Abstract
Background:
Various treatment methods exist to treat obstructive sleep apnea (OSA); continuous positive airway pressure (CPAP) is considered the gold standard. It is however a clinical reality that the use of CPAP is often cumbersome. CPAP treatment is considered compliant when used ≥ 4 h per night as an average over all nights observed. Surgery, on the other hand, is regarded as successful when the apnea hypopnea index (AHI) drops at least 50% and is reduced below 20/h postoperatively in patients whose preoperative AHI was > 20/h.
The effectiveness of CPAP compliance criteria can be questioned, just as the effectiveness of surgical success criteria has often been questioned.
Study Objectives:
The aim of the study was to compare non optimal use of optimal therapy (CPAP) with the continuous effect (100%) of often non optimal therapy (surgery).
Design:
Using mathematical function formulas, the effect on the AHI of various treatment modalities and their respective compliance and success criteria were calculated.
Results:
The more severe the AHI, the more percentage of total sleep time (TST) CPAP must be used to significantly reduce the AHI. Patients with moderate OSA reduce the AHI by 33.3% to 48.3% when using CPAP 4 h/ night (AHI 0–5, respectively). The required nightly percentage use rises as one reduces the AHI target to < 5. CPAP must be used 66.67% to 83.33% per night to reduce the AHI below 5 (AHI of 0 while using CPAP).
Conclusion:
Using a mean AHI in CPAP therapy is more realistic than using arbitrary compliance rates, which, in fact, hide insufficient reductions in AHI.
Citation:
Ravesloot MJL; de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. SLEEP 2011;34(1):105-110.
Keywords: OSAS, apnea, treatment effectiveness, CPAP, surgery
OBSTRUCTIVE SLEEP APNEA (OSA) IS THE MOST PREVALENT SLEEP DISORDERED BREATHING PROBLEM, AFFECTING 2% TO 26% OF THE GENERAL population, depending on gender, age, and definition.1,2 The severity of OSA is expressed in the apnea hypopnea index (AHI): an AHI of 5-15 is considered mild OSA, 15-30 moderate OSA, and > 30 severe OSA.
Continuous positive airway pressure (CPAP), introduced in 1981 by Sullivan, is in many countries regarded as the gold standard in treatment of OSA, with oral device therapy (mandibular repositioning appliances) or surgery reserved for CPAP failures.3 In other countries, in mild to moderate OSA, oral devices and surgery are considered first-line treatment in well-selected patients. Heated and sometimes emotional discussions exist between CPAP protagonists, defenders of the merits of oral devices, and surgeons. Some CPAP advocates, (un)intentionally provocative, go as far as to state that there is no place for surgery in the treatment of OSA; lack of evidence from randomized controlled trials supporting surgical practices strengthens their defense.4 At the other, more nuanced, end of the spectrum, it is believed that in well-selected patients with obvious anatomical correctable features, surgery is a viable alternative to lifelong CPAP therapy. The issue is confused by the fact that different definitions of successful therapy are being used for the different treatment options. Surgeons are being forced by the AHI classification to compete on an uneven playing field.
In surgery, traditionally, success was defined as a postoperative reduction of the AHI to < 20 and > 50% postoperative reduction of AHI.5 Others have later proposed to tighten these criteria to a postoperative AHI to < 15 (regarded as “clinically relevant” OSA), < 10, and recently even < 5 (as in CPAP therapy).6 Some have added “response” as reduction of the AHI between 20% and 50%.7 The same discussion regarding success criteria has surfaced in oral device therapy. Without external validation, any arbitrary cut-off definition of success will be incorrect. Furthermore, the point at which the AHI becomes harmful remains unclear. He et al. reported an acceleration of harm when the AHI rises above 20-25.8 Whether a patient is “cured” when the AHI falls below 5 is questionable. CPAP therapy is regarded as successful if the AHI drops below 5 when CPAP is used. It is, however, a clinical reality that the use of CPAP is often cumbersome, and that CPAP is often not used 8 h/night for 7 days/ week. Patients seem to either tolerate the device well or not at all—a bimodal distribution, with an average of approximately 4 hours.9 Hence the term “compliance” was introduced. Current trends define compliance as 4 h/ night as an average over all nights observed.
