Abstract
Background and objectives: Serum levels of galactose-deficient IgA1 (Gd-IgA1) are elevated and heritable in Caucasian and Asian patients with IgA nephropathy (IgAN), but have not been characterized in African Americans (AA). Our objective was to determine whether serum Gd-IgA1 levels are increased in AA patients with IgAN and whether this is a heritable trait in this group.
Design, setting, participants, & measurements: Blood and urine samples were obtained from 18 adult and 11 pediatric AA patients with biopsy-proven IgAN and from 34 of their first-degree relatives. Healthy controls included 150 Caucasian adults, 65 AA adults, 45 Caucasian children, and 49 AA children. Serum total IgA and Gd-IgA1 levels were measured in patients and controls. Significant differences between patient and control groups for serum total IgA, Gd-IgA1, and ratio of Gd-IgA1/total IgA were determined by the Mann-Whitney U test. Heritability was calculated using SOLAR.
Results: After stratifying by age, 7 of 11 pediatric and 9 of 18 adult AA patients with IgAN had serum Gd-IgA1 levels above the 95th percentile for age-appropriate AA controls. For first-degree relatives, the serum Gd-IgA1 level was >95th percentile for 1 of 8 when the patient's level was <95th percentile and 12 of 26 when the patient's level was >95th percentile (P = 0.116, Fisher exact test). Heritability was 0.74 (P = 0.007).
Conclusions: Serum levels of Gd-IgA1 are often elevated in AA patients with IgAN and their first-degree relatives. Thus, aberrant IgA1 glycosylation is a heritable risk factor for IgAN in African Americans.
IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide (1). Nonetheless, the condition is rarely diagnosed in sub-Saharan Africans (2,3) and, on the basis of biopsy series, is uncommon in some African-American (AA) cohorts (4–7). Population-based data from central and eastern Kentucky, however, have shown an incidence for IgAN in AA adults similar to that in Caucasians adults (8), raising the possibility that, in some regions of the United States, IgAN may be underdiagnosed in AAs. A lower incidence of IgAN in AA patients could be due to factors such as the lack of early detection because of infrequent testing by urinalysis, delayed referral to nephrology, and decreased likelihood of renal biopsy.
The pathogenesis of IgAN is related to aberrant glycosylation of O-linked glycans in the hinge region of IgA1. Rather than terminating with galactose, the aberrant glycans end with N-acetylgalactosamine (GalNAc). This GalNAc moiety, in turn, is recognized by anti-glycan antibodies (9), leading to formation of nephritogenic circulating immune complexes that subsequently deposit in the glomerular mesangium to induce renal injury (10). In vitro studies have shown that these complexes stimulate cultured mesangial cells to proliferate and secrete extracellular-matrix proteins, whereas uncomplexed galactose-deficient IgA1 (Gd-IgA1) or galactose-replete IgA1 does not (11).
Serum Gd-IgA1 levels are elevated in Caucasian and Asian patients with IgAN (12–14). Elevated serum Gd-IgA1 levels have also been found to be heritable in a dominant pattern for Caucasian and Chinese patients, although most affected relatives have no clinical manifestation of IgAN (12,15). The purpose of this study was to determine whether the serum levels of Gd-IgA1 in AA patients are increased and are heritable, as is the case for Caucasian and Asian patients with IgAN.
Materials and Methods
Patients
The diagnosis of IgAN requires renal biopsy showing IgA as the dominant or co-dominant Ig in a typical mesangial distribution in the absence of clinical and laboratory evidence for systemic disease (16). Patients with IgAN included 18 AA (8 men, 10 women) adults ≥18 years of age at time of initial diagnostic biopsy and 11 AA (6 boys, 5 girls) children <18 years of age at time of diagnostic biopsy. Patients who had received a kidney transplant or who required dialysis were excluded from the study. This study also included 34 first-degree relatives of 20 patients with IgAN. Both parents were studied for four families.
