Abstract
Study Objective:
Traffic noise disturbs sleep and may impair recuperation. There is limited information on single and combined effects of air, road, and rail traffic noise on sleep and recuperation.
Design:
Repeated measures.
Setting:
Polysomnographic laboratory study.
Participants:
72 healthy subjects, mean ± standard deviation 40 ± 13 years, range 18-71 years, 32 male.
Interventions:
Exposure to 40, 80, or 120 rail, road, and/or air traffic noise events.
Measurement and Results:
Subjects were investigated for 11 consecutive nights, which included 8 noise exposure nights and one noise-free control night. Noise effects on sleep structure and continuity were subtle, even in nights with combined exposure, most likely because of habituation and an increase in arousal thresholds both within and across nights. However, cardiac arousals did not habituate across nights. Noise exposure significantly affected subjective assessments of sleep quality and recuperation, whereas objective performance was unaffected, except for a small increase in mean PVT reaction time (+4 ms, adjusted P < 0.05). Road traffic noise led to the strongest changes in sleep structure and continuity, whereas subjective assessments of sleep were worse after nights with air and rail traffic noise exposure. In contrast to daytime annoyance, cortical arousal probabilities and cardiac responses were significantly lower for air than for road and rail traffic noise (all P < 0.0001). These differences were explained by sound pressure level rise time and high frequency (> 3 kHz) noise event components.
Conclusions:
Road, rail, and air traffic noise differentially affect objective and subjective assessments of sleep. Differences in the degree of noise-induced sleep fragmentation between traffic modes were explained by the specific spectral and temporal composition of noise events, indicating potential targets for active and passive noise control. Field studies are needed to validate our findings in a setting with higher ecologic validity.
Citation:
Basner M; Müller U; Elmenhorst EM. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. SLEEP 2011;34(1):11-23.
Keywords: Aircraft noise, road traffic noise, railway noise, traffic noise, awakening, arousal, heart rate, health, memory, reaction time
TRAFFIC NOISE IS PERCEIVED AS A MAJOR ENVIRONMENTAL STRESSOR BY THE AFFECTED POPULATION. ALTHOUGH NOISE EMITTED FROM SINGLE VEHICLES was significantly reduced in the past, this effect was outweighed by steadily increasing traffic volumes. The effects of traffic noise are manifold: It may disturb communication, cause annoyance, and impair recreation.1 Most of the complaints about traffic noise are received during the night,2 that is, when people try to sleep and regenerate mental and physical powers depleted during the day. In a representative German survey, when asked for reasons for existing sleep problems, external noise sources were mentioned in third position, outnumbered only by somatic disorders and problems of getting away from the strains of the day.3
Environmental noise may elevate the organism's arousal level, fragment sleep, and consequently lead to a redistribution of time spent in the different sleep stages, typically increasing wake and stage 1 sleep and decreasing slow wave sleep (SWS) and REM sleep.4,5 Although these global alterations are not specific for traffic noise, there is an ample number of laboratory and field studies showing that traffic noise causally disturbs sleep and, depending on number and acoustic properties of noise events, may impair behavior and well-being during the subsequent wake period.5–10 In addition, recent epidemiological research suggests that long-term traffic noise exposure increases the risk for cardiovascular disease, especially if people are exposed during the night.11,12
It has been repeatedly shown that the degree of noise annoyance depends on traffic mode. At the same average noise level, the percentage of highly annoyed residents decreases in the order aircraft noise, road traffic noise, and rail traffic noise.13 Possible explanations for these differences in annoyance have been brought forward, among them the potential threat of aircraft crashes, the problem of escaping from aircraft noise that, in contrast to road and rail traffic noise, is not restricted to one façcade of a building, and the positive environmental image of rail traffic. These annoyance differences have lead to the implementation of a rail bonus in the legislation of some countries. In several countries (e.g., Germany) average rail traffic noise levels may be 5 dB higher than those of other traffic modes before legal consequences are invoked.
Compared to traffic noise effects on annoyance, much less is known on the differences in physiological effects of air, road, and rail traffic noise, especially during the night. There has been no balanced study that would allow a “fair” comparison of the 3 traffic modes regarding their potential to disturb sleep. As noise events are evaluated even while asleep,14,15 we hypothesized that the same order found for annoyance effects would be found for sleep disturbance, i.e., that aircraft noise disturbs sleep more than road traffic noise, which itself is more disturbing than rail traffic noise. The results of a systematic comparison of the different traffic modes would deliver important information for legislative bodies, and help decide whether a rail bonus is also justified for the nighttime.
In most countries, limit values are set for each traffic mode separately. However, parts of the population are exposed to more than one traffic mode simultaneously, and, although individual limit values may not be exceeded, the overall noise load may nevertheless be unacceptable because of the combined effects induced by the exposure to multiple noise sources. We were therefore interested in whether the effects of combined exposure to multiple traffic modes were consistently higher compared to a scenario with exposure to a single traffic noise source.
Finally, if there are differences in the effects of the 3 traffic modes on sleep, we were interested to find out what acoustic properties of single noise events are responsible for these differences. This knowledge would allow mitigating the sleep disturbing effects of traffic noise by, in an engineering approach, either actively (at the source) or passively (sound insulation), targeting those acoustic properties responsible for the stronger effects.
This polysomnographic study investigated single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Both neurobehavioral tests and questionnaires were used to assess next day performance and well-being after nights with and without noise exposure. A word-pair test was used to investigate whether memory consolidation, representing one very important function of sleep, was affected by nocturnal noise exposure.
METHODS
Subjects and Protocol
Seventy-two subjects (40 ± 13 years, range 18-71 years, 32 male) were selected in a multistage selection process. They had to be healthy sleepers with an average time in bed of 6 to 10 h, and habitual time of retiring no earlier than 21:00 and no later than 01:00 during weekdays. Average habitual weekday sleep time of participating subjects was 7.9 h (SD 0.8 h), with an average habitual time of retiring of 23:09 and of getting up of 07:01. Subjects reporting apneas, loud snoring, or symptoms typical for restless legs syndrome (RLS) or periodic limb movement in sleep (PLMS) in our screening questionnaire were ineligible to participate. Hemoglobin oxygen saturation and heart rate were measured during one night at the subjects' home prior to study participation. Subjects with oxygen saturation profiles suspicious of sleep disordered breathing were excluded from study participation. Also, subjects needed to have normal hearing thresholds according to age, defined as a maximum hearing loss on the weaker ear no greater than 10% (18-33 years), 15% (34-49 years), or 20% (≥ 50 years). One subject discontinued for personal reasons and was replaced after the first study night. Another subject was excluded from the study after night 6 because of a viral infection. The study was approved by the local ethics committee. Subjects gave written informed consent prior to study participation and were free to discontinue any time without explanation.
We tried to make sure that the group was balanced according to prior annoyance. However, due to the vicinity of the German Aerospace Center to Airport Cologne/Bonn and the general dominance of road traffic exposure, we only partly managed to balance prior annoyance (percent with highest annoyance caused by: air 34.7%, road 45.8%, and rail 19.4%).
The subjects were investigated polysomnographically for 11 consecutive nights in groups of 8 in the underground sleep facility of the German Aerospace Center (DLR). Physiological variables included the electroencephalogram (EEG: C3-A2, C4-A1), the electrooculogram (EOG), the electromyogram (EMG), the electrocardiogram (ECG), respiratory movements of rib cage and abdomen, and finger pulse amplitude. Additionally, subjects wore actigraphs 24 h a day. The first night served as adaptation. It was noise-free and excluded from the analyses. In nights 2-10, different noise exposure patterns were played back, including a silent control night (see below). Night 11 served as backup, i.e., if signals of relevant electrodes were lost and sleep stage classification was impossible for a subject in nights 2 to 10, the respective noise scenario was presented in night 11 again; otherwise, it served as a noise-free recovery night. Data from night 11 were only used for the event-related analysis on awakening probability; less than 4.4% of the data stemmed from night 11.
During the study period, subjects stayed in the laboratory from 19:00 until 08:00. During the day they were free to go on with their usual daily activities, with the exception that naps were not allowed. A large part of the evening was granted for applying electrodes and sensors. At 21:00, subjects conducted the Advisory Group for Aerospace Research and Development (AGARD) performance test battery16 and the evening part of the word pair test (see below). Shortly before going to bed, subjects filled in a short questionnaire. Lights off was scheduled for 23:00. Lights were turned on again exactly after a time in bed (TIB) of 8 h. Immediately after getting up and detaching the electrodes, the subjects filled in various questionnaires and performed the morning part of the word pair test and the AGARD tests. Afterwards, the subjects were allowed to shower and to have breakfast. Caffeinated or alcoholic drinks should only be consumed in moderation during the day and were prohibited after 15:00. The study was conducted in a double-blind fashion, i.e., neither the investigators nor the subjects were aware of the exposure pattern of the following night.