The effectiveness of CPAP compliance criteria can be questioned, just as the effectiveness of surgical success criteria has often been questioned. For example, simple calculations show that given an average sleeping time of 8/24 h, the TST/week is 56 h. With a minimal compliance of 4 h/night, the mean AHI of a patient with an original AHI of 60 would only drop to 32.5 (during 50% of the TST, the AHI would be 5; but during the remaining 50%, the AHI would still be 60). In a similar way, an AHI of 40 would only decrease to 22.5, an AHI of 30 to 17.5, and an AHI of 20 to 12.5. Such moderate decreases can easily be reached with contemporary surgical techniques in well-selected patients, and are not even remotely in the range of the traditional liberal surgical success criteria of Sher (success would be reduction of the AHI of 60 to < 20, AHI of 40 to < 20, AHI of 30 to < 15, and AHI of 20 to < 10).10–13
It is clear that there is need for objective criteria to compare the effects of successful surgery, oral device therapy, and CPAP therapy. Such equations are lacking. We were interested to see if we could develop mathematical formulas and graphs to compare the expected mean drop in AHI in case of surgery and CPAP therapy. In other words: how to compare non optimal use of optimal therapy (CPAP) with the continuous effect (100%) of often non optimal therapy (surgery).
In this paper, we provide evidence that the suboptimal use of “highly effective CPAP treatment” can be compared with the 100% of the TST effect of “sub-therapeutic” surgical treatment effect.
FORMULAS
Using mathematical function formulas, the effect on the AHI of various treatment modalities and their respective compliance and success criteria, were calculated.
Formula 1: Calculation of mean AHI and percentage AHI reduction during compliant CPAP use
Firstly, a patient must use CPAP > 4 h/night to be deemed compliant. While using CPAP (HOURSonCPAP) ideally, the AHI is reduced to 0, 1, 2, 3, 4, or 5 (AHIonCPAP). While not using CPAP (HOURSoffCPAP = TST-HOURSonCPAP), we assume the AHI remains status quo (AHIoffCPAP). Ideally, we sleep 8 h each night (TST); the average AHI per night can be calculated using the following formula:
 |
For example: if we take a patient with an AHI of 19 (AHIoffCPAP). Using the compliance criteria cut-off discussed, our patient sleeps using CPAP 7 nights per week (NIGHTSonCPAP). The AHI (AHIoffCPAP) is reduced to 5 (AHIonCPAP) during 4 h, again using the compliance cut-off criteria discussed (HOURSonCPAP). During the residual 4 h (HOURSoffCPAP), the AHI remains 19. Using the generalized formula above and the parameters for this patient, we can calculate the mean AHI during compliant use of CPAP:
The mean AHI is 12, so the AHI is reduced by 36.84%.
Formula 2: Percentage of TST during which CPAP must be used to reduce AHI to < 5
To be cured of OSAS, the mean AHI must be < 5. What is the minimum percentage of TST during which CPAP must be used to reduce the AHI to < 5?
Multiplied by 100 and divided by the TST, one can calculate the percentage of time (AHIonCPAP%).
For example: in a patient with an AHI of 19, the AHI is reduced to 4 while wearing CPAP. Using the following general and specific parameters for this patient: TST: 8 h, AHIonCPAP: 4, AHIoffCPAP: 19, and AHI < 5:, one can calculate that CPAP must be used ≥ 7 h 27 min (which is equal to ≥ 93.33% of TST) to reach a mean AHI < 5.
One can use this formula to measure the minimum percentage of TST during which CPAP must be used to reduce the initial AHI below another number by simply replacing AHI < 5 by the other number, e.g., AHI < 10 (9.9).
Formula 3: Percentage of TST during which CPAP must be used to reduce AHI by a certain percentage
Examining CPAP from another angle, what is the minimum percentage of TST during which CPAP must be used to reduce the initial AHI by a certain percentage (%reduction):
For example: in a patient with an initial AHI of 77 (AHIoffCPAP), AHI is reduced to 5 while wearing CPAP (AHIonCPAP): He or she must use CPAP ≥ 96.25% of TST to reduce the mean AHI by 90% (%reduction).