Healthy adult controls were ≥18 years of age and included 150 Caucasians (74 men, 76 women) and 65 AAs (21 men, 44 women). Healthy pediatric (<18 years of age) controls included 45 Caucasian children (26 boys, 19 girls) and 49 AA children (29 boys, 20 girls). The healthy controls resided in Alabama, Kentucky, or Tennessee.
The study was approved by the Institutional Review Boards of the University of Tennessee Health Science Center and the University of Alabama at Birmingham. All patients or their parents/guardians provided written informed consent. Signed assent was obtained from all patients aged 8 to 18 years.
Clinical and Laboratory Measures and Analysis
Blood samples were collected from patients and controls on one occasion for determination of total serum IgA and Gd-IgA1. Serum creatinine and spot urinary protein/creatinine ratios were measured for patients and adult controls, but not for healthy pediatric controls. Urinalysis for blood and protein determination was performed for patients and all controls using Bayer (Frankfurt, Germany) Multistix reagent test strips. All healthy controls had urines that tested negative for blood and protein. Estimated GFR was calculated with the four-variable Modification of Diet in Renal Disease (MDRD) formula (17) for adult patients and the Schwartz formula (18) for pediatric patients.
Serum total IgA and Gd-IgA1 levels were determined by ELISA, as described previously (13). The Gd-IgA1 ELISA used biotinylated Helix aspersa lectin (Sigma-Aldrich, St. Louis, MO) that binds to GalNAc. Results for levels of Gd-IgA1 were expressed as U/ml serum, with 1 U (unit) being considered 1 μg of a galactose-deficient IgA1 myeloma protein (Ale) used as the standard in this assay.
Statistical Analyses
The Mann Whitney U test was used to compare patient and control groups for serum concentrations of total IgA and Gd-IgA1, and percentage of Gd-IgA1 of total IgA (SAS v9.1, Cary, NC).
Heritabilities were calculated using SOLAR (19). Values were log-transformed to achieve a normal distribution. Heritabilities were modeled using age, sex, body mass index (BMI), and IgAN status as covariates.
Results
For the adult patients, estimated GFR was ≥90 ml/min per 1.73 m2 for four patients, ≥60 but <90 ml/min per 1.73 m2 for three patients, ≥30 but <60 ml/min per 1.73 m2 for five patients, and <30 ml/min per 1.73 m2 for six patients. For the pediatric patients, estimated GFR was ≥90 ml/min per 1.73 m2 for nine patients and ≥60 but <90 ml/min per 1.73 m2 for two patients.
The age (mean and range) of the AA adult controls in this study did not differ from that of our previously published Caucasian adult controls (Table 1). For the adult control groups, there was an equal gender distribution in the Caucasian group (51% female) as compared with a female preponderance (68%) in the AA group.
Table 1.
Demographic characteristics and serum total IgA, Gd-IgA1, and percentage of Gd-IgA1 of total IgA for control groups
| Caucasian Adult (n = 150) | AA Adult (n = 65) | Caucasian Pediatric (n = 45) | AA Pediatric (n = 49) | |
|---|---|---|---|---|
| Women/girls, n (%) | 76 (50.7) | 44 (67.7) | 19 (42.2) | 20 (40.8) |
| Age, years, mean, SD (range) | 36.4 ± 12.9 (18.1 to 80.9) | 35.5 ± 10.7 (18.1 to 55.6) | 12.7 ± 3.3 (7.3 to 17.9) | 12.3 ± 2.8 (6.8 to 17.6) |
| Total IgA, mg/ml, median (range) | 2813 (886 to 8185) | 2789 (659 to 5244) | 1524 (511 to 3593) | 1736 (949 to 3372) |
| 95th percentile | 5230 | 4988 | 3030 | 3012 |
| Gd-IgA1, U/ml, median (range) | 615 (264 to 1807) | 486 (122 to 1541) | 287 (109 to 1009) | 251 (81 to 666) |
| 95th percentile | 1145 | 1010 | 954 | 478 |
| % Gd-IgA1 of total IgA, median (range) | 24.8 (5.6 to 66.5) | 17.1 (5.9 to 41.6) | 19.9 (6.7 to 48.8) | 14.4 (2.8 to 31.5) |
| 95th percentile | 45.0 | 39.7 | 43.1 | 30.7 |
Serum levels for total IgA and Gd-IgA1 and percentage Gd-IgA1 of total IgA are shown for the patient and control groups in Table 1. As described previously for Caucasian adult controls (13), serum Gd-IgA1 levels in AA controls were not normally distributed. For adult controls, serum total IgA level did not significantly differ between AA and Caucasian patients. However, serum Gd-IgA1 levels were significantly lower for the AA controls as compared with those for the Caucasian controls (P < 0.0001). The percentage of total IgA comprised of Gd-IgA1 was lower for AA adult controls than for Caucasian adult controls, with a median of 17.1 and 24.8%, respectively (P < 0.0001), but higher for AA pediatric controls than for Caucasian pediatric controls, with a median of 19.9 and 14.4%, respectively (P = 0.0001). Because of these racial differences for serum Gd-IgA1 levels, we used the 95th percentile value in ethnicity-matched controls for definition of an elevated level in patients with IgAN. Serum levels for total IgA and Gd-IgA1 did not differ on the basis of sex within the Caucasian and AA control groups.