Description of Noise Scenarios
There were 9 different noise scenarios with a noise-free control night and single, double, and triple exposure nights. Traffic noise events were recorded with class-1 sound level meters in bedrooms of residents living close to a road, a railway track, or an airport. We tried to choose representative noise events for each traffic mode, and used various measuring sites for recording. The 3 single exposure nights each consisted of 40 noise events from one traffic mode only, i.e.; aircraft (AI), road (RO), or rail (RA). Acoustic properties of these 40 noise events are shown in Table 1 for each traffic mode. Event rise time was analyzed automatically with a MatLAB algorithm and inspected visually for plausibility.
Table 1.
Acoustic properties of noise events depending on traffic mode; each traffic mode category consists of N = 40 noise events
| AIR | ROAD | RAIL | P (Mann-Whitney-U test) |
|||
|---|---|---|---|---|---|---|
| Variable | Mean (SD, Range) | Mean (SD, Range) | Mean (SD, Range) | AIR vs. ROAD | AIR vs. RAIL | ROAD vs. RAIL |
| SPL rise time [dB/s] | 3.6 (1.1, 1.2 - 5.8) | 6.3 (1.9, 3.1 – 13.6) | 7.1 (2.6, 2.3 – 12.7) | < 0.0001 | < 0.0001 | 0.0798 |
| Noise duration [s] | 66.0 (16.6, 36.8 – 109.5) | 20.5 (7.2, 9.1 – 38.1) | 25.9 (7.9, 14.0 – 46.4) | < 0.0001 | < 0.0001 | 0.0030 |
| Octave energy 31.5 Hz [dB] | 44.6 (6.9, 34.4 – 63.4) | 43.4 (6.2, 32.8 – 61.2) | 62.5 (4.8, 52.9 – 70.1) | 0.4914 | < 0.0001 | < 0.0001 |
| Octave energy 63 Hz [dB] | 47.8 (5.9, 37.9 – 60.4) | 48.6 (8.4, 33.1 – 68.6) | 59.9 (5.9, 43.2 – 71.0) | 0.8549 | < 0.0001 | < 0.0001 |
| Octave energy 125 Hz [dB] | 45.4 (5.9, 35.9 – 63.3) | 40.9 (9.7, 24.8 – 67.5) | 52.3 (6.8, 36.6 – 66.8) | 0.0084 | < 0.0001 | < 0.0001 |
| Octave energy 250 Hz [dB] | 46.1 (5.6, 37.7 – 62.6) | 37.6 (6.8, 26.2 – 51.4) | 51.1 (7.7, 36.7 – 64.6) | < 0.0001 | 0.0029 | < 0.0001 |
| Octave energy 500 Hz [dB] | 46.0 (5.2, 37.9 – 56.6) | 40.0 (7.0, 29.1 – 54.0) | 47.1 (7.2, 33.1 – 61.4) | 0.0002 | 0.4558 | 0.0001 |
| Octave energy 1 kHz [dB] | 41.8 (32.6, 32.4 – 52.7) | 45.0 (5.8, 36.2 – 55.0) | 42.0 (6.5, 28.5 – 54.9) | 0.0289 | 0.8061 | 0.0377 |
| Octave energy 2 kHz [dB] | 32.6 (6.9, 21.3 – 45.1) | 40.6 (6.3, 30.6 – 51.9) | 39.4 (7.2, 25.9 – 53.9) | < 0.0001 | 0.0003 | 0.4052 |
| Octave energy 4 kHz [dB] | 18.1 (6.6, 9.5 – 30.6) | 30.1 (5.5, 22.0 – 42.2) | 32.6 (8.2, 18.3 – 46.1) | < 0.0001 | < 0.0001 | 0.1019 |
| Octave energy 8 kHz [dB] | 11.4 (1.8, 7.9 – 15.5) | 18.6 (5.8, 9.7 – 32.7) | 18.1 (5.6, 11.6 – 37.0) | < 0.0001 | < 0.0001 | 0.7508 |
SD refers to standard deviation; mid frequencies are given for octaves
Noise events belonged to one of 5 maximum sound pressure level categories (A-weighted with time constant set to slow): 45, 50, 55, 60, or 65 dB. Therefore, single exposure nights consisted of 8 noise events from each of the 5 sound pressure level (SPL) categories. For rail traffic noise, each SPL category was divided into 4 noise events from freight trains and 4 noise events from passenger trains. For road traffic noise, each category was divided into 5 noise events from passenger cars with dry roads, one noise event from passenger cars with wet roads, one noise event from motorcycles, and one noise event from trucks. Aircraft noise was not divided further.
There were 3 double exposure nights: Aircraft plus road traffic noise (AIRO), aircraft plus rail traffic noise (AIRA), and road plus rail traffic noise (RORA). Each of the double exposure nights consisted of 40 noise events from each of the respective single exposure nights, i.e., 80 noise events in total. There was one triple exposure night (AIRORA), consisting of all 120 noise events from the single exposure nights.
With this study design, exposures with different traffic modes were comparable according to number and maximum SPL of noise events. Additionally, we managed that both AI and RA exposure nights calculated to an average sound level LA,eq of 39.7 dB. Because of the shorter duration of road traffic noise events, the LA,eq of the road traffic single exposure night was lower than 39.7 dB. In order to achieve an LA,eq of 39.7 dB, the number of road traffic noise events was doubled in exposure night RORO. In that way, it was possible to compare single exposure nights based on LA,eq as well. In the noise-free control night the LA,eq of about 30 dB was caused by the constant sound of the A/C system.
Within a single exposure night, the length of the time interval between the start of 2 noise events differed depending on the number of noise events per night. The length of the interval differed in nights with 40 noise events between 3 and 21 min; in nights with 80 noise events between 3 and 9 min; and in nights with 120 noise events between 3 and 5 min. Using block randomization we assured that the length of intervals between noise events and maximum SPLs were evenly distributed throughout the night. In single, double and triple exposure nights playback of noise events started after 12, 6, and 4 minutes, respectively. Playback always started at the beginning of a full minute to coincide with the beginning of a 30-s sleep epoch.
In order to be able to balance the study design, i.e., applying every exposure scenario in every study night position exactly once, there were 9 study periods with 8 subjects each. We assured that no more than 2 double or triple exposure nights (AIRO, AIRA, RORA, RORO, AIRORA) followed each other. Because sound insulation of sleep cabins was not absolute, in each study period, all 8 subjects received the same noise pattern in the same night. Sleep cabins were acoustically calibrated before each study period. There were no noise-free nights interposed between 2 exposure nights, i.e., there were no wash-out nights (except for the noise-free control night).
Questionnaires
Morning questionnaires were administered after electrode detachment and included a variety of sleep related questions. This analysis concentrates on visual analogue scales dealing with 6 different aspects of sleep quality and recuperation: (1) falling asleep (anchors easy-hard), (2) sleep continuity (anchors calm-disturbed), (3) sleep depth (anchors deep-light), (4) recuperation (anchors high-low), (5) feeling sleepy after wake-up, and (6) feeling sleepy before retiring in the evening (anchors fresh-tired). The scales ranged from 0 to 1000, with higher values always indicating worse sleep quality.
Performance Tests
After questionnaire administration in the morning, subjects conducted a 10-min psychomotor vigilance test (PVT)17 and a 4-letter memory and search task (MST)16 that were implemented on the test-software ERTS (Berisoft Company). During the PVT, the subjects had to respond to a white stopwatch appearing in irregular intervals (1.5 s to 10 s) on the dark screen by pressing a key as fast as possible. In contrast to the PVT described by Dinges and Powell,17 the AGARD version times out after only 850 ms compared to the original 30 s. We used mean reaction time (RT) and the number of lapses (defined as RT ≥ 500 ms) as our main PVT outcome variables. For the purpose of statistical analyses, we performed a square root transform on the number of lapses to better reflect a normal distribution. In the MST, 4 letters had to be memorized at the beginning of the task without time pressure. In the 3-min recall phase, single letters were randomly presented, 50% of them belonging to the learning set. Subjects were asked to decide as quickly and accurately as possible whether the current letter belonged to the learning set or not by pressing 2 different keys. The MST timed out after 4 s. We used detection accuracy A' and mean RT as our MST outcome variables.18 The signal detection measure A' reveals the extent to which subjects are able to differentiate signal (letter belongs to the learning set) from noise. A' varies between 50% (performance at chance level) to 100% (perfect accuracy). RTs < 130 ms were excluded from PVT and MST analysis as false starts. Performance tests were repeatedly practiced before the study until stable performance levels were achieved.
