Abstract
Background and objectives: The introduction of new therapies, including agents that block the renin-angiotensin system, may have affected progression of autosomal dominant polycystic kidney disease (ADPKD). We investigated whether the age when reaching ESRD and survival during renal replacement therapy in Danish patients with ADPKD changed from January 1, 1990, through December 31, 2007.
Design, setting, participants, & measurements: According to the Danish National Registry on Regular Dialysis and Transplantation, 693 patients with ADPKD reached ESRD in the study period. The 18 years were divided into three consecutive 6-year intervals.
Results: The incidence of reaching ESRD for patients with ADPKD increased from 6.45 per million people in 1990 through 1995 to 7.59 per million people in 2002 through 2007, and the mean age at onset of ESRD increased by 4.7 years. The age-adjusted male-to-female ratio for onset of ESRD changed from 1.6 to 1.1, indicating a trend toward similar progression in both genders. From onset of ESRD, a Cox regression analysis to compare the first and second 6-year intervals, adjusted for age, gender, and treatment modality, showed that patient survival improved by 38%. Although NS, a similar trend was found during the second and third time intervals.
Conclusions: This study demonstrates that in Danish patients with ADPKD, the prognosis had significantly improved during the study period. Furthermore, the results indicate that male gender may be losing its importance as a risk factor for progression in ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder that affects an estimated 4 to 6 million people worldwide (1). In Denmark, the prevalence of ADPKD has been estimated to be one per 1000 people, and it accounts for approximately 8% of patients who are on renal replacement therapy (RRT) (2,3). ADPKD is a genetically heterogeneous disease with mutations in two genes, PKD1 and PKD2, which accounts for 85 and 15% of cases, respectively (4,5). Mutations in PKD1 lead to more severe disease as a result of earlier development of cysts, compared with PKD2 mutations (6). Hypertension is an important contributor to progression in ADPKD, and cardiovascular morbidity and mortality are the main causes of death (7–9). Activation of the intrarenal renin-angiotensin system may be associated with progression in ADPKD (10), and treatment with angiotensin-converting enzyme inhibitors (ACEIs) has been shown to reduce proteinuria and reduce the left ventricular mass index (LVMI) in patients with ADPKD (11,12). Statins may also have beneficial effects in ADPKD (13), and several ongoing clinical trials are investigating various strategies to slow disease progression in ADPKD (14).
This study describes the epidemiology of Danish patients with ADPKD who reached ESRD between 1990 and 2007. Our objective was to investigate how the increasing knowledge of factors that influence progression in ADPKD has affected the prognosis for events such as ESRD and death in the Danish population of patients with ADPKD. This has not previously been described in a national study, but others have demonstrated a significant slowing of renal demise in patients with ADPKD between 1985 and 2001 (15).
Materials and Methods
Data Collection
The analyses were based on data from the Danish National Registry on Regular Dialysis and Transplantation (NRDT) established in 1990. NRDT contains data on all patients who are actively being treated for ESRD in Denmark. Once a year, all 14 Danish departments of nephrology transmit data to the NRDT, and no private clinics exist. The NRDT is used in scientific studies, in disease monitoring, and for evaluation of treatment quality and activity. Furthermore, the NRDT forms the basis of the Danish section of the European Dialysis and Transplantation Association Registry (2,16–18). The quality of data in the NRDT has been validated, and for the diagnosis of ADPKD a sensitivity of 100% and a specificity of 99% was found (19).