When the AHI while using CPAP (AHIonCPAP) is reduced to 0, the formula reduces to:
Therefore, for example, to reach an 80% reduction in AHI, CPAP must be worn 80% of the time. But when the AHIonCPAP is > 0, the HOURSonCPAP% will be higher than the %reduction.
Formula 4: Percentage of TST during which CPAP must be used to meet Sher's success criteria
A 50% of the AHI is a straightforward division calculation, but from an AHI of 40 onwards, a 50% reduction is insufficient to meet surgical success criteria; the AHI must be lower than 20.
What is the minimum percentage of TST during which CPAP must be used to reach Sher's surgical success criteria?
Using various formulas shown above:
In case of an initial AHI < 40, one can use the following formula:
AND from an initial AHI of 40:
GRAPHS
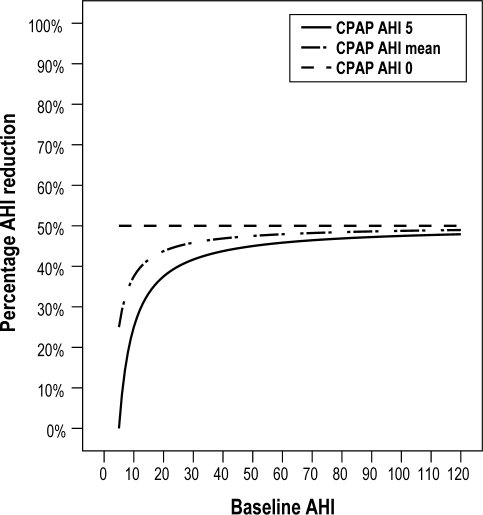
Percentage AHI reduction during compliant use of CPAP (Figure 1)
Figure 1.
Percentage AHI reduction when using CPAP 4 h/night. This graph depicts the results when applying formula 1 “Calculation of percentage AHI (apnea hypopnea index) reduction while compliantly using CPAP (continuous positive airway pressure).” While using CPAP, ideally the AHI is reduced to 0-5.
Assuming that on average, people sleep 8 h/ night, and using the 4 h/ night compliance criteria, patients must use CPAP during ≥ 50% of TST. As the initial AHI increases, so does the percentage of AHI reduction. Maximum AHI reduction stagnates at 50% when the AHI while using CPAP is 0 or initial AHI nears infinity. The lower the AHI while using CPAP (0-5), the more AHI reduction is attained (as shown in Graph 2). Patients with mild OSA (AHI 5-15) reduce their AHI by a minimum of 0% to 46.7% (AHI while using CPAP 1-5); those with moderate OSA (15-30) reduce their AHI by 33.3% to 48.3% (AHI while using CPAP 1-5); and those with severe OSA (AHI > 30) reduce their AHI by 41.7% (AHI while using CPAP 1-5).
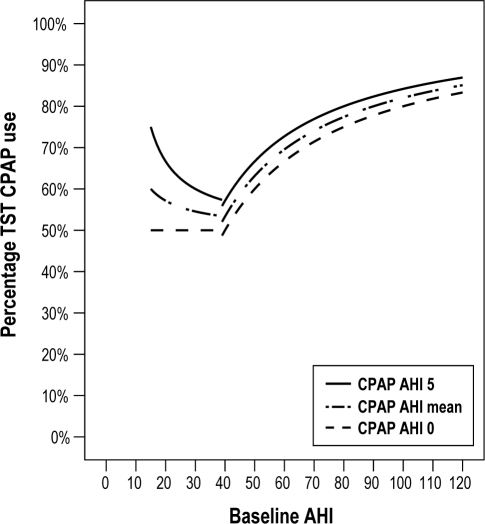
Percentage of TST during which CPAP must be used to meet Sher's success criteria (Figure 2)
Figure 2.