Word Pair Test
Noise effects on declarative memory consolidation were investigated with a word pair test.19 Eleven different word lists were used, each consisting of 24 associate pairs of German nouns (e.g., car - trunk). In addition to the 24 word pairs, 4 dummy word pairs at the beginning and 4 dummy word pairs at the end of each list served as primacy and recency effect buffers. The word pair sequence within a list was randomized over repeated trials in both presentation and recall phases in order to prevent serial learning. Each word pair was presented for 5 sec. The recall phase started directly after presentation. During the recall phase, the first word of a pair was presented and the subject was asked to type the second word without time pressure on a laptop keyboard. If the subject memorized at least 60% (i.e., 15 word pairs) correctly, the evening part of the test was finished. Otherwise, presentation and recall phases were repeated, but not more than twice. The recall phase was repeated in the morning after the performance tests. The difference between the last evening and the morning recall phase in the number of word pairs correctly remembered was the primary outcome variable.
Data Analysis
The 40 noise events of each traffic mode (AI, RO, RA) were analyzed for differences in SPL rise time, event duration, and octave band energy with nonparametric Mann-Whitney-U tests.
Experienced technicians scored the polysomnograms according to the criteria of Rechtschaffen and Kales20 (for sleep staging) and the American Sleep Disorders Association21 (for EEG arousals). Movement time was scored as wake and stages S3 and S4 were combined to SWS. Both the beginning and the duration of EEG arousals were noted. A total of 37 nights were excluded from the analysis because TIB was < 480 min because of signal loss. Another 12 nights were excluded because the whole night could not be analyzed because of technical problems or medical problems (e.g., toothaches). Hence, 599 of 648 nights (92.4%) contributed to the final analysis. The following variables were subjected to descriptive and inferential analyses:
The sleep structure variables included sleep onset latency (SOL), SWS latency, and REM latency, defined as the first occurrence of stage S1 and the first occurrence of SWS or REM after sleep onset, respectively; duration of wake, S1, S2, SWS, and REM in minutes as part of a constant TIB of 480 min; total sleep time (TST), wake after sleep onset (WASO), and sleep efficiency (TST/TIB). The sleep continuity variables included number of awakenings per h TST (the termination of an awakening was defined as the first occurrence of a sleep stage other than wake or S1) and number of ASDA EEG arousals per h TST, the number of sleep stage changes per hour SPT (sleep period time, i.e., sleep onset until final awakening), and the physiologic variable average heart rate in SPT. Subjective sleep quality and recuperation were assessed with 6 visual analogue scales. Neurobehavioral performance was assessed with mean RT and number of lapses on the PVT, and mean RT and accuracy on the MST; and finally the number of word pairs forgotten in the memory test.
The means of these variables were estimated with a random subject effect regression model22 (MIXED procedure of SAS, SAS Institute, Version 9.2) for the 8 different exposure nights, for the noise-free control night, and for pooled data of single (AI, RO, RA), double (AIRO, AIRA, RORA), triple (AIRORA), and all exposure nights. Proc MIXED was also used to contrast all exposure categories (11 in total) individually to the noise-free control night. We adjusted for multiple testing by limiting the false discovery rate, i.e., the expected fraction of null hypotheses rejected mistakenly, to 0.05 (*), 0.01 (**), and 0.001 (***), respectively.23 Pooled data of single, double, and triple exposure nights were contrasted with Proc MIXED to investigate cumulative effects of noise. Finally, within-subject differences between study night 10 and study night 2 were calculated to investigate a time-in-study effect on the outcome variables. A one-sample t-test was used to test whether these differences were statistically significant from zero.
Event-Related Analysis
An event-related analysis establishes a temporal association between the occurrence of a noise event and the reaction of the investigated subject.24–26 This analysis was facilitated by sampling electrophysiological signals and acoustic data synchronously. Our primary outcomes of interest were EEG awakenings20 and EEG arousals21 (representing 2 degrees of cortical arousal) and changes in heart rate (representing vegetative [autonomic] arousals). Awakenings were defined as sleep stage changes from any sleep stage other than wake to stage wake. By design, noise events started exactly at the beginning of a sleep epoch, which was then defined as the first epoch under the influence of noise. An ANE (aircraft noise event) was excluded from the analysis if the subject was already awake in the epoch preceding the first noise epoch. Therefore, noise events outside of SPT, i.e., before sleep onset or after final awakening, were also excluded from the analysis.
The reactions of sleeping subjects to noise are nonspecific, because they are also observed spontaneously in undisturbed nights or between noise events. Two important implications follow. First, if the sleeper reacts while exposed to aircraft noise, it is unclear whether this reaction was induced by noise or whether it was spontaneous, because there is currently no method to identify the underlying cause of the reaction.27 Second, a certain interval after the beginning of the noise event is usually screened for a reaction of the sleeper. On the one hand, this interval should be long enough to detect all noise-related reactions. On the other hand, if the interval is too long, too many spontaneous (i.e., non–noise-related) reactions are picked up, and repeated activations within the same subject are possible. In this study, the first noise epoch and the epoch following it were screened for an EEG awakening, as this maximized the difference in awakening probability with and without noise exposure (i.e., signal to noise ratio). Spontaneous reaction probability was determined in noise-free control nights. Here, for each noise event onset, the respective interval in the control night of the same subject was screened for spontaneous reactions with the above mentioned methodology.
For EEG arousals, a 60-s window following the beginning of a noise event was screened for arousal onset. This way, noise events from the 3 traffic modes were compared on an equal footage, and comparisons with the analysis based on EEG awakenings were facilitated. The noise event was only included if it fell within SPT and if the 10-s interval preceding noise onset was free of EEG arousals. In the same way, spontaneous EEG arousal probability was determined in noise-free control nights.
For heart rate analysis, heart beats with inter-beat intervals (IBI) > 2 s or < 500 ms (corresponding to heart rates of < 30 bpm and > 120 bpm) were considered invalid (less than 0.2% of all beats). Nights where valid heart beats covered less than 95% of SPT were excluded from the analysis (N = 28). For each noise event, maximum heart rate was identified in a 60-s time window following noise onset. Then, average heart rate was calculated for an interval ± 10 s relative to the moment when maximum heart rate occurred. The same procedure was repeated for a 30-s time window preceding noise onset. The difference between average heart rate after and before noise onset was calculated and constituted the outcome variable for the event related analysis. This difference increases both with amplitude and duration of a noise-induced heart rate response. Noise events were excluded from analysis if the screening window contained > 10% invalid heart beats (see above) or a single heart beat with an IBI > 6 s.
Altogether, 31,266 noise events contributed to the analysis of awakening probability, 29,151 noise events contributed to the analysis of arousal probability, and 30,224 noise events contributed to the event-related heart rate analysis. Proc NLMIXED was used to perform random subject effect regressions on the noise event data only. This type of regression takes the clustered nature of the data into account, i.e., that each subject was exposed to several noise events.22 Also, in contrast to classic repeated-measures ANOVA, it can to some extent deal with missing data. For the awakening and arousal data, random subject effect logistic regression was performed. The dichotomous dependent variable was classified as 1 for an awakening or arousal and 0 for no awakening or no arousal. For the heart rate data, random subject effect linear regression was performed.
Several independent variables were considered as predictors or mediators. They were entered hierarchically into the regression model. The first model contained two indicator variables for traffic mode, contrasting air and rail traffic with road traffic (reference), and maximum SPL. The second model contained, additional to the variables of model one, individual and situational moderators, i.e., age and gender, sleep stage in the epoch preceding the noise event with stage 2 as reference, elapsed sleep time since sleep onset, study night (2-11), the number of noise events per night with single exposure nights serving as reference, and noise-free interval between the end of the last (or, in case of the first noise-event, sleep onset) and the beginning of the current noise event. The third model contained, additional to the variables of model two, acoustical moderators, i.e., noise event duration, SPL rise time, and octave band energy for mid frequencies from 31.5 Hz to 8 kHz. We were primarily interested in whether traffic modes differed in their influence on the outcome variables, and if so, whether other variables, especially acoustic variables, would account for these differences.