Median serum Gd-IgA1 and total IgA levels were significantly lower for the pediatric control group as compared with levels in the adult control group. Thus, we developed separate normal ranges for patients <18 years of age and ≥18 years of age for serum Gd-IgA1 levels. The 95th percentile for AA pediatric controls was 478 U/ml, substantially lower than the corresponding level of 1010 U/ml for adult AA controls. The mean age for the adult AA patients of 42.4 years was older than that of the AA adult controls (35.5 years, P = 0.044). For the AA children, the mean age for patients and controls was similar at 12.0 and 12.3 years, respectively.
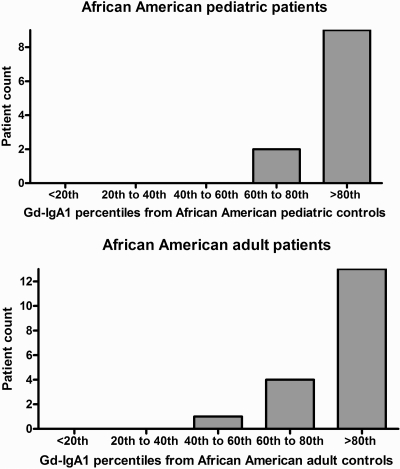
Frequency distributions by quintile derived for pediatric and adult controls showed that 82% of the pediatric patients and 68% of adult patients had serum Gd-IgA1 levels in the highest (80 to 100%) quintile (Figure 1). The median serum Gd-IgA1 level of 1059 U/ml for the 18 AA adults with IgAN was significantly higher than that for the AA adult controls (P < 0.0001; Table 1). Median serum total IgA level was 3966 μg/ml for AA adult patients as compared with 2789 μg/ml for AA adult controls (P = 0.057). The median serum Gd-IgA1 level of 749 U/ml for the 11 AA pediatric patients with IgAN was significantly higher than that for the AA pediatric controls (P < 0.0001). Median serum total IgA level was significantly higher for AA pediatric patients as compared with AA pediatric controls (2526 versus 1736 μg/ml, P = 0.038).
Figure 1.
Frequency distributions by quintile derived for pediatric and adult controls.
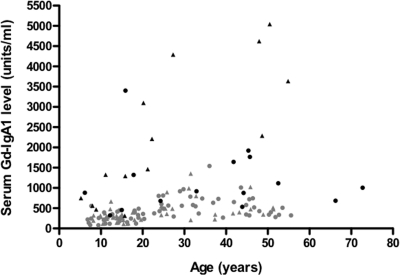
Serum Gd-IgA level was plotted against age for all patients in the study (Figure 2). The level for controls of all ages significantly correlated with age (r = 0.41; P < 0.0001). Serum Gd-IgA1 levels were significantly elevated compared with age-group (i.e., pediatric or adult) controls for 7 of the 11 pediatric patients and 9 of the 18 adult patients.
Figure 2.