The minimum percentage of TST during which CPAP must be used to reach Sher's surgical success criteria. The traditional criteria for a successful surgical outcome, was defined by Sher et al. as a decrease ≥ 50% in postoperative AHI and a postoperative AHI < 20 in patients whose preoperative AHI was > 20. This graph depicts the results of formula 4: “Percentage TST (total sleeping time) during which CPAP must be used to meet Sher's success criteria”
The traditional criteria for a successful surgical outcome, was defined by Sher et al. as a decrease ≥ 50% in postoperative AHI, and a postoperative AHI < 20 in patients whose preoperative AHI was > 20.5
Keeping these traditional “succesful surgery” criteria into consideration, what is the minimal percentage of CPAP use needed per night to attain the same result? Up to an AHI of 40, CPAP must be used at least 50% of the time; thereafter, the percentage quickly curves upwards to reach a minimal percentage of 83.3% (initial AHI 120, AHI while using CPAP 0). These percentages surpass the 35.71% of the CPAP compliance criteria considerably and are therefore significantly harder to reach.
Elshaug et al. debate whether the definition for surgical success should be redefined as a post-operative AHI < 10 or even 5.
Percentage of TST during which CPAP must be used to reduce AHI to below 10
Concentrating on the first definition, to reach an average AHI < 10, CPAP must be used at least 33.3% to 66.7% per night, in patients with moderate OSA and an AHI of 0 whilst using CPAP. When the AHI is 5 while using CPAP, CPAP must be used minimally 50% of the TST. In patients with severe OSA, a minimum of 67.4% of nightly use is necessary when the AHI while using CPAP is 0, but 80% when the AHI whilst using CPAP is 5.
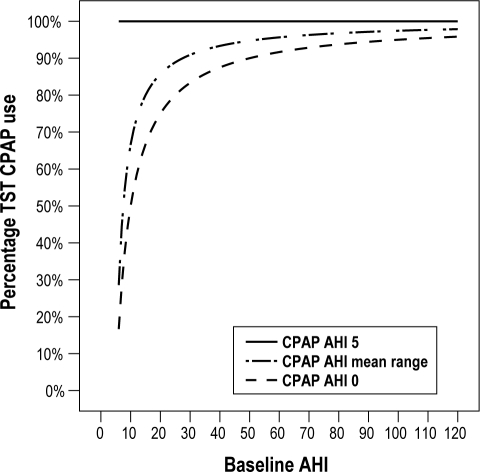
Percentage of TST during which CPAP must be used to reduce AHI to below 5 (Figure 3)
Figure 3.
The minimum percentage of TST during which CPAP must be used to reach a mean AHI < 5. To be cured of OSAS, the mean AHI must be < 5. This graph represents formula 2: “Percentage of TST during which CPAP must be used to reduce AHI to < 5.”
Focusing on the second definition (average AHI < 5), CPAP must be used 100% at night (when the AHI is 5 while using CPAP). In patients with moderate OSAS and an AHI of 0 while using CPAP, CPAP must be worn at least 66.7% to 83.3% of the TST (Figure 3). In patients with severe OSA, a minimum of 83.9% of nightly use is necessary when the AHI while using CPAP is 0, but 96.2% when the AHI while using CPAP is 4.
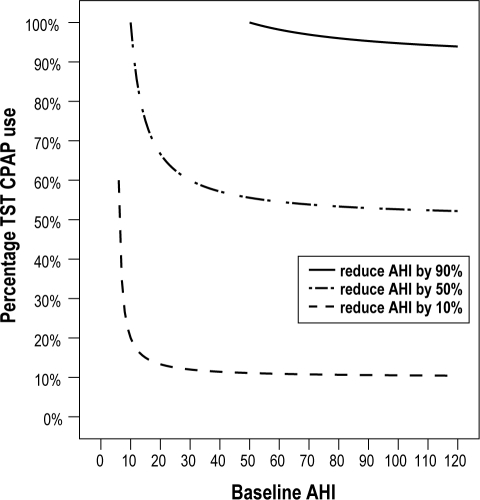
Percentage of TST during which CPAP must be used to reduce AHI by a certain percentage (Figure 4)
Figure 4.
