8 | 52 | Sarcoidosis | 52 | 0 | 14 hours | − | CNI/MMF/CT | − | + | 18 | 31 | 0.5 |
| 9 | 21 | Sarcoidosis | 18 | 0 | 20 hours | − | CNI/AZA/CT | − | + | 196 | 22 | 0.7 |
| 10 | 32 | Sarcoidosis | 30 | 0 | 2 hours | − | CNI/MMF/CT | + | + | 36 | 29 | 0 |
| 11 | 44 | Renal agenesis | 29 | 0 | 33 hours, 30 minutes | + | CNI/MMF/CT | − | + | 67 | 86 | 0 |
| 12 | 53 | Focal segmental glomerulosclerosis | 54 | 0 | 21 hours | + | CNI/MMF/CT | − | − | 40 | 31 | 0.26 |
| 13 | 40 | Diabetic nephropathy | 17 | 0 | 8 hours | − | CNI/MMF/CT | + | − | 32 | 86 | 0 |
| 14 | 55 | Undetermined | 55 | 0 | 16 hours, 45 minutes | − | CNI/MMF/CT | − | − | 38 | 58 | 0.6 |
| 15 | 39 | AA amyloidosis | 25 | 0 | 20 hours | − | CNI/MMF/CT | − | − | 108 | 74 | 1.2 |
| 16 | 44 | Alport syndrome | 28 | 0 | 24 hours | − | CNI/MMF/CT | − | − | 72 | 76 | 0 |
| 17 | 44 | Polycystic kidney disease | 45 | 0 | 14 hours, 45 minutes | − | CNI/MMF | − | − | 24 | 60 | 0 |
| 18 | 57 | Polycystic kidney disease | 55 | 30% | 12 hours | − | CNI/SRL/CT | − | − | 24 | 32 | 0 |
AZA, azathioprine; CNI, calcineurin inhibitors; CT, corticosteroids; DGF, delayed graft function; IS, immunosuppressor; MMF, mycophenolate mofetil; PRA, panel reactive antibody; SRL, sirolimus; +, yes; −, no.
The median follow-up after transplantation was 42 (18 to 196) months. At last follow-up, patient survival and death-censored graft survival were 94.4%, and the mean GFR was 60 (±25) ml/min per 1.73 m2. Only one patient died from pulmonary embolism 196 months after transplantation. Three patients (16%) exhibited acute graft rejection, which was treated with methylprednisolone pulses in all cases; one steroid-refractory patient was also treated with orthoclone OKT3. Cytomegalovirus infection occurred in two patients (11%). Patient and graft survivals, median GFR, and incidence of acute rejection were not statistically different among patients with sarcoidosis as the primary cause of ESRD (n = 10) and those with other causes (n = 8).
A recurrence of sarcoidosis after renal transplantation was observed in five patients (27%) (patients #5, #8, #9, #10, and #11). A detailed analysis of each case of recurrence is depicted in Table 3. Three were of black African or Native Caribbean descent and two were of North-African descent. The median period between last sarcoidosis episode and renal transplantation was 42 (8 to 96) months. Two of these patients remained on immunosuppressive therapy for sarcoidosis at the time of transplantation (patients #9 and #11), one received steroids for severe asthma, and the other received azathioprine for hepatic sarcoidosis. The median period between renal transplantation and recurrence was 13 (7 to 192) months and four of five patients exhibited a recurrence during the first 18 months after renal transplantation. Renal sarcoidosis was the primary renal disease in four of five of these patients. Clinical recurrence involved a renal allograft in two patients, extra-renal organs in two patients, and both renal and extra-renal organs in one patient.
Table 3.