On the basis of the International Classification of Disease, 10th Revision diagnosis DQ61.2, we retrieved data only on patients who had ADPKD. The following parameters were registered on each patient: Date of birth, gender, renal diagnosis, start date of ESRD, treatment modality (hemodialysis, peritoneal dialysis, or transplantation), and end date (death, alive or lost to follow-up). Patients who had ever received a renal transplant were classified as “transplanted” patients, even if the transplant never functioned. The remaining patients who were on hemodialysis and peritoneal dialysis were classified as dialysis patients. A total of 694 patients with ADPKD reached ESRD during the follow-up period. One patient with onset of ESRD at age 5 was excluded because of concern about the diagnosis. A total of 180 patients who had ADPKD; were registered in the NRDT with ESRD before January 1, 1990; and were living on RRT were included in the analysis of prevalence. For the analyses of incidence and prevalence, population data were also retrieved from the Statistical Yearbook, Statistics Denmark (20). By January 1, 2008, Denmark had a population of 5,475,791 people. Less than 3% were immigrants from either Africa or Asia. The study was approved by the Danish Data Protection Agency and the Danish Society of Nephrology, which administers the Danish NRDT.
Statistical Analysis
For a more accurate differentiation of the developments during the follow-up period, the 18 years were divided into three consecutive 6-year intervals. The statistical analyses comparing incidence, prevalence, and numbers of patients across time intervals were made using a logistic regression model accounting for Poisson distributed data. One-way ANOVA was used when comparing the mean age at onset of ESRD across time intervals.
Survival plots were made using the Kaplan-Meier method, and these results were unadjusted for traditional risk factors. Log-rank tests were performed for comparisons between the three 6-year intervals. For estimation of the two and five year survival a life table analysis was performed. The Cox proportional hazards model was adjusted for time intervals, gender, treatment modalities, and age at ESRD, with time since onset of ESRD as the underlying time scale. These results are presented as hazard ratios (HR) with corresponding 95% confidence intervals (CI). HR <1 denotes a better survival rate in the first group than in the second group, and HR >1 denotes a poorer survival rate in the first group than in the second group. The estimation of the odds ratio between male and female patients who had received a transplant was made using a logistic regression analysis for binomial distributed data. All statistical analyses were performed using SAS 9.1 software, and figures were generated using SAS Enterprise Guide 4; both products are from the SAS Institute (Cary, NC). P < 0.05 was considered significant.
Results
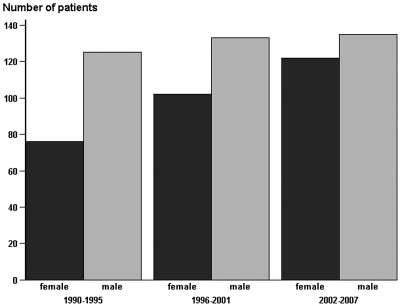
The 18 years of follow-up were divided into three consecutive 6-year intervals, in which the number of patients who had ADPKD and reached ESRD gradually increased from 201 in 1990 through 1995 to 257 in 2002 through 2007. As illustrated in Figure 1, this was mostly due to a significant increase in female patients reaching ESRD from 76 in 1990 through 1995 to 122 in 2002 through 2007 (P < 0.01).
Figure 1.
Number of patients with ADPKD, by gender, with onset of ESRD in Denmark, 1990 through 2007.
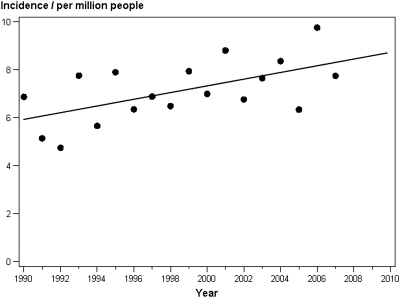
To rule out the possibility that the increased number of patients could be explained by a general increase in the Danish population, we analyzed the incidence per million people (pmp). As shown in both Table 1 and Figure 2, the incidence changed from 6.45 pmp (95% CI 4.8 to 8.8) in 1990 through 1995 to 7.59 pmp (95% CI 6.9 to 8.9) in 2002 through 2007 (P < 0.05). The observed increase in number of patients who had ADPKD and reached ESRD was therefore independent of changes in population size.
Table 1.