All models were re-run with air traffic as the reference category for traffic mode to also obtain estimates for the contrast of rail versus air traffic noise. Also, all models were re-run with fast instead of slow time constants for the calculation of maximum SPLs. As the results of both series of models (slow vs. fast) did not differ relevantly, only the results for slow time constant are presented here. All continuous variables were mean centered and inspected for linearity in the logit of awakening and arousal probability, and in heart rate change, respectively. Based on the results of this analysis, maximum SPL was the only variable to enter the models non-linearly. The variables “noise-free interval,” “noise event duration,” and octave bands with mid frequencies of 250 Hz, 1 kHz, and 2 kHz were statistically nonsignificant (P > 0.05) and thus removed from the final models.
Facilitating data on reactions with and without noise exposure, we used random subject effect logistic regression models to estimate the difference between awakening and arousal probability with and without noise exposure, i.e., the probability in excess of spontaneous probability. Altogether, 31,266 noise events and 30,655 control events contributed to the analysis of awakening probability, while 29,151 noise events and 29,584 control events contributed to the analysis of arousal probability. Average heart rate in a 60-s interval after noise onset was compared to average heart rate in a 30-s interval before noise onset with a linear regression model with random subject effect.
Additionally, the number of awakenings and the number of arousals were counted separately for periods with and without noise exposure and for control, single, double, and triple exposure nights to derive the average number of awakenings/arousals per h TST and the average duration of awakenings/arousals for the above mentioned conditions. Again, the 60-s interval following noise onset was defined as being influenced by noise for both awakenings and arousals. We were especially interested in whether spontaneous awakening/arousal frequency between noise events would change compared to noise-free control nights. For the investigation of cumulative effects on sleep, Proc MIXED was used to estimate differences between control, single, double, and triple exposure nights. Eleven subjects were excluded only from this analysis because the control or the triple exposure night, or all of the single (AI, RO, RA) or double (AIRO, AIRA, RORA) exposure nights were missing.
RESULTS
Acoustic Properties
Comparisons of acoustic properties of the different traffic modes are shown in Table 1. SPL rise time was significantly faster in road and rail compared to aircraft noise, while noise duration increased significantly in the order road, rail, and air traffic noise. Sound energy in the low frequency octave bands was significantly higher for rail compared to road and aircraft noise, while sound energy in the high-frequency octave bands was significantly lower for aircraft noise than for road and rail traffic noise, while it did not differ significantly between the latter.
Noise Effects on Sleep, Performance, and Memory Consolidation
The results of the analysis of sleep variables, subjective assessment of sleep quality, performance, and memory consolidation are shown in Table 2. Compared to the pooled data of all exposure nights, SWS latency and amounts of stage 1 were significantly lower in the noise-free control nights, while amounts of SWS were significantly higher. The frequency of arousals and sleep stage changes was significantly higher in exposure nights. There was no difference in average heart rate. Subjects assessed their sleep as being significantly more disturbed, lighter, and with a lower recuperative value. They also felt significantly more tired after waking up, but not in the next evening.
Table 2.
Analysis of sleep structure, sleep continuity, subjective assessment, performance, and memory consolidation according to noise exposure
| Control |
All Exposure Nights |
Single Exposure Nights |
Double Exposure Nights |
Triple E.N. |
N10 - N2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO | Pooled | AI | RO | RA | Pooled | AIRO | AIRA | RORA | RORO | Pooled | AIRORA | ||
| Equivalent SPL LA,eq [dB] | 30.0 | - | 39.7 | 36.9 | 39.7 | - | 41.2 | 42.5 | 41.2 | 39.7 | - | 43.3 | - |
| Sleep Structure | |||||||||||||
| Sleep onset latency [min] | 14.6 (12.5) | 14.1 (8.9) | 12.8 (13.9) | 14.7 (12.3) | 13.5 (10.7) | 13.7 (9.8) | 13.1 (11.0) | 14.5 (12.5) | 16.1 (12.5) | 13.5 (18.2) | 14.6 (10.6) | 14.6 (13.2) | -5.4 (17.9)* |
| SWS latency [min] | 26.3 (10.7) | 34.6 (31.3)** | 30.5 (32.7) | 33.4 (21.9)* | 30.8 (14.2) | 31.7 (28.7)* | 35.6 (45.3)* | 32.3 (30.5) | 34.4 (18.7)* | 43.3 (35.8)*** | 34.2 (33.4)** | 36.9 (27.4)** | -1.0 (21.8) |
| REM latency [min] | 77.3 (25.9) | 86.6 (21.2) | 81.1 (29.7) | 80.0 (34.2) | 84.5 (34.2) | 81.8 (27.6) | 87.6 (35.6) | 92.8 (34.7)* | 92.1 (44.5)* | 86.1 (39.5) | 90.9 (27.3)* | 88.0 (32.4) | -25.6 (44.8)*** |
| Wake [min] | 53.3 (36.7) | 57.2 (25.2) | 50.3 (31.9) | 57.6 (34.9) | 53.8 (29.1) | 53.8 (29.5) | 58.8 (32.9) | 57.2 (39.8) | 59.0 (27.8) | 60.3 (35.5) | 58.3 (25.6) | 60.5 (37.1) | -13.2 (37.1)** |
| S1 [min] | 21.5 (11.3) | 25.5 (10.9)*** | 24.7 (11.6)* | 26.3 (13.3)*** | 25.8 (10.8)** | 25.6 (10.8)*** | 25.1 (14.4)** | 25.8 (12.1)** | 25.8 (12.7)** | 25.9 (12.7)** | 25.6 (11.1)*** | 24.9 (12.6)** | -0.5 (11.3) |
| S2 [min] | 243.7 (37.6) | 244.4 (29.7) | 246.5 (36.3) | 243.5 (34.2) | 247.3 (34.8) | 245.8 (31.5) | 242.3 (38.9) | 243.9 (43.2) | 245.0 (40.8) | 240.9 (35.1) | 243.9 (33.1) | 245.9 (37.1) | -0.0 (36.9) |
| SWS [min] | 64.7 (32.3) | 58.7 (28.8)** | 61.4 (32.0) | 58.0 (31.9)** | 58.6 (27.9)* | 59.4 (29.5)** | 61.8 (30.5) | 58.9 (33.4)* | 54.8 (28.5)*** | 56.7 (30.8)** | 58.5 (29.4)** | 59.5 (31.3)* | 1.9 (21.5) |
| REM [min] | 96.5 (20.0) | 94.1 (16.0) | 97.1 (20.3) | 94.3 (21.5) | 94.4 (20.8) | 95.3 (18.2) | 91.9 (19.8) | 93.9 (21.5) | 95.5 (25.4) | 96.2 (19.4) | 93.8 (16.8) | 89.2 (24.5) | 11.9 (22.0)*** |
| WASO [min] | 38.7 (31.9) | 43.0 (22.8) | 37.4 (26.3) | 43.0 (33.3) | 40.2 (25.9) | 40.1 (25.9) | 45.7 (32.0) | 42.7 (37.0) | 42.8 (24.4) | 46.8 (28.4) | 43.6 (23.0) | 45.9 (31.7) | -8.2 (30.6)* |
| Sleep efficiency [%] | 88.9 (7.6) | 88.1 (5.2) | 89.5 (6.6) | 88.0 (7.3) | 88.8 (6.1) | 88.8 (6.1) | 87.7 (6.9) | 88.1 (8.3) | 87.7 (5.8) | 87.4 (7.4) | 87.9 (5.3) | 87.4 (7.7) | 2.8 (7.7)** |
| Sleep Continuity | |||||||||||||
| Awakenings per h TST [N] | 3.2 (2.1) | 3.4 (1.0) | 3.0 (1.0) | 3.3 (1.2) | 3.4 (1.2) | 3.3 (1.0) | 3.4 (1.7) | 3.5 (1.1) | 3.4 (1.2) | 3.6 (1.1) | 3.4 (1.1) | 3.5 (1.3) | -0.0 (1.3) |
| ASDA Arousals per h TST [N] | 14.5 (6.0) | 16.2 (6.3)** | 14.9 (7.1) | 16.2 (6.8)* | 15.7 (6.0) | 15.6 (6.5) | 16.8 (8.0)** | 16.2 (8.0)* | 16.3 (6.1)* | 16.1 (6.8)* | 16.4 (6.3)** | 17.2 (7.6)*** | -1.4 (4.6)* |
| Stage changes per h SPT [N] | 17.0 (5.6) | 18.2 (4.6)** | 17.1 (4.5) | 18.0 (5.0)* | 18.4 (5.1)** | 17.8 (4.4)* | 18.2 (5.7)* | 18.3 (4.6)** | 18.3 (5.4)** | 18.5 (5.6)** | 18.3 (4.8)** | 18.6 (5.5)** | 0.4 (4.4) |