Sarcoidosis recurrence after renal transplantation
| No. | Initial Nephropathy | Delay between Last Relapse and RT (months) | Delay between Treatment Cessation and RT (months) | Delay between RT and Recurrence (months) | Organ Involvement | Renal Presentation | Treatment | Evolution | Follow-up Duration (months) | GFR at Last Follow-up (ml/min per 1.73 m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | Sarcoidosis | 96 | 29 | 12 | Kidney | Nephrotic proteinuria and acute renal failure | CT increase | GFR improvement | 21 | 90 |
| 8 | Sarcoidosis | 60 | 0 | 7 | Kidney | None (protocol renal biopsy) | CT increase | Chronic renal failure | 18 | 31 |
| 9 | Sarcoidosis | 24 | NA | 192 | Lung, kidney | Acute renal failure | CT increase (pulses) | Death: pulmonary embolism | 196 | 22 |
| 10 | Sarcoidosis | NA | NA | 13 | Neurologic | No recurrence | 36 | 29 | ||
| 11 | Renal agenesis | 8 | 0 | 18 | Lung, liver, skin | CT increase and switch MMF to AZA | CT dependent (20 mg/d) | 67 | 86 |
AZA, azathioprine; CT, corticosteroids; MMF, mycophenolate mofetil; NA, not available; RT, renal transplantation.
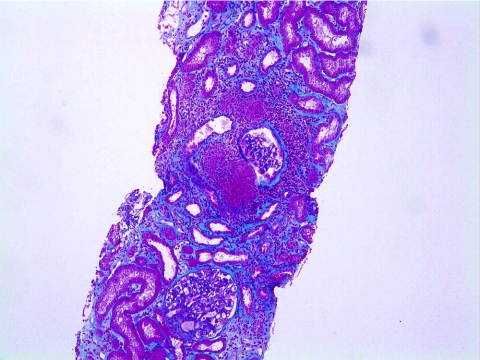
Patients characteristics at the time of renal recurrence of sarcoidosis are shown in Table 3. Two patients had acute renal failure (patients #5 and #9), which was associated with heavy proteinuria and leucocyturia in one case (patient #5). Recurrence in one patient was detected in a protocol biopsy sample performed 6 months after transplantation (patient #8). Sarcoidosis recurrence in renal allografts manifested itself as tubulo-interstitial nephritis associated with noncaseating granulomas in all patients (Figure 1). At the time of recurrence, all five patients received maintenance steroid therapy (5 mg of prednisone per day). Treatment for recurrence was limited to an increase in corticosteroid therapy in all patients between 0.3 and 0.5 mg/kg per day and patients were maintained at this dosage for 1 month. The steroid taper schedule was progressive: 5 mg/d per month. Clinical outcomes after recurrence included one fatality (patient #9) attributed to a pulmonary embolism occurring 1 month after receiving therapy. The renal outcome in patient #5 was favorable, with a return of renal function to baseline levels. A control renal biopsy performed 18 months after recurrence showed a complete disappearance of the granulomatous lesions. Patient #8 had no improvement in renal function and histologic analysis performed 6 months after recurrence showed persisting granulomas and tubulo-interstitial nephritis. The median GFR was 31 (22 to 90) ml/min per 1.73 m2 in patients with renal recurrence n = 3 versus n = 60 (±25) ml/min per 1.73 m2 for the entire cohort. Finally, in patient #11, multiple relapses involving the skin occurred despite steroid treatment and he became corticodependent (20 mg of oral prednisone per day).
Figure 1.
Recurrence of renal sarcoidosis in patient #5 including tubulo-interstitial nephritis associated with noncaseating granuloma (trichrome of Masson [magnification, ×200]).
Discussion
We report here the first series of 18 patients with sarcoidosis who underwent renal transplantation. Our findings indicate that renal transplantation can be carried out safely in patients with sarcoidosis with excellent graft and patient survival (94%), after a median follow-up of 4 years. These findings are observed in renal transplant recipients in which primary renal disease is related and unrelated to sarcoidosis; these findings are also consistent with a previous report on liver and lung transplantation showing similar graft and patient survival when compared with control groups (15,16,33).