Serum levels for Gd-IgA1 are plotted versus age for all patients. Men/boys are denoted by triangles and women/girls by circles. The symbols for healthy controls are gray and those for patients with IgAN are black.
We found no association of serum Gd-IgA1 level with disease severity, as judged by estimated GFR or spot urinary protein/creatinine ratio, in this small cohort of AA patients.
For six patients with serum Gd-IgA1 levels <95th percentile, seven of eight of their first-degree relatives also had Gd-IgA1 levels <95th percentile. However, for 14 patients with serum Gd-IgA1 levels >95th percentile almost half of their first-degree relatives also had levels >95th percentile (Table 2). Heritabilities were modeled using age, sex, BMI, and IgAN status as covariates. The heritability of the serum Gd-IgA1 level was 0.74 (P = 0.007), with IgAN status as the only significant covariate (P < 0.05). The heritability of total IgA level was 0.66 (P = 0.01) and the heritability of Gd-IgA1/IgA ratio was 0.66 (P = 0.007); however, that of BMI of 0.47 was not statistically significant (P = 0.08).
Table 2.
Serum Gd-IgA1 levels for first-degree relatives of AA patients with IgAN with respect to the level in healthy controls (< or ≥95th percentile)
| Patient Gd-IgA1 Level | Relative Gd-IgA1 Level |
||
|---|---|---|---|
| <95th Percentile | ≥95th Percentile | ||
| <95th percentile | 6 | 7 | 1 |
| ≥95th percentile | 14 | 14 | 12 |
P = 0.116, Fisher exact test.
Discussion
This study strengthens the evidence that the serum Gd-IgA1 level is a good candidate for a disease marker for IgAN across multiple ethnic populations. Serum levels of Gd-IgA1 were elevated in 75% of Caucasian adult patients with IgAN in our previous report (13). A Japanese report showed that 49% of 41 adult patients had significantly elevated serum Gd-IgA1 levels (14). Serum Gd-IgA1 level was not associated with severity of disease in these reports (13,14). Significantly elevated serum Gd-IgA1 levels have also been found in 77% of a cohort of 22 pediatric IgAN patients that included Caucasians and AAs (20). In that publication, the data were not analyzed by race, but four of the six AA patients had significantly elevated serum Gd-IgA1 levels. These six patients have been included in this study. In this study, the percentages of pediatric (64%) and adult (50%) AA patients with significantly elevated Gd-IgA1 levels were lower than the frequencies documented in our Caucasian adult cohort (13), but were similar to those in the Japanese cohort. Our data suggest that the pathogenic feature of undergalactosylated serum IgA1 typically found in Caucasians and Asians with IgAN is frequently present in AA patients.
Development of a disease marker for IgAN would provide a better gauge to assess the prevalence of this renal disease. Although some centers have reported that IgAN was rare in AA cohorts (4–7), others have shown incidence rates in Caucasians and AA populations to be similar (8,21). Data from the African American Study of Kidney Disease also suggest that IgAN may be rare in African Americans (22). Analysis of renal biopsies of the enrolled patients found only one patient with possible IgAN. However, merely 6 of the 39 biopsied patients had significant proteinuria.
Because of the attendant risks, many patients with proteinuria and hypertension forego a renal biopsy to establish a histologic diagnosis; the renal damage is usually ascribed to an unidentified glomerulonephritis or to complications of hypertension. According to the 2008 United States Renal Data System report, ESRD was attributed to glomerulonephritis without a documented histologic diagnosis for 3.1% of patients and to hypertension (not otherwise specified) for an additional 25% of patients (http://www.usrds.org/2008). As the features of undiagnosed cases of IgAN would easily fit in these categories, it is likely that IgAN accounts for a greater portion of the total burden of severe renal disease in this country than currently appreciated.
Our data are similar to published results that found serum levels of total IgA did not differ significantly between healthy adult Caucasians and AAs (23,24). Serum Gd-IgA1 level was significantly lower in adult AA controls as compared with those of Caucasian controls. However, serum Gd-IgA1 levels did not differ by race for the pediatric controls.