| Mean Heart Rate in SPT [1/min] | 63.9 (8.4) | 63.3 (7.5) | 63.6 (8.4) | 63.2 (7.3) | 63.8 (7.1) | 63.2 (7.5) | 63.4 (8.4) | 63.7 (8.4) | 62.8 (7.0) | 63.3 (7.2) | 63.3 (7.5) | 63.4 (7.6) | 0.8 (4.1) |
| Subjective Assessment VAS | |||||||||||||
| Falling asleep (hard) | 345 (259) | 392 (138) | 368 (297) | 342 (261) | 365 (266) | 359 (183) | 378 (288) | 381 (265) | 388 (280) | 465 (293) | 382 (164) | 447 (289) | -200 (373)*** |
| Sleep continuity (disturbed) | 316 (222) | 472 (150)*** | 422 (267)** | 396 (241)* | 442 (232)*** | 420 (167)*** | 456 (256)*** | 503 (255)*** | 524 (257)*** | 490 (255)*** | 495 (164)*** | 546 (260)*** | -115 (317)** |
| Sleep depth (light) | 358 (248) | 455 (166)*** | 414 (267) | 383 (247) | 426 (245) | 408 (186) | 428 (252) | 496 (237)*** | 490 (281)*** | 478 (252)** | 472 (195)*** | 529 (263)*** | -140 (337)** |
| Recuperation (low) | 332 (209) | 491 (162)*** | 455 (245)*** | 435 (225)** | 455 (239)*** | 448 (172)*** | 468 (248)*** | 533 (245)*** | 531 (291)*** | 494 (249)*** | 511 (191)*** | 559 (248)*** | -125 (312)** |
| Sleepiness morning (sleepy) | 387 (235) | 503 (183)*** | 513 (248)*** | 460 (235)* | 503 (233)*** | 492 (181)*** | 478 (255)** | 536 (261)*** | 514 (265)*** | 483 (246)** | 510 (211)*** | 535 (276)*** | -72 (308) |
| Sleepiness evening (sleepy) | 607 (246) | 621 (171) | 616 (242) | 582 (246) | 622 (227) | 607 (191) | 621 (256) | 630 (237) | 655 (242) | 639 (203) | 635 (186) | 605 (245) | -17 (238) |
| Performance | |||||||||||||
| PVT mean reaction time [ms] | 254 (30) | 258 (31)* | 261 (35)** | 256 (31) | 256 (31) | 258 (31)* | 257 (33) | 258 (33)* | 259 (32)* | 258 (34) | 258 (32)* | 258 (34)* | 4 (13)* |
| PVT lapses [N] | 0.6 (1.5) | 0.7 (1.9) | 0.9 (1.4) | 0.7 (1.3) | 0.6 (2.3) | 0.7 (2.0) | 0.6 (2.1) | 0.8 (1.6) | 0.6 (2.7) | 0.8 (1.5) | 0.7 (1.6) | 0.7 (1.6) | 0.4 (1.5) |
| MS4 mean reaction time [ms] | 581 (111) | 572 (105) | 572 (107) | 572 (122) | 572 (110) | 572 (108) | 570 (112) | 565 (110) | 574 (98) | 575 (128) | 569 (106) | 577 (113) | -14 (68) |
| MS4 accuracy A' [%] | 98.7 (1.4) | 98.9 (0.9) | 98.9 (1.1) | 99.0 (1.1) | 98.9 (1.2) | 98.9 (0.9) | 99.0 (1.6) | 98.7 (0.9) | 98.9 (1.5) | 98.8 (1.6) | 98.9 (1.1) | 98.9 (1.4) | -0.6 (1.6)** |
| Memory Consolidation | |||||||||||||
| Word pairs forgotten [N] | 2.1 (2.0) | 2.5 (1.4) | 2.5 (2.2) | 3.0 (2.4) | 2.2 (2.0) | 2.6 (1.7) | 2.5 (2.2) | 2.6 (2.0) | 2.6 (2.0) | 2.7 (2.2) | 2.6 (1.6) | 2.3 (1.9) | 1.2 (2.5)*** |
Estimated means and standard deviation (in parenthesis) are shown. For the calculation of standard deviations in the pooled categories, outcomes were averaged within subjects first. N10-N2 refers to the difference in outcome variables between night 10 and night 2
E.N., Exposure Nights; SPL, sound pressure level; PVT, psychomotor vigilance test; MS4, memory and search task with 4 letters; AI, aircraft noise; RO, road traffic noise; RA, rail traffic noise; NO, noise-free control night; All Exposure Nights Pooled: AI, RO, RA, AIRO, AIRA, RORA, RORO, AIRORA; Single Exposure Nights Pooled: AI, RO, RA; Double Exposure Nights Pooled: AIRO, AIRA, RORA;
P < 0.05
P < 0.01
P < 0.001 after controlling for a false discovery rate, i.e., the expected fraction of null hypotheses rejected mistakenly, of 0.05(*), 0.01(**), and 0.001(***), respectively.
Mean RT on the PVT was significantly increased by 4 ms after exposure nights, while the number of lapses did not differ statistically significantly between noise and noise-free nights. Performance on the MST and the memory test did not differ significantly between exposure and control nights.
In a comparison of single exposure nights, road traffic noise showed the strongest adverse effects on sleep structure and continuity (strongest effect in 9 of 12 categories) followed by rail traffic noise (3 of 12 categories), while aircraft noise never showed the strongest adverse effect. Similar results were found in double exposure nights, where the greatest adverse effects on sleep were found for the road traffic noise exposure nights, with RORO showing the greatest adverse effects in 7 of 12 categories alone.
These findings did not replicate for the subjective assessment of sleep and its recuperative effects. In single exposure nights, the strongest adverse effects were found for air (2 of 5 categories) and rail (3 of 5 categories), while road traffic noise never showed the greatest adverse effect. Similar results were found in double exposure nights, were the strongest adverse effects were found for air and rail traffic noise (both 4 of 5 categories), while road traffic noise only contributed to 2 of 5 categories. The effects on performance were small, and the 3 traffic modes did not differ relevantly in their effect on performance. However, the strongest adverse effect on memory consolidation was again observed for nights with road traffic noise exposure both in single and double exposure nights.
The double exposure night RORO was consistently associated with stronger adverse effects on sleep compared to the single exposure nights AI and RA, although equivalent sound levels did not differ. This applies to both objective and subjective indicators of sleep quality and quantity. Fifty-six percent of the outcome variables showed a significant time-in-study effect (see last column of Table 2).
Cumulative Effects
SWS latency was 5.2 min longer in triple than in single exposure nights (P = 0.034). REM latency was 9.0 min longer in double than in single exposure nights (P = 0.005), and time spent in REM was 6.1 min (P = 0.010) and 4.7 min (P = 0.050) shorter in triple compared to single and double exposure nights, respectively. For the other sleep structure variables, there were no statistically significant differences between single, double, and triple exposure nights.
Cumulative effects were observed for all sleep continuity variables. The frequency of awakenings (+0.24/h TST, P = 0.010), arousals (+1.61/h TST, P = 0.003), and sleep stage changes (+0.79/h SPT, P = 0.031) was significantly higher in triple than single exposure nights. Additionally, awakening frequency (+0.16/h TST, P = 0.016) and arousal frequency (+0.84/h TST, P = 0.030) were significantly higher in double than in single exposure nights. Double and triple exposure nights did not show statistically significant differences.
Cumulative effects were also observed for the subjective assessments of sleep. Falling asleep was assessed harder (+89, P = 0.013), sleep was assessed more disturbed (+126, P < 0.001), lighter (+121, P < 0.001), and less recuperative (+111, P < 0.001) in triple compared to single exposure nights. Additionally, sleep was reported to be more disturbed (+75, P = 0.001), lighter (+64, P = 0.002), and less recuperative (+62, P = 0.002) in double than in single exposure nights. Finally, sleep was assessed to be significantly lighter (+57, P = 0.046) in triple compared to double exposure nights.