Development in epidemiologic parameters in Danish patients who had ADPKD and reached ESRD between 1990 and 2007
| Characteristic | 1990 to 1995 | 1996 to 2001 | 2002 to 2007 | P |
|---|---|---|---|---|
| Incidence pmp (95% CI) | ||||
| total | 6.45 (4.80 to 8.80) | 7.39 (5.50 to 8.00) | 7.59 (6.90 to 8.90) | <0.05 |
| female | 2.44 (1.50 to 3.90) | 3.21 (2.10 to 5.00) | 3.76 (3.20 to 4.50) | <0.01 |
| male | 4.01 (2.70 to 6.10) | 4.08 (2.80 to 6.30) | 4.13 (3.50 to 4.90) | NS |
| Prevalence pmp (95% CI) | 39.80 (35.50 to 44.60) | 50.40 (45.30 to 56.20) | 64.20 (61.50 to 67.00) | <0.0001 |
| Age at onset of ESRD (mean [95% CI]) | ||||
| all patients | 55.90 (52.20 to 59.60) | 58.50 (55.00 to 62.10) | 60.60 (59.10 to 62.00) | <0.001 |
| female | 56.00 (50.60 to 61.50) | 60.00 (54.90 to 65.20) | 60.40 (58.30 to 62.40) | <0.01 |
| male | 55.80 (50.80 to 60.80) | 57.40 (52.40 to 62.40) | 60.80 (58.70 to 62.80) | <0.01 |
Figure 2.
The incidence pmp of ESRD in patients with ADPKD in Denmark, 1990 through 2007.
As shown in Table 1 the patients who had ADPKD and reached ESRD got older during the 18 years of follow-up. The mean age increased from 55.9 years in 1990 through 1995 to 60.6 years in 2002 through 2007 (P < 0.001). In female patients, most of this increase was found during the first 12 years, whereas the male patients had the highest increase between the second and third time intervals. The mean age at onset of ESRD increased on average by 4.4 years for female patients and by 5.0 years for male patients.
The age-adjusted gender ratio combines both the increasing number of female patients who had ADPKD and reached ESRD and the increase in age at onset of ESRD described already. From 1990 through 1995, the age-adjusted male-to-female ratio was 1.6. From 1996 through 2001, it was 1.2, and from 2002 through 2007, it had decreased to 1.1.
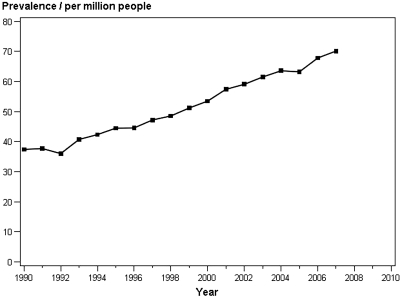
Figure 3 illustrates the prevalence of Danish patients who had ADPKD and were on RRT. During the follow-up period, this increased continuously from 39.8 pmp in 1990 through 1995 to 64.2 pmp in 2002 through 2007 (P < 0.0001; Table 1).
Figure 3.
The prevalence of patients who had ADPKD and were on RRT in Denmark, 1990 through 2007.
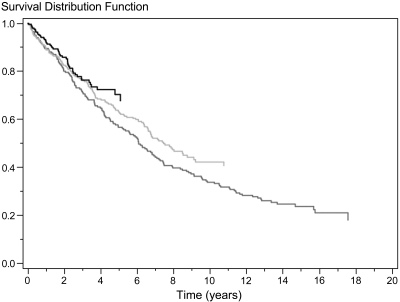
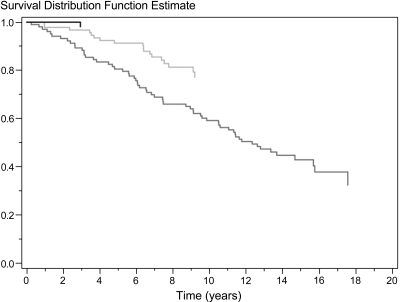
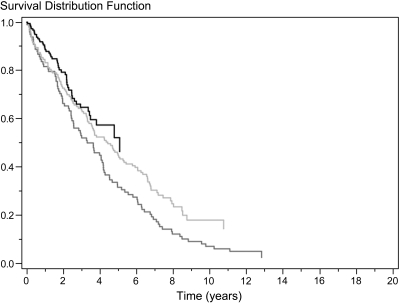
The overall unadjusted survival rate of Danish patients who had ADPKD and were on RRT is illustrated by each 6-year interval in Figure 4 using the Kaplan-Meier method. Although the follow-up periods were not equally long, Figure 4 shows that patient survival improved by each 6-year interval (P < 0.05). From 1990 through 1995 to 2002 through 2007, patient survival after 2 years improved from 80 to 86% and after 5 years from 56 to 68%. During the follow-up period, 330 of 693 patients died. The significant improved survival rate found during the study period was identified in both patients who had received a transplant (Figure 5) and dialysis patients (Figure 6).