Importantly, we also found a high incidence of sarcoidosis recurrence after transplantation (27%). This had previously been reported in the context of lung transplantation, with a recurrence rate of up to 47% (16). Because all patients did not undergo systematic protocol posttransplant biopsies, some cases of “subclinical” recurrences might have been missed; thus, the incidence of recurrence might have been underestimated. This has been observed in other diseases with a propensity for renal recurrence after transplant, such as systemic lupus erythematosus or IgA nephropathy (34,35). Recurrences involved the same organ in four of five patients and included renal involvement in three patients and lung and liver in one patient. Sarcoidosis recurrence had no clear detrimental effect on both graft and patient survival, as previously shown in the context of other organ transplants (15,16,25). However, graft function appeared to be influenced by renal sarcoidosis recurrence, as the median GFR at the end of the follow-up was significantly lower in the three patients with recurrence than that for the entire cohort (31 versus 60 (±25) ml/min per 1.73 m2, respectively). Thus, whether recurrence affects long-term graft survival or not remains to be established.
The next main question was to determine the risk factors for recurrence. Analysis of our recurrent cases clearly showed that (1) recurrence occurred shortly after transplantation (during the first 18 months after transplantation in four of five patients), (2) primary renal disease related to sarcoidosis was strongly associated with recurrence (40% in the group with renal sarcoidosis versus 12.5% in the group with another primary nephropathy), and (3) the median period between the last episode of sarcoidosis and renal transplantation was shorter in the case of sarcoidosis recurrence (42 versus 78 months, respectively). No risk factors for recurrence have previously been reported in series from lung, cardiac, or liver transplantation (15,16,25,36). An analysis of previous case reports of sarcoidosis recurrence occurring after renal transplantation showed that primary renal disease was related to interstitial granulomatous nephritis in all patients (26–30). Our findings are consistent with these results and suggest that patients with initial renal involvement display a specific sensitivity to renal recurrence. Reasons for this finding remain to be determined, as correlations between environmental, genetic, or immune conditions and clinical phenotype have yet to be established in patients with sarcoidosis (2,5). The incidence of recurrence was also significant, as all patients were maintained on triple immunosuppressive therapy, including steroids and mycophenolate mofetil, at the time of relapse. Steroids have been effectively used as a first-line therapy for sarcoidosis, first relapse, or renal graft recurrence (5,10,26–30). Also, mycophenolate mofetil has been used as steroid-sparing agent in cases of steroid-dependent sarcoidosis (37–41). Sarcoidosis is associated with an increase in peripheral regulatory T lymphocytes (Treg) cells, accounting for the anergic state usually observed in these patients; however, these levels are still unable to control local inflammation and granuloma formation (42). Moreover, it has recently been shown that Treg may participate in the early stages of granuloma formation (43). Although CNI are likely to affect both Treg expansion and function both in vivo and in vitro (44), their ability to promote sarcoidosis recurrence is unlikely because all transplant recipients received CNI-based immunosuppresive regimen. Similarly, the role of induction therapy remains confusing because two patients with recurrence receive rabbit anti-thymocyte globulin and three IL-2 receptor antagonists. Finally, and in absence of Treg monitoring during the course of transplantation including recurrence, involvement of these T cell subsets in the recurrence process is only speculative.