No significant cumulative effects were observed for average heart rate, performance, or memory consolidation.
Event-Related Analysis
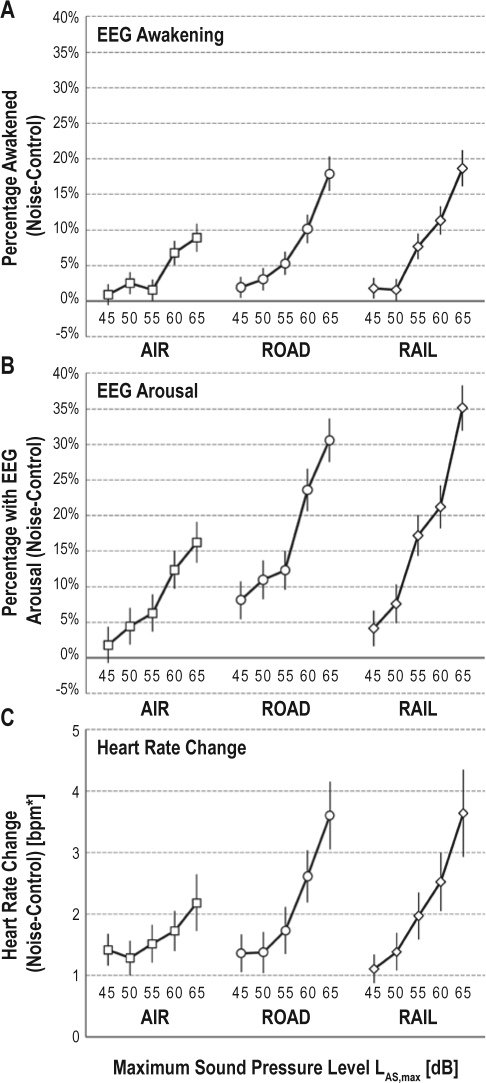
Differences in awakening and arousal probability between nights with and without noise exposure depending on SPL and traffic mode are shown in Figure 1A and Figure 1B. For all 3 traffic modes, both excess awakening and arousal probability were > 0 and increased in a more or less monotonous fashion with maximum SPL, more pronounced for noise levels ≥ 55 dB. Excess arousal probability was on average 2.6 times higher than excess awakening probability. Excess awakening and arousal probability of road and rail traffic noise were higher compared to aircraft noise. Awakening and arousal probability were significantly higher in exposure than in control nights in all categories, with the exception of the 45 dB aircraft noise category (P = 0.230 for awakenings, P = 0.152 for arousals). Average heart rate was significantly greater during periods of noise exposure compared to control periods for all traffic modes and exposure categories (all P < 0.0001). The heart rate increase was comparable between road and rail noise exposure categories, but lower for the aircraft noise category, especially for maximum SPLs ≥ 55 dB(A).
Figure 1.
Probability of awakenings (A) and arousals (B) in noise exposure nights in excess of spontaneous probability observed in noise-free control nights is shown. The change in heart rate between periods with and without noise exposure is shown in (C) depending on maximum SPL and traffic mode. Estimates for (A), (B), and (C) were derived from random subject effect regression models and include 95% confidence intervals. *bpm, beats per minute.
The results of the regression models are shown in Table 3. According to the results of Model 1, awakening probability increased significantly and in a nonlinear fashion (on the logit scale) with maximum SPL. Aircraft noise lead to significantly lower awakening probabilities than road and rail traffic noise (both P < 0.0001), while the latter did not differ statistically significantly (P > 0.05), corroborating the findings presented in Figure 1A.
Table 3.
Regression model results
| EEG Awakening |
EEG Arousal |
Heart Rate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Traffic Mode and Maximum SPL | |||||||||
| Intercept | -2.1434 (0.0622)**** | -2.0792 (0.0854)**** | -2.0677 (0.0903)**** | -0.7729 (0.0633)**** | -0.5397 (0.0824)**** | -0.6634 (0.0836)**** | 1.8771 (0.1399)**** | 2.1583 (0.1767)**** | 2.0927 (0.1877)**** |
| Traffic mode Air vs. Road | -0.3369 (0.0450)**** | -0.3630 (0.0461)**** | 0.2234 (0.0848)* | -0.4675 (0.0328)**** | -0.4865 (0.0335)**** | 0.1173 (0.0573)* | -0.4983 (0.0926)**** | -0.4923 (0.0921)**** | 0.3220 (0.1753) |
| Traffic mode Rail vs. Road | 0.0585 (0.0414) | 0.0546 (0.0425) | -0.4300 (0.0878)**** | -0.0083 (0.0314) | -0.0019 (0.0321) | -0.0742 (0.0352)* | -0.0144 (0.0916) | -0.0063 (0.0911) | -0.2978 (0.1529) |
| Traffic mode Rail vs. Air | 0.3954 (0.0445)**** | 0.4176 (0.0456)**** | -0.6534 (0.1226)**** | 0.4592 (0.0329)**** | 0.4846 (0.0336)**** | -0.1915 (0.0656)** | 0.4838 (0.0923)**** | 0.4860 (0.0918)**** | -0.6198 (0.1943)** |
| Lmax [dB] | 0.0632 (0.0026)**** | 0.0656 (0.0027)**** | 0.0067 (0.0071) | 0.0521 (0.0019)**** | 0.0545 (0.0019)**** | 0.0184 (0.0034)**** | 0.0936 (0.0053)**** | 0.0941 (0.0053)**** | -0.0018 (0.0146) |
| Lmax2 [dB2] | 0.0023 (0.0004)**** | 0.0023 (0.0004)**** | 0.0011 (0.0005)* | 0.0014 (0.0003)**** | 0.0014 (0.0003)**** | 0.0004 (0.0003) | 0.0051 (0.0009)**** | 0.0052 (0.0009)**** | 0.0028 (0.0009)** |
| Individual Moderators | |||||||||
| Age [years] | 0.0033 (0.0040) | 0.0033 (0.0040) | 0.0090 (0.0042)* | 0.0090 (0.0042)* | -0.0198 (0.0081)* | -0.0199 (0.0081)* | |||
| Male gender | 0.2158 (0.1067)* | 0.2153 (0.1070)* | 0.2971 (0.1123)* | 0.2991 (0.1132)* | 0.6234 (0.2189)** | 0.6208 (0.2187)** | |||
| Situational Moderators | |||||||||
| Prior sleep stage S1 vs. S2 | 1.570 (0.0581)**** | 1.5754 (0.0583)**** | 0.8968 (0.0600)**** | 0.8978 (0.0602)**** | -2.0758 (0.1674)**** | -2.0937 (0.1670)**** | |||
| Prior sleep stage SWS vs. S2 | -0.4928 (0.0626)**** | -0.4934 (0.0628)**** | -0.9064 (0.0455)**** | -0.9159 (0.0457)**** | 0.4977 (0.1142)**** | 0.4963 (0.1140)**** | |||
| Prior sleep stage REM vs. S2 | -0.3815 (0.0496)**** | -0.3839 (0.0497)**** | -0.6433 (0.0361)**** | -0.6496 (0.0363)**** | -0.5614 (0.0952)**** | -0.5586 (0.0950)**** | |||
| Elapsed sleep time [h] | 0.0294 (0.0084)*** | 0.0296 (0.0085)*** | 0.0119 (0.0063) | 0.0111 (0.0064) | 0.2244 (0.0180)**** | 0.2229 (0.0180)**** | |||
| Study night [d] | -0.0202 (0.0069)** | -0.0206 (0.0069)** | -0.0118 (0.0054)* | -0.0122 (0.0054)* | 0.0140 (0.0143) | 0.0136 (0.0142) | |||
| Noise events per night 80 vs. 40 | -0.1654 (0.0430)*** | -0.1671 (0.0432)*** | -0.1902 (0.0325)**** | -0.1912 (0.0326)**** | -0.3837 (0.0918)**** | -0.3826 (0.0916)**** | |||
| Noise events per night 120 vs. 40 | -0.3266 (0.0518)**** | -0.3299 (0.0520)**** | -0.3025 (0.0380)**** | -0.3056 (0.0382)**** | -0.6285 (0.1069)**** | -0.6313 (0.1067)**** | |||
| Acoustical Moderators | |||||||||
| SPL rise time [dB/s] | 0.0289 (0.0099)** | 0.0262 (0.0073)*** | 0.0451 (0.0208)* | ||||||
| Octave energy 31.5 Hz [dB] | 0.0149 (0.0037)*** | ||||||||
| Octave energy 63 Hz [dB] | -0.0208 (0.0088)* | ||||||||
| Octave energy 125 Hz [dB] | 0.0251 (0.0091)** | ||||||||
| Octave energy 500 Hz [dB] | 0.0151 (0.0062)* | 0.0278 (0.0137)* | |||||||
| Octave energy 4 kHz [dB] | 0.0271 (0.0056)**** | 0.0231 (0.0039)**** | 0.0219 (0.0107)* | ||||||
| Octave energy 8 kHz [dB] | 0.0352 (0.0057)**** | 0.0344 (0.0044)**** | 0.1027 (0.0126)**** | ||||||
| Variance random subject effect | 0.1753 (0.0336)**** | 0.1751 (0.0337)**** | 0.1763 (0.0339)**** | 0.2349 (0.0416)**** | 0.2089 (0.0373)**** | 0.2121 (0.0379)**** | 0.9561 (0.1778)**** | 0.7402 (0.1408)**** | 0.7390 (0.1406)**** |
Regression coefficient estimates and standard errors (in parenthesis) are shown.