Figure 4.
Unadjusted survival rate after onset of ESRD in 693 patients with ADPKD from 1990 through 1995 (dark gray), 1996 through 2001 (light gray), and 2002 through 2007 (black). P < 0.05 for difference among the three groups.
Figure 5.
Unadjusted survival rate after onset of ESRD in 264 patients who had ADPKD and received a transplant from 1990 through 1995 (dark gray), 1996 through 2001 (light gray), and 2002 through 2007 (black). P < 0.001 for difference among the three groups.
Figure 6.
Unadjusted survival rate after onset of ESRD in 429 dialysis patients with ADPKD from 1990 through 1995 (dark gray), 1996 through 2001 (light gray), and 2002 through 2007 (black). P < 0.01 for difference among the three groups.
For further differentiation of how different risk factors influenced patient survival, a multivariate Cox regression analysis with time since ESRD as underlying time scale was conducted. It was adjusted for time intervals, gender, treatment modality (transplantation/dialysis), and age at onset of ESRD. The results are shown in Table 2 as HRs with the corresponding 95% CIs. From the first to the second time intervals, survival improved by 38% (P < 0.001), and from the second to the third time intervals, it improved by 20%. Although the comparison between the second and third time intervals was NS, it is in accordance with the survival plots in Figures 4 through 6.
Table 2.
Multivariate Cox regression analysis in 693 Danish patients who had ADPKD and reached ESRD between 1990 and 2007
| Comparisons | HR (95% CI) | P |
|---|---|---|
| Time periods | ||
| 1996 to 2001 versus 1990 to 1995 | 0.62 (0.49 to 0.80) | <0.001 |
| 2002 to 2007 versus 1996 to 2001 | 0.80 (0.56 to 1.15) | NS |
| Gender | ||
| male versus female | 1.34 (1.07 to 1.68) | <0.01 |
| Treatment | ||
| transplantation versus dialysis | 0.30 (0.22 to 0.42) | <0.0001 |
| Age at onset of ESRD | ||
| age per year | 1.05 (1.04 to 1.07) | <0.0001 |
A total of 330 events, 52% censored, mean follow-up time 5.1 years. HR <1 denotes a lower risk for death, HR >1 denotes a higher risk for death.
During the follow-up period, 393 male and 300 female patients with ADPKD reached ESRD. Of the 693 patients, 264 received a transplant (165 male and 99 female patients). More male than female patients received a renal transplant (odds ratio 1.5; 95% CI 1.1 to 2.0; P < 0.05). Mean follow-up time on RRT was 5.0 years for female patients and 5.2 years for male patients. In this adjusted model, the risk for death was reduced by as much as 70% in patients who received a transplant compared with dialysis patients (P < 0.0001), and this is consistent with the different survival rates found when comparing Figure 5 and Figure 6. Although the results were adjusted for treatment modality, the male patients had a significant poorer survival after onset of ESRD compared with female patients (HR 1.34; P < 0.01). As might be expected, the age at onset of ESRD was also associated with patient survival, and by each year of age the patients were older at onset of ESRD, the risk for death increased by 5% (P < 0.0001).