For both pediatric and adult controls, the percentage of total IgA that is comprised of Gd-IgA1 is significantly lower in AAs than in Caucasians. It is possible that some cytokines affect transcription of various glycosyltransferases in human B cells and, consequently, affect O-glycosylation of IgA1. As some cytokines are hyperexpressed in AAs (25–27), this specific cytokine milieu may consequently affect galactosylation of IgA1 in AA and, thus, explain the generally lower serum Gd-IgA1 levels and, maybe, the lower incidence of IgAN in this ethnic group.
Serum Gd-IgA1 levels also varied by age in our cohort. These data are consistent with the known effect of age on serum Ig levels and emphasize the need to utilize rigorous age- and ethnicity-matched reference populations for comparison of Ig parameters (23,24,28,29).
Serum Gd-IgA1 levels for AA and Caucasian controls are not normally distributed because about 3% of normal individuals from each ethnic group had a level considerably >2 SD above the mean. One might speculate that such individuals have subclinical renal deposits of IgA. Necropsy studies in Singapore and Germany have indicated that the prevalence of histologic features of IgAN in persons dying without evidence of renal disease was 2 to 4% (30,31). The prevalence was much higher, 16%, in living Japanese kidney donors when the allograft was biopsied at implantation (32). These findings have led some transplant centers to be reluctant to consider first-degree relatives as candidates for kidney donors for patients with IgAN. Serum Gd-IgA1 level may prove useful in guiding evaluation of potential donors. Whether individuals with very high serum Gd-IgA1 levels are at increased risk to develop IgAN in the future will require study of a large number of controls followed over many years.
We previously showed that serum Gd-IgA1 levels were heritable in Caucasians with sporadic and familial forms of IgAN (15). In that study, serum Gd-IgA1 levels were high in 65 of 84 (78%) patients with sporadic IgAN and 50 of 202 (25%) of their first-degree relatives. Heritability of the serum Gd-IgA1 level was estimated at 0.54 (P < 0.0001), and segregation analysis suggested the presence of a major dominant gene on a polygenic background. A Chinese study found mean serum Gd-IgA1 levels to be significantly higher for first-degree relatives of patients with IgAN (12). Our study indicates that heritability of the serum Gd-IgA1 level is a likely pathogenetic factor for IgAN in AAs.
Conclusions
In summary, serum Gd-IgA1 levels are often elevated in AA patients with IgAN and their first-degree relatives. Aberrant IgA1 glycosylation appears to be a heritable trait in AAs. Thus, findings related to serum Gd-IgA1 level are similar in all ethnic groups studied to date.
Disclosures
None.
Acknowledgments
Dr. Hastings was supported by a research career development award from the Clinical and Translational Research Institute of the University of Tennessee Health Science Center. This work was also supported by National Institutes of Health grants DK061525, DK082753, DK078244, DK077279, and DK075868 and by grants to the General Clinical Research Centers of the University of Tennessee Health Science Center (M01 RR00211) and the University of Alabama at Birmingham (M01 RR00032), by National Kidney Foundation and Strides for IgA Nephropathy Fellowship Award (Dr. Sanders), and by a generous gift to the University of Tennessee Pediatric Nephrology Research Support Fund from Anna and Donald Waite. We thank Catherine V. Barker, Sandra Grimes, Susan Y. Woodford, Olivia Hancox, and Sherrie Walker for assistance with collection of blood samples and management of clinical data.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.D'Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Borok MZ, Nathoo KJ, Gabriel R, Porter KA: Clinicopathological features of Zimbabwean patients with sustained proteinuria. Cent Afr J Med 43: 152–158, 1997 [PubMed] [Google Scholar]