Conclusions
In summary, our findings indicate that renal transplantation may be carried out safely in renal transplant candidates with sarcoidosis. However, the high incidence of recurrence observed in patients with primary renal disease related to sarcoidosis and the effect of sarcoidosis recurrence on graft function warrant specific clinical and histologic monitoring, to detect recurrences that mostly occur during the early period after transplantation. To establish whether shorter periods between the last episode of sarcoidosis and renal transplantation in patients experiencing recurrence should have an implication on renal transplant candidate management, a study with a larger cohort is required.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rybicki BA, Major M, Popovich J, Jr., Maliarik MJ, Iannuzzi MC: Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol 145: 234–241, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Rybicki BA, Teirstein AS: Sarcoidosis. N Engl J Med 357: 2153–2165, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Pietinalho A, Hiraga Y, Hosoda Y, Lofroos AB, Yamaguchi M, Selroos O: The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis 12: 61–67, 1995 [PubMed] [Google Scholar]
- 4.Newman LS, Rose CS, Maier LA: Sarcoidosis. N Engl J Med 336: 1224–1234, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Mahevas M, Lescure FX, Boffa JJ, Delastour V, Belenfant X, Chapelon C, Cordonnier C, Makdassi R, Piette JC, Naccache JM, Cadranel J, Duhaut P, Choukroun G, Ducroix JP, Valeyre D: Renal sarcoidosis: Clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 88: 98–106, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Chapelon C, Uzzan B, Piette JC, Jacques C, Coche E, Godeau P: Sarcoidosis in internal medicine. A cooperative study of 554 cases. Ann Med Interne (Paris) 135: 125–131, 1984 [PubMed] [Google Scholar]
- 7.Mayock RL, Bertrand P, Morrison CE, Scott JH: Manifestations of sarcoidosis. Analysis of 145 patients, with a review of nine series selected from the literature. Am J Med 35: 67–89, 1963 [DOI] [PubMed] [Google Scholar]
- 8.Wirnsberger RM, de Vries J, Wouters EF, Drent M: Clinical presentation of sarcoidosis in The Netherlands an epidemiological study. Neth J Med 53: 53–60, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Longcope WT, Freiman DG: A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltimore) 31: 1–132, 1952 [PubMed] [Google Scholar]
- 10.Rajakariar R, Sharples EJ, Raftery MJ, Sheaff M, Yaqoob MM: Sarcoid tubulo-interstitial nephritis: Long-term outcome and response to corticosteroid therapy. Kidney Int 70: 165–169, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Hannedouche T, Grateau G, Noel LH, Godin M, Fillastre JP, Grunfeld JP, Jungers P: Renal granulomatous sarcoidosis: Report of six cases. Nephrol Dial Transplant 5: 18–24, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Robson MG, Banerjee D, Hopster D, Cairns HS: Seven cases of granulomatous interstitial nephritis in the absence of extrarenal sarcoid. Nephrol Dial Transplant 18: 280–284, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Brause M, Magnusson K, Degenhardt S, Helmchen U, Grabensee B: Renal involvement in sarcoidosis–a report of 6 cases. Clin Nephrol 57: 142–148, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Bergner R, Hoffmann M, Waldherr R, Uppenkamp M: Frequency of kidney disease in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 20: 126–132, 2003 [PubMed] [Google Scholar]
- 15.Casavilla FA, Gordon R, Wright HI, Gavaler JS, Starzl TE, Van Thiel DH: Clinical course after liver transplantation in patients with sarcoidosis. Ann Intern Med 118: 865–866, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padilla ML, Schilero GJ, Teirstein AS: Sarcoidosis and transplantation. Sarcoidosis Vasc Diffuse Lung Dis 14: 16–22, 1997 [PubMed] [Google Scholar]
- 17.Zaidi AR, Zaidi A, Vaitkus PT: Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplant 26: 714–717, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Arcasoy SM, Christie JD, Pochettino A, Rosengard BR, Blumenthal NP, Bavaria JE, Kotloff RM: Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest 120: 873–880, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Shorr AF, Davies DB, Nathan SD: Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest 124: 922–928, 2003 [PubMed] [Google Scholar]