The minimum percentage of TST during which CPAP must be used to reduce the initial AHI by a certain percentage (when the AHI is reduced to 5 while using CPAP). This graph shows the minimum percentage of TST during which CPAP must be used (when the AHI is 5 while using CPAP) to reduce the initial AHI by 10%, 50%, and 90%.
The initial AHI must be greater than 50 to be reduced by 90%; if the initial AHI is below 50, a 90% reduction is not possible. When the AHI is 50, the patient must use CPAP 100% of the TST. The percentage TST slowly dips to a maximum 90% as the AHI increases. When trying to reduce the AHI by 10% at an AHI of 6, CPAP must be worn 60% of the TST, the percentage TST slowly decreases to 10%.
DISCUSSION
Even the most effective medical devices are only effective when they are used. The effect may be 100% when always used, nil when never used, and partial when used sometimes but not always. This is particularly true for CPAP use in OSA.
Approximately 15% to 30% patients refuse CPAP; patients are often discouraged by the aesthetic aspect or the thought of being bound to CPAP for the rest of their lives.14 In patients who are willing to try CPAP, pressure is titrated to reduce the individual patient's AHI to < 5 events per hour.15 Most patients who discontinue CPAP treatment do so after a few months. In spite of advances in CPAP technology, such as auto-PAP, bi-level PAP, heated humidification, a large range of interfaces, and mask comfort improvement, as well as educational and behavioral support, patient adherence has only marginally improved in the last 23 years.15,17–19 Approximately 20% to 40% will discontinue CPAP after 3 months.19
Surgery, on the other hand, is slowly gaining momentum, because of improvements in patient selection, through use of the Friedman classification system, by means of drug-induced sleep endoscopy, and by applying more site-specific surgical techniques compared to earlier days (when the only surgical technique available was uvulopalatopharyngoplasty). Nevertheless, the Sher surgical success criteria are difficult to fulfill, especially in cases with severe OSA. It is generally easier to reach surgical success in patients with a low AHI, which is somewhat unjust. For example, a reduction of a mere 15 is needed to reach Sher's criteria in a patient with an initial AHI of 30, while a far larger decrease would be necessary in a patient with an AHI of 80 and CPAP failure. A reduction of 60 points would be insufficient (mandatory) to qualify for success; even though it goes without saying that the gain in quality of life and reduction of cardiovascular risk is likely to be more dramatic than less serious cases with a reduction of only 19 points.
Our formulas have various limitations. Assumptions were made to simplify and construct the formulas. Firstly, we acknowledge 8 hours of sleep per night to be ideal; others may appoint a different ideal (e.g., 7 h). In clinical practice, despite recording extensive CPAP use data, it is often difficult to acquire accurate total nightly hours of sleep in actual CPAP patients. Clinicians may want to request patients to record the latter parameter when applying the formula. We also assume that the AHI reverts to baseline as soon as CPAP is removed. CPAP is thought to play a role in reducing edema resulting from snoring-associated vibration and apnea-induced suction of the upper airway. The baseline AHI may be reduced by a fraction in chronic CPAP use. As the effect is minimal, we considered this point negligible.20 But we also make the assumption that the “AHIoffCPAP” matches the AHI of the diagnostic polysomnogram (PSG), and we consider the AHI to be uniform across the night, which raises the controversial question: how effective is a PSG as a gold standard for the diagnosis of OSA? PSG has many limitations. Besides night-to-night variability, the AHI does not have a uniform distribution over the night. The AHI fluctuates because of the cyclic alternating pattern of the sleep stages, body position of the patient, medication and alcohol use, nasal congestion, and external factors influencing sleep efficiency, such as a sleep laboratory vs home recording, etc.21–23 This is not only a hurdle for the present study, but a handicap for research and clinical management of OSA in general. Some clinicians argue that other PSG variables could be used as an outcome measure; e.g., desaturation index (DI) as a measure of intermittent hypoxia. The latter is also considered to be less susceptible to nightly variability.24,25 One could generalize these formulas to other PSG outcomes such as the apnea index or DI. Others argue that clinical outcomes may be more appropriate. There are more dimensions to consider in clinical management of OSA than AHI alone, e.g., side-effects, partner acceptance, or cost-effectiveness.