- 3.Seedat YK, Nathoo BC, Parag KB, Naiker IP, Ramsaroop R: IgA nephropathy in blacks and Indians of Natal. Nephron 50: 137–141, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Galla JH, Kohaut EC, Alexander R, Mestecky J: Racial difference in the prevalence of IgA-associated nephropathies. Lancet 2(8401): 522, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Hall YN, Fuentes EF, Chertow GM, Olson JL: Race/ethnicity and disease severity in IgA nephropathy. BMC Nephrol 5: 10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennette JC, Wall SD, Wilkman AS: Low incidence of IgA nephropathy in blacks. Kidney Int 28: 944–950, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Nair R, Walker PD: Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int 69: 1455–1458, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Wyatt RJ, Julian BA, Baehler RW, Stafford CC, McMorrow RG, Ferguson T, Jackson E, Woodford SY, Miller PM, Kritchevsky S: Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. Central Kentucky Region of the Southeastern United States IgA Nephropathy DATABANK Project. J Am Soc Nephrol 9: 853–858, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J: Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104: 73–81, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J: IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67: 504–513, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Ding J, Zhu L, Shi S, Jiang L, Zhao M, Zhang H: Aberrant galactosylation of IgA1 is involved in the genetic susceptibility of Chinese patients with IgA nephropathy. Nephrol Dial Transplant 24: 3372–3375, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Shimozato S, Hiki Y, Odani H, Takahashi K, Yamamoto K, Sugiyama S: Serum under-galactosylated IgA1 is increased in Japanese patients with IgA nephropathy. Nephrol Dial Transplant 23: 1931–1939, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA: Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19: 1008–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyatt RJ, Julian BA, Bhathena DB, Mitchell BL, Holland NH, Malluche HH: IgA nephropathy: Presentation, clinical course, and prognosis in children and adults. Am J Kidney Dis 4: 192–200, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Feld LG, Langford DJ: A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104: 849–854, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Almasy L, Blangero J: Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62: 1198–1211, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau KK, Wyatt RJ, Moldoveanu Z, Tomana M, Julian BA, Hogg RJ, Lee JY, Huang WQ, Mestecky J, Novak J: Serum levels of galactose-deficient IgA in children with IgA nephropathy and Henoch-Schönlein purpura. Pediatr Nephrol 22: 2067–2072, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sehic AM, Gaber LW, Roy S, 3rd, Miller PM, Kritchevsky SB, Wyatt RJ: Increased recognition of IgA nephropathy in African-American children. Pediatr Nephrol 11: 435–437, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R: Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: A report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int 51: 244–252, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Albandar JM, DeNardin AM, Adesanya MR, Winn DM, Diehl SR: Associations of serum concentrations of IgG, IgA, IgM and interleukin-1beta with early-onset periodontitis classification and race. J Clin Periodontol 29: 421–426, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Susskind BM, Kerman RH, Browne BJ, Hartwell BA, Davis BG, Katz SM, Van Buren CT, Kahan BD: The impact of elevated serum IgA and race on primary recipient renal allograft survival. Transplantation 61: 205–211, 1996 [DOI] [PubMed] [Google Scholar]
- 25.August P, Leventhal B, Suthanthiran M: Hypertension-induced organ damage in African Americans: Transforming growth factor-beta(1) excess as a mechanism for increased prevalence. Curr Hypertens Rep 2: 184–191, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS: TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Report of the ASHI Minority Workshops: Part IV. Hum Immunol 65: 1413–1419, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Suthanthiran M, Khanna A, Cukran D, Adhikarla R, Sharma VK, Singh T, August P: Transforming growth factor-beta 1 hyperexpression in African American end-stage renal disease patients. Kidney Int 53: 639–644, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Buckley RH, Dees SC, O'Fallon WM: Serum immunoglobulins. I. Levels in normal children and in uncomplicated childhood allergy. Pediatrics 41: 600–611, 1968 [PubMed] [Google Scholar]
- 29.West CD, Hong R, Holland NH: Immunoglobulin levels from the newborn period to adulthood and in immunoglobulin deficiency states. J Clin Invest 41: 2054–2064, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinniah R: Occurrence of mesangial IgA and IgM deposits in a control necropsy population. J Clin Pathol 36: 276–279, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldherr R, Rambausek M, Duncker WD, Ritz E: Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant 4: 943–946, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y: Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294, 2003 [DOI] [PubMed] [Google Scholar]