Discussion
This study of the Danish population of patients who had ADPKD and reached ESRD from 1990 through 2007 clearly demonstrates that the prognosis has improved during the follow-up period. Both the incidence rate and the age at onset of ESRD had increased, indicating an improved survival in patients with ADPKD before the onset of ESRD. Although the patients who had ADPKD and reached ESRD are now older, they also live longer on RRT. A multivariate Cox regression analysis showed that patient survival markedly improved, by 38%. Although not statistically significant, a similar trend was found from the second to the third time periods. The age-adjusted sex-gender ratio for patients with onset of ESRD fell from 1.6 in the first interval to 1.1 in the last interval, and it indicates that male gender may not be a risk factor for progression in ADPKD; however, in the multivariate Cox regression analysis, male gender was associated with a significant poorer survival after onset of ESRD.
Many studies have identified patients who have ADPKD and are at risk for more severe disease, and several risk factors have been identified (6,8,21–23). The most important risk factors are probably mutations in the PKD1 gene and hypertension, but other risk factors that predict more severe disease include younger age, proteinuria >1 g/d, hematuria, increased number and volume of renal cysts, black race, and increased LVMI.
The enlargements of renal cysts have been associated with stimulation of the renin-angiotensin system in ADPKD. A randomized, prospective study found a significant reduction in LVMI in hypertensive patients who had ADPKD after BP control <120/80 mmHg (12). In another study of patients who had ADPKD and were treated to the same BP level, proteinuria decreased significantly only on treatment with ACEIs, when compared with treatment with calcium channel blockers (11). With the introduction of newer antihypertensive treatments such as the ACEIs, it seems that more patients with ADPKD reach BP control (24), and this may influence the age at onset of ESRD in these patients (15). Whether treatment with either an ACEI or angiotensin receptor blocker is superior to other antihypertensive drugs in slowing disease progression in ADPKD has not been clearly demonstrated (25,26).
In the 2009 annual report by the United States Renal Data System (USRDS) (27), incidence rates of reported ESRD increased in patients with cystic kidneys from 3.3 pmp in 1980 to 8.6 pmp in 2007. Studies from Europe (28) and Japan (29) have demonstrated similar trends in incidence rates of patients with cystic kidney diseases. Although these studies and annual reports described patients with unspecified cystic kidney diseases, it must be assumed that the majority of patients had ADPKD, and our results are consistent with these data.
Although an increasing number of patients with ADPKD reached ESRD in Denmark, their age at onset of ESRD also increased significantly throughout the period. This is in accordance with data from the USRDS: The 2009 annual report showed an increasing age at onset of RRT from 51.5 years in 1980 to 54.8 years in 2007 in patients with cystic kidney diseases (27).
The epidemiologic studies from Japan and Europe reported age-adjusted gender ratios of 1.2 to 1.4 at onset of ESRD in patients with ADPKD (28,29), and male gender has been associated with earlier onset of hypertension and more severe progression to ESRD in patients with ADPKD (8,30); however, not all studies could show that male gender was in fact a risk factor of progression (22,31). If male gender were not a risk factor of progressive ADPKD, then we would expect to find approximately the same incidence and age at onset of ESRD in male and female patients. Between 2002 and 2007, the age at onset of ESRD was similar in male and female patients, and after the significant increase in numbers of female patients who reached ESRD, the age-adjusted gender ratio was only 1.1 instead of 1.6 found in the first 6-year interval. It therefore seems that male and female patients who reached ESRD from 2002 through 2007 on average had the same progression of ADPKD.
The lower incidence rate of female patients with onset of ESRD could reflect a better predialysis survival rate in male patients with ADPKD rather than female patients; however, a limitation of this study is the lack of survival data on all patients with ADPKD in Denmark before onset of ESRD, and, to our knowledge, such data have not been presented. Whether a lower predialysis mortality rate in female patients with ADPKD explains the increased incidence found during the follow-up period therefore remains unclear.