We assume that there is a linear dose-response between CPAP use and actual patient outcomes; so the more hours a patient uses CPAP, the greater the therapeutic effect. We question whether the dose-response curve is indeed linear, but unfortunately very little information is available in literature.26 Furthermore information is to be found concerning the outcome of patients who reject CPAP completely and are left untreated.27,28 As patients cannot “try” surgery, surgical literature does not include patients who chose not to have surgery and are also left untreated.
To continue the debate initiated by Elshaug et al., who contend that “current notions of surgical “success” are overdue for reevaluation,” we contend that the same holds true for CPAP compliance.7 CPAP compliance rates vary greatly. Weaver and Grunstein report in their review that 29% to 83% of patients are nonadherent and use their CPAP less than 4 hours per night.19 We recently analyzed the compliance of 232 CPAP users.15 Only 138 (59.5%) of these 232 patients were “compliant,” with compliance liberally defined as CPAP use > 4 h/night, > 5 days /week. Even in this compliant group, mean CPAP use was 6.5 h/night (81% with a given 8 h/night, SD 1.5), and 6.4 nights/week (91%, SD 1.4). With a CPAP use of 81% per night and 91% nights/ week, compliant patients on average still only use the device 74% of TST.15 Patients seem to either tolerate the device well or not at all—a bimodal distribution.9
Elshaug et al. have pointed out that when the traditional surgical definition (50% reduction in AHI and/or ≤ 20) is applied, the pooled success rate for Phase I procedures (soft palate) is 55%, but with AHI ≤ 10 as a cut-off point, success rate decreases to 31.5%; and at AHI ≤ 5, success is reduced to 13%. According to these definitions, Phase II (hard palate) success (fail) rates decrease from 86% (14%) to 45% (55%) and 43% (57%), respectively.6 But, if we use the mean AHI in CPAP therapy, instead of the AHI reduction while using CPAP and aim at a mean AHI ' 5, the % of the TST during which CPAP should be used increases as a result.
In conclusion, a goal of a mean AHI ≤ 5, for both surgery and CPAP therapy, is rarely achievable. As soon as CPAP is not regularly used, one should be aware that a cut-off point is easily reached when surgery could be more effective in reducing the mean AHI. To “cure” OSA, the AHI should be reduced to below 5, but even with CPAP this seems to be an idealistic goal. Nightly percentage use rises as one reduces the target to below 5 (see Graph 3). CPAP must be used at least 66.67% to 83.33% per night, in patients with moderate OSA and an AHI of 0 while using CPAP. In patients with severe OSA, a minimum of 83.87% of nightly use is necessary when the AHI while using CPAP is 0, but 100% when the AHI while using CPAP is 5.
Each patient is unique and reacts to treatment differently; there is no guarantee of success, despite success rates and results reported in literature. Success rates report mean improvements, but for yet unexplained reasons, some patients do better than others with comparable clinical features.
After surgery, the AHI remains relatively consistent during the night, but with CPAP it may dip below 5 during a certain percentage of TST and increase during the rest of the night when CPAP is not being used. Both may achieve the same mean AHI, but potential disparity between these treatment modalities on clinical symptoms and cardiovascular risk remains uncertain.