P < 0.05
P < 0.01
P < 0.001
P < 0.0001
variables were centered at Lmax = 55 dB, Age = 40 years, Elapsed sleep time = 4 hours, Study night = 6th night, SPL rise time = 5.7 dB/s, Octave 31.5 Hz = 50 dB, Octave 63 Hz = 52 dB, Octave 125 Hz = 46 dB, Octave 500 Hz = 44 dB, Octave 4 kHz = 27 dB, Octave 8 kHz = 16 dB.
According to the results of Model 2, male subjects were significantly more likely than female subjects to wake up due to traffic noise. Compared to sleep stage S2, awakening probability was significantly higher from stage S1 and significantly lower from REM and SWS. The lowest probability was observed from SWS. Awakening probability increased significantly with elapsed sleep time and it decreased significantly across study nights. Awakening probability (per noise event) decreased continuously with an increasing number of noise events per night. As expected, adjusting for individual and situational moderators did not lead to a qualitative change in the difference in awakening probability between traffic modes.
According to the results of Model 3, awakening probability increased significantly with SPL rise time, energy in the 31.5-Hz and 500-Hz octave bands, and even more pronounced with energy in the 4-kHz and 8-kHz octave bands. Adjusting for acoustic moderators not only resolved the differences in awakening probability between traffic modes, but reversed it. In the fully adjusted model, awakening probability decreased in the order air, road, and rail traffic noise.
These findings were replicated in models where EEG arousals served as the dependent variable, with the exception that arousal probability now increased significantly with age, and that only energy in the highest octave bands (4 kHz and 8 kHz) contributed significantly to arousal probability. The findings were also very similar for the models where changes in heart rate served as the dependent variable, except for the following differences: The change in heart rate decreased significantly with age, and it was significantly lower out of S1 or REM sleep and significantly higher out of SWS compared to S2. There was no significant change in heart rate response across study nights. Heart rate increased significantly with increasing energy in octave bands with mid frequencies of 125 Hz, 500 Hz, 4 kHz, and 8 kHz; while it significantly decreased with energy in the 63-Hz octave band.
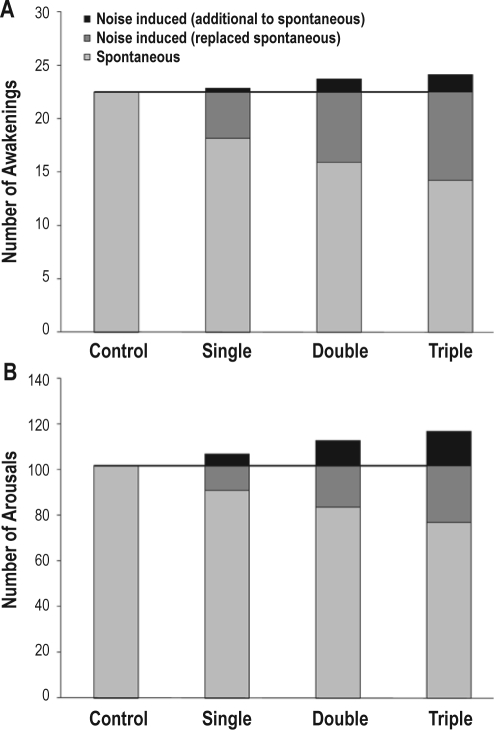
Figure 2 shows the average number of awakenings (A) and arousals (B) for control nights and for periods with and without noise in exposure nights. It illustrates that 84% to 93% of awakenings observed under the influence of noise merely replaced spontaneous awakenings, while this was true for only 62% to 67% of EEG arousals observed under the influence of noise. Results of the analysis of awakening and arousal frequency and duration in periods with and without noise exposure are shown in Table 4. Spontaneous awakening frequency between noise events was significantly lower than in control nights, while spontaneous arousal frequency between noise events did not differ compared to control nights. Neither the duration of spontaneous awakenings nor the duration of spontaneous arousals differed significantly between exposure and control nights. The frequency of awakenings and arousals during noise exposure decreased significantly with increasing number of noise events per night, thus corroborating the findings of the regression models. The duration of awakenings and arousals during noise exposure did not differ between single, double, or triple exposure nights.
Figure 2.
The average number of awakenings (A) and arousals (B) is shown for control, single, double, and triple exposure nights. In the exposure nights, spontaneous reactions, reactions under the influence of noise exposure replacing spontaneous reactions, and reactions under the influence of noise exposure additional to spontaneous reactions are differentiated. The horizontal line represents the average number of awakenings/arousals in noise-free control nights.
Table 4.
Analysis of frequency and duration of awakenings and EEG arousals in periods with and without noise exposure during both exposure and noise free control nights
| Mean (SE) |
Difference (SE) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | SINGLE | DOUBLE | TRIPLE | CTRL-SGL | CTRL-DBL | CTRL-TPL | SGL-DBL | SGL-TPL | DBL-TPL | |
| Periods without noise exposure | ||||||||||
| Awakenings per h TST [N] | 3.2 (0.2) | 2.8 (0.1) | 2.7 (0.1) | 2.7 (0.2) | 0.4 (0.1)** | 0.5 (0.1)** | 0.5 (0.2)** | 0.1 (0.1) | 0.1 (0.1) | 0.0 (0.1) |
| Arousals per h TST [N] | 14.2 (0.8) | 14.0 (0.7) | 14.3 (0.7) | 14.6 (0.8) | 0.3 (0.5) | -0.0 (0.5) | -0.0 (0.6) | -0.3 (0.4) | -0.7 (0.5) | -0.4 (0.5) |
| Awakening duration [s] | 154.8 (18.2) | 145.0 (12.8) | 156.1 (12.8) | 167.4 (18.2) | 9.9 (18.7) | -1.2 (18.7) | -12.6 (22.8) | -11.1 (13.5) | -22.5 (18.7) | -11.4 (18.7) |
| Arousal duration [s] | 20.3 (1.8) | 19.6 (1.4) | 20.1 (1.4) | 19.4 (1.8) | 0.7 (1.6) | 0.1 (1.6) | 0.9 (1.9) | -0.6 (1.1) | 0.2 (1.6) | 0.8 (1.6) |
| Periods with noise exposure | ||||||||||
| Awakenings per h TST [N] | 8.9 (0.5) | 7.2 (0.5) | 6.0 (0.7) | 1.7 (0.5)*** | 2.9 (0.6)*** | 1.2 (0.6) | ||||
| Arousals per h TST [N] | 28.9 (1.3) | 25.6 (1.3) | 23.3 (1.6) | 3.2 (0.8)*** | 5.5 (1.2)*** | 2.3 (1.2) | ||||
| Awakening duration [s] | 131.7 (15.7) | 174.4 (15.6) | 150.8 (24.2) | -42.7 (19.2) | -19.1 (26.7) | 23.6 (26.7) | ||||
| Arousal duration [s] | 20.3 (1.7) | 22.9 (1.7) | 20.9 (2.5) | -2.5 (2.0) | -0.5 (2.7) | 2.0 (2.7) | ||||
SE refers to standard error; TST, total sleep time
P < 0.05
P < 0.01
P < 0.001 after controlling for a false discovery rate, i.e., the expected fraction of null hypotheses rejected mistakenly, of 0.05(*), 0.01(**), and 0.001(***), respectively.