According to the USRDS (32) 2007 annual data report, the prevalence of patients with PKD and ESRD increased by 20.9% from 58.8 pmp in 1996 through 1997 to 71.1 pmp in 2004 through 2005. In this study, the prevalence of Danish patients with ADPKD and ESRD increased by 61% from 39.8 pmp in 1990 through 1995 to 64.2 pmp in 2002 through 2007. Between 1996 and 2005, the Danish prevalence increased by 42%, and this is twice the increase found by the USRDS. Several factors may have contributed to the increased prevalence of patients with ADPKD and ESRD found in this study. Both the significant increased incidence of patients who had ADPKD and reached ESRD and the significant improved survival on RRT have contributed. The increased incidence may also reflect an improved patient survival before onset of ESRD, some of which may be explained by improved antihypertensive therapy. Improvements in radiologic imaging may also have detected more patients with ADPKD. Both the unadjusted survival plots and the multivariate Cox regression analysis demonstrated that patient survival on RRT improved during the study period; however, only the change in survival from the first to the second 6-year interval was significant in the adjusted model. This can be explained by the shorter follow-up period in the last 6-year interval with fewer events. The multivariate Cox regression analysis was adjusted for time periods, gender, age at onset of ESRD, and treatment modality. It clearly demonstrated that each of these factors influenced patient survival in Danish patients with ADPKD. Although our results demonstrate that the age at onset of ESRD was now the same in both genders, male gender was still associated with an increased risk for death of 34% after onset of ESRD. It is possible, however, that this difference in patient survival between male and female patients will be reduced with the new trends in incidence and age at onset of ESRD in patients with ADPKD.
During the study period, unadjusted patient survival in the Danish ESRD population without ADPKD did not improve (data not shown), and the mean age at onset of ESRD increased by 8.3 years. This reflects an increasing number of elderly patients who initiate dialysis in the general Danish ESRD population and probably explains why patient survival did not improve during the follow-up period.
Conclusions
This study demonstrated that the prognosis for renal and patient survival in Danish patients with ADPKD significantly improved during the study period. The age-adjusted male-to-female ratio for onset of ESRD fell from 1.6 to 1.1, indicating that male gender may be losing its importance as a risk factor for progression in ADPKD.
Disclosures
None.
Acknowledgments
This study was supported by grants from the Research Fund of the Danish Society of Nephrology, the Research Fund of the Danish Kidney Association, the Helen and Ejnar Bjørnow Foundation, and the Hoerslev Foundation.
Part of this work was presented as an abstract at the annual meeting of the American Society of Nephrology; October 28 through November 1, 2009; San Diego, CA.