- 20.Muller C, Briegel J, Haller M, Vogelmeier C, Bittman I, Welz A, Fürst H, Dienemann HThe Munich Lung Transplant Group: Sarcoidosis recurrence following lung transplantation. Transplantation 61: 1117–1119, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Cengiz C, Rodriguez-Davalos M, deBoccardo G, Fiel MI, Rodriguez-Laiz G, Kovacevic M, Emre S, Schiano T: Recurrent hepatic sarcoidosis post-liver transplantation manifesting with severe hypercalcemia: A case report and review of the literature. Liver Transpl 11: 1611–1614, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Fidler HM, Hadziyannis SJ, Dhillon AP, Sherlock S, Burroughs AK: Recurrent hepatic sarcoidosis following liver transplantation. Transplant Proc 29: 2509–2510, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Hunt J, Gordon FD, Jenkins RL, Lewis WD, Khettry U: Sarcoidosis with selective involvement of a second liver allograft: Report of a case and review of the literature. Mod Pathol 12: 325–328, 1999 [PubMed] [Google Scholar]
- 24.Pescovitz MD, Jones HM, Cummings OW, Lumeng L, Leapman SB, Filo RS: Diffuse retroperitoneal lymphadenopathy following liver transplantation–a case of recurrent sarcoidosis. Transplantation 60: 393–396, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Milman N, Andersen CB, Mortensen SA, Sander K: Cardiac sarcoidosis and heart transplantation: A report of four consecutive patients. Sarcoidosis Vasc Diffuse Lung Dis 25: 51–59, 2008 [PubMed] [Google Scholar]
- 26.Shen SY, Hall-Craggs M, Posner JN, Shabazz B: Recurrent sarcoid granulomatous nephritis and reactive tuberculin skin test in a renal transplant recipient. Am J Med 80: 699–702, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Kukura S, Viklicky O, Lacha J, Voska L, Honsova E, Teplan V: Recurrence of sarcoidosis in renal allograft during pregnancy. Nephrol Dial Transplant 19: 1640–1642, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Beaufils H, Gompel A, Gubler MC, Lucsko M, Guedon J: Pre- and posttransplant glomerulonephritis in a case of sarcoidosis. Nephron 35: 124–129, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Vargas F, Gedalia A, Craver RD, Matti Vehaskari V: Recurrence of granulomatous interstitial nephritis in transplanted kidney. Pediatr Transplant 28, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Brown JH, Jos V, Newstead CG, Lawler W: Sarcoid-like granulomata in a renal transplant. Nephrol Dial Transplant 7: 173, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160: 736–755, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Wille KM, Gaggar A, Hajari AS, Leon KJ, Barney JB, Smith KH, Pajaro O, Wang W, Oster RA, McGiffin DC, Young KR: Bronchiolitis obliterans syndrome and survival following lung transplantation for patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 25: 117–124, 2008 [PubMed] [Google Scholar]
- 34.Goral S, Ynares C, Shappell SB, Snyder S, Feurer ID, Kazancioglu R, Fogo AB, Helderman JH: Recurrent lupus nephritis in renal transplant recipients revisited: It is not rare. Transplantation 75: 651–656, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Odum J, Peh CA, Clarkson AR, Bannister KM, Seymour AE, Gillis D, Thomas AC, Mathew TH, Woodroffe AJ: Recurrent mesangial IgA nephritis following renal transplantation. Nephrol Dial Transplant 9: 309–312, 1994 [PubMed] [Google Scholar]
- 36.Shah L: Lung transplantation in sarcoidosis. Semin Respir Crit Care Med 28: 134–140, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Bhat P, Cervantes-Castaneda RA, Doctor PP, Anzaar F, Foster CS: Mycophenolate mofetil therapy for sarcoidosis-associated uveitis. Ocul Immunol Inflamm 17: 185–190, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Kouba DJ, Mimouni D, Rencic A, Nousari HC: Mycophenolate mofetil may serve as a steroid-sparing agent for sarcoidosis. Br J Dermatol 148: 147–148, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Moravan M, Segal BM: Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology 72: 337–340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moudgil A, Przygodzki RM, Kher KK: Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 21: 281–285, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Hobbs DJ, Barletta GM, Chung JY, Bunchman TE: Isolated sarcoid granulomatous interstitial nephritis in pediatrics: A case report and review of literature. Clin Nephrol 72: 410–413, 2009 [PubMed] [Google Scholar]
- 42.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Kambouchner M, Valeyre D, Chapelon-Abric C, Debré P, Piette JC, Gorochov G: The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 203: 359–370, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taflin C, Miyara M, Nochy D, Valeyre D, Naccache JM, Altare F, Salek-Peyron P, Badoual C, Bruneval P, Haroche J, Mathian A, Amoura Z, Hill G, Gorochov G: FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol 174: 497–508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS: Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108: 390–399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]