In conclusion, we hope this paper serves as motivation for further debate and discussion, initiated by Elshaug et al.6,29 Using a mean AHI in CPAP therapy is more realistic than using arbitrary compliance rates, which in fact hide insufficient reductions in AHI.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Kryger MW. Diagnosis and management of sleep apnea syndrome. Clin Cornerstone. 2000;2:39–47. doi: 10.1016/s1098-3597(00)90039-5. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Hutton R, Finn L, Baddr S, Palta M. The gender basis in sleep apnea diagnosis: are women missed because they have different symptoms? Arch Intern Med. 1996;156:2445–51. [PubMed] [Google Scholar]
- 3.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 4.Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea--a systematic review. Sleep. 2009;32:27–36. [PMC free article] [PubMed] [Google Scholar]
- 5.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 6.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep. 2007;30:461–7. doi: 10.1093/sleep/30.4.461. [DOI] [PubMed] [Google Scholar]
- 7.Richard W, Kox D, den Herder C, van Tinteren H, de Vries N. One stage multilevel surgery (uvulopalatopharyngoplasty, hyoid suspension, radiofrequent ablation of the tongue base with/without genioglossus advancement), in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2007;264:439–44. doi: 10.1007/s00405-006-0182-z. [DOI] [PubMed] [Google Scholar]
- 8.He J, Kryger M, Zorick F, et al. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 9.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 10.Kezerian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg. 2006;132:206–13. doi: 10.1001/archotol.132.2.206. [DOI] [PubMed] [Google Scholar]
- 11.Den Herder C, van Tinteren H, de Vries N. Hyoidthyroidpexia: a surgical treatment for sleep apnea syndrome. Laryngoscope. 2005;115:740–5. doi: 10.1097/01.mlg.0000156464.37681.BF. [DOI] [PubMed] [Google Scholar]
- 12.Hessel NS, de Vries N. Results of uvulopalatopharyngoplasty after diagnostic workup with polysomnography and sleep endoscopy: a report of 136 snoring patients. Eur Arch Otorhinolaryngol. 2003;260:91–5. doi: 10.1007/s00405-002-0511-9. [DOI] [PubMed] [Google Scholar]
- 13.Lin HC, Friedman M, Chang HW, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2008;118:902–8. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 14.Olsen S, Smith S, Oei TP. Adherence to continuous positive airway pressure therapy in obstructive sleep apnoea sufferers: a theoretical approach to treatment adherence and intervention. Clin Psychol Rev. 2008;28:1355–71. doi: 10.1016/j.cpr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Richard W, Venker J, den Herder C, et al. Acceptance and long-term compliance of nCPAP in obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2007;264:1081–6. doi: 10.1007/s00405-007-0311-3. [DOI] [PubMed] [Google Scholar]
- 16.Collard P, Pieters T, Aubert G, Delguste P, Rodenstein DO. Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med Rev. 1997;1:33–44. doi: 10.1016/s1087-0792(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–72. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 18.Rubins JB, Kunisaki KM. Contemporary issues in the diagnosis and treatment of obstructive sleep apnea. Postgrad Med. 2008;120:46–52. doi: 10.3810/pgm.2008.07.1790. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan CF, Lowe A, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144:939–44. doi: 10.1164/ajrccm/144.4.939. [DOI] [PubMed] [Google Scholar]
- 21.Wittig RM, Romaker A, Zorick FJ, Roehrs TA, Conway WA, Roth T. Night-to-night consistency of apneas during sleep. Am Rev Respir Dis. 1984;129:244–6. [PubMed] [Google Scholar]
- 22.Kramer M. Obstructive sleep apnea-one night is not enough. Sleep Res. 1988;17:205. [Google Scholar]
- 23.Portier F, Portmann A, Czernichow P, et al. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162:814–8. doi: 10.1164/ajrccm.162.3.9908002. [DOI] [PubMed] [Google Scholar]
- 24.Fietze I, Dingli K, Diefenbach K, et al. Night-to-night variation of the oxygen desaturation index in sleep apnoea syndrome. Eur Respir. 2004;24:987–93. doi: 10.1183/09031936.04.00100203. [DOI] [PubMed] [Google Scholar]
- 25.Aber WR, Block AJ, Hellard DW, Webb WB. Consistency of respiratory measurements from night to night during the sleep of elderly men. Chest. 1989;96:747–51. doi: 10.1378/chest.96.4.747. [DOI] [PubMed] [Google Scholar]
- 26.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily function. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 28.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 29.Elshaug AG, Moss JR, Hiller JE, et al. Upper airway surgery should not be first line treatment for obstructive sleep apnoea in adults. BMJ. 2008;336:44–5. doi: 10.1136/bmj.39381.509213.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]