We thank the current head of the Danish National Registry on Regular Dialysis and Transplantation, Dr. James Heaf.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wilson PD: Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Danish Society of Nephrology: Danish National Registry Report on Dialysis and Transplantation in Denmark, 2007. Available at: https://www.sundhed.dk/Fil.ashx?id=6959&ext=pdf&navn=Nefrologisk_aarsrapport_2007.pdf Accessed January 14, 2010
- 3.Dalgaard OZ: Bilateral polycystic disease of the kidneys: A follow-up of two hundred and eighty-four patients and their families. Acta Med Scand Suppl 328: 1–255, 1957 [PubMed] [Google Scholar]
- 4.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S: PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 5.The European Polycystic Kidney Disease Consortium: The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77: 881–894, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Fick GM, Johnson AM, Hammond WS, Gabow PA: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT: Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis 2: 630–639, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Loghman-Adham M, Soto CE, Inagami T, Cassis L: The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol 287: F775–F788, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ecder T, Chapman AB, Brosnahan GM, Edelstein CL, Johnson AM, Schrier RW: Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 35: 427–432, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Schrier R, McFann K, Johnson A, Chapman A, Edelstein C, Brosnahan G, Ecder T, Tison L: Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: Results of a seven-year prospective randomized study. J Am Soc Nephrol 13: 1733–1739, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Namli S, Oflaz H, Turgut F, Alisir S, Tufan F, Ucar A, Mercanoglu F, Ecder T: Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail 29: 55–59, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrier RW, McFann KK, Johnson AM: Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int 63: 678–685, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Sorensen VR, Hansen PM, Heaf J, Feldt-Rasmussen B: Stabilized incidence of diabetic patients referred for renal replacement therapy in Denmark. Kidney Int 70: 187–191, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Sorensen VR, Mathiesen ER, Heaf J, Feldt-Rasmussen B: Improved survival rate in patients with diabetes and end-stage renal disease in Denmark. Diabetologia 50: 922–929, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Vestergaard P, Lokkegaard H: Predicting future trends in the number of patients on renal replacement therapy in Denmark. Nephrol Dial Transplant 12: 2117–2123, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Hommel K, Rasmussen S, Madsen M, Kamper AL: The Danish Registry on Regular Dialysis and Transplantation: Completeness and validity of incident patient registration. Nephrol Dial Transplant 25: 947–951, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Statistics Denmark: Statistical Yearbook 2010. Available at: http://www.dst.dk/HomeUK/Statistics/ofs/Publications/Yearbook.aspx Accessed January 15, 2010
- 21.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW: Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8: 1292–1297, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Dicks E, Ravani P, Langman D, Davidson WS, Pei Y, Parfrey PS: Incident renal events and risk factors in autosomal dominant polycystic kidney disease: A population and family-based cohort followed for 22 years. Clin J Am Soc Nephrol 1: 710–717, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Yium J, Gabow P, Johnson A, Kimberling W, Martinez-Maldonado M: Autosomal dominant polycystic kidney disease in blacks: Clinical course and effects of sickle-cell hemoglobin. J Am Soc Nephrol 4: 1670–1674, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Ecder T, Edelstein CL, Fick-Brosnahan GM, Johnson AM, Duley IT, Gabow PA, Schrier RW: Progress in blood pressure control in autosomal dominant polycystic kidney disease. Am J Kidney Dis 36: 266–271, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Jafar TH, Stark PC, Schmid CH, Strandgaard S, Kamper AL, Maschio G, Becker G, Perrone RD, Levey AS: The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int 67: 265–271, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lawson CR, Doulton TW, MacGregor GA: Autosomal dominant polycystic kidney disease: Role of the renin-angiotensin system in raised blood pressure in progression of renal and cardiovascular disease. J Renin Angiotensin Aldosterone Syst 7: 139–145, 2006 [DOI] [PubMed] [Google Scholar]
- 27.US Renal Data System: USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Minneapolis, USRDS Coordinating Center, 2009. Available at: http://www.usrds.org/2009/slides/indiv/INDEX_ESRD.HTML Accessed January 14, 2010 [Google Scholar]
- 28.Stengel B, Billon S, van Dijk PC, Jager KJ, Dekker FW, Simpson K, Briggs JD: Trends in the incidence of renal replacement therapy for end-stage renal disease in Europe, 1990–1999. Nephrol Dial Transplant 18: 1824–1833, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Wakai K, Nakai S, Kikuchi K, Iseki K, Miwa N, Masakane I, Wada A, Shinzato T, Nagura Y, Akiba T: Trends in incidence of end-stage renal disease in Japan, 1983–2000: Age-adjusted and age-specific rates by gender and cause. Nephrol Dial Transplant 19: 2044–2052, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kelleher CL, McFann KK, Johnson AM, Schrier RW: Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S. population. Am J Hypertens 17: 1029–1034, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hateboer N, Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 32.US Renal Data System: USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Minneapolis, USRDS Coordinating Center; Available at: http://www.usrds.org/adr_2007.htm Accessed January 14, 2010 [Google Scholar]