DISCUSSION
This polysomnographic study was designed to systematically compare the effects of air, road, and rail traffic noise on sleep. The study design was carefully balanced according to the number of noise events, maximum SPL, equivalent noise level Leq, study night, and prior noise annoyance to enable a “fair” comparison. Compared to similar studies conducted in the past,28–30 the age range (18 to 71 years) of our subjects was wide.
The effects of traffic noise on sleep macrostructure were subtle, corroborating earlier findings.4,31 Small changes in SWS latency (+8.3 min), stage 1 sleep (+4 min), and SWS (−6 min) were the only statistically significant effects. Changes is sleep continuity were more pronounced and statistically significant for all indicators except awakening frequency, but compared to clinical sleep disorders like obstructive sleep apnea (OSA) the changes were still marginal. These small noise-induced changes in sleep structure and continuity sufficed to significantly affect subjective assessments of sleep quality and recuperation, but they failed to affect objective measures of daytime performance (except for a small significant increase in mean PVT reaction time).
In a comparison of the effects of the different traffic modes on sleep structure, a striking difference was found in the objective and subjective evaluation of sleep. While road traffic noise clearly led to the most prominent changes in sleep structure and continuity, nights with air and rail traffic noise exposure were scored as being more disturbing than road traffic noise on the subjective rating scales. While the acoustic properties of road traffic noise may be responsible for the changes in sleep structure and continuity (see below), it is possible that road traffic noise events were too short to be consciously perceived by the subjects that were woken up by the noise event, while rail and air traffic noise events may have been long enough (see Table 1). This would corroborate earlier speculations that consciously perceived noise events determine the subjective assessment of sleep quality.32
Cumulative effects of noise on sleep structure were only observed for REM latency, SWS latency, and time spent in REM, and they were relatively moderate. In contrast to this, small but statistically significant cumulative effects were seen for all sleep continuity variables and for the subjective assessments of sleep. This supports the existence of a mechanism that, despite of the increased fragmentation of sleep, preserves sleep structure. Increased sleep fragmentation in double and triple compared to single exposure nights were not accompanied by similar changes in performance or memory consolidation. The degree of sleep fragmentation may simply have been too small even in double and triple exposure nights to result in neurobehavioral consequences.
Although most of the night is spent in an unconscious state, subjects were not only able to differentiate between nights with and without noise, but also between nights with low and high degrees of traffic noise exposure. Hence, if these findings extend to the field, morning questionnaires, although prone to manipulation, may be a very cost-effective way for the investigation of traffic noise effects on sleep.
Regression model M1 showed that in spite of the balanced study design and after adjusting for maximum SPL, awakening probability decreased in the order rail, road, and air traffic noise. After adjusting for an unbalanced study design, the same order was found by Marks et al. in a polysomnographic study on the effects of air, road, and rail traffic noise on sleep.30 Two smaller studies by Hofman et al.33 and by Muzet et al.29 also support this finding, which contradicts our hypothesis and shows that the order observed for annoyance during the day is reversed for sleep disturbance during the night. Obviously, these findings do not support a rail bonus for nighttime.
A significant habituation effect was found for cortical arousals across study nights, and for both cortical and cardiac arousals within the same study night. This habituation is most likely caused by a decrease in the importance of noise events due to repeated stimulation, and it seems biologically plausible in terms of sleep homeostasis and energy conservation. It is unclear whether it represents true habituation or whether it can be, at least in part, explained by increased arousal thresholds due to noise-induced sleep fragmentation in previous exposure nights or in preceding parts of the same night. According to Bonnet,34 both are probably true. It is certainly one reason that the cumulative effects on sleep were only moderate, and that the effects of noise on sleep structure and sleep continuity were also only moderate. The observation that the degree of sleep disturbance found in field studies, i.e., after months or years of noise exposure, is usually much lower compared to laboratory studies suggests that habituation continues beyond the periods usually investigated in the laboratory.35,36
The fact that cardiac arousals habituated within but not across nights suggests either that the mechanisms responsible for habituation across nights differ from those responsible for habituation within nights or that the two mechanisms outlined above contribute differentially to habituation across and within nights, at least for vegetative arousals. The hierarchical nature of the arousal response37 may explain why habituation across nights was seen for cortical but not for cardiac arousals as, depending on the analysis of the content of the acoustic stimulus, thalamo-cortical gating may prevent the cortex from being aroused,38,39 while there may still be a subcortical response independent of information processing of higher central nervous system structures. The fact that cardiac arousals did not habituate across nights stresses their potential relevance for the genesis of long-term cardiovascular consequences of noise-induced sleep disturbance.6,8,9 However, the noise-induced changes in heart rate observed in this study were again subtle and not able to increase average heart rate based on SPT.
In addition to the habituation effects, Figure 2A illustrates that most of the noise induced awakenings (up to 93%) merely replaced awakenings that otherwise would have occurred spontaneously either in or after the 60-s screening window. The fact that awakening frequency between noise events was significantly reduced compared to control nights shows that at least some noise induced awakenings truly replaced spontaneous awakenings that otherwise would have occurred after the 60-s screening window. This mechanism keeps the number of additional awakenings low and preserves sleep structure and continuity at the same time. Up to 67% of noise induced arousals replaced arousals that would otherwise have occurred spontaneously. In contrast to awakenings, noise-induced arousals did not replace spontaneous arousals that would otherwise have occurred after the 60-s screening window, as spontaneous arousal frequency between noise events did not differ significantly from spontaneous arousal frequency in control nights. This explains the significantly increased arousal frequency in exposure nights. While sleep macrostructure seems to be preserved to a large extent during nights with noise exposure, our results hint at more prominent fragmentation on the level of sleep microstructure. Duration of awakenings and arousals in control nights during noise exposure and between noise events did not differ significantly. Therefore, habituation processes apply to the incidence of awakenings and arousals only, and not to their duration.
Cortical arousal probability and the degree of cardiac arousals increased significantly with SPL rise time as well as with energy especially in the high frequency ranges (mid frequency ' 4 kHz). These effects were able to explain the differences between traffic modes. In fact, as aircraft noise events had the lowest SPL rise times and (due to atmospheric absorption) low energies especially in the high frequency range, the rank order was reversed in the fully adjusted models. These results provide important insights for the optimization of mitigation measures. For example, SPL rise times could be lowered by nocturnal speed control, while energy reductions in the relevant frequency bands could be accomplished either by passive sound insulation of bedrooms or by improved sound engineering of the vehicles.
Limitations
This study had the primary goal to investigate differences in the sleep disturbing properties of air, road, and rail traffic noise, and not to assess the impact of traffic noise on the population. The latter should not be attempted due to several limitations of the study, including the healthy adult study population, the laboratory setting, the restricted representativeness of the noise scenarios, and the limited number of days subjects were exposed to noise. Therefore, although the effects of traffic noise on sleep and performance were subtle, it cannot be excluded that the consequences would be more severe in other settings, especially under the following conditions: chronic noise exposure, high traffic densities, high sound pressure levels, or vulnerable populations, (e.g., children, shift workers, or subjects with premorbid conditions, sleep disorders, a high sensitivity to noise, or problems adapting to noise).6,7,12 Field studies are needed to validate our findings in a setting with higher ecologic validity.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors declare no conflicts of interest concerning the study presented. Dr. Basner has received compensation for consulting from Purdue University for the FAA PARTNER Center of Excellence Project 25B and from Aéroports de Montréal. Dr. Basner has made paid presentations to the World Health Organization (WHO, German office), to the American Academy of Sleep Medicine (AASM), and Université de Montréal. Dr. Basner is an Associate Editor of the journal SLEEP.
ACKNOWLEDGMENTS
Thanks go to the subjects participating in the study and to our colleagues at the German Aerospace Center who helped sampling the data during many weekend and night shifts. We would like to thank Jan Wagner and Werner Plihal from Lübeck University for providing us with word pairs for the memory test. The AIRORA study was internally funded by the German Aerospace Center (DLR). The study was performed at the German Aerospace Center (DLR), Institute of Aerospace Medicine, Cologne, Germany.
Footnotes
A commentary on this article appears in this issue on page 7.
REFERENCES
- 1.Stansfeld SA, Berglund B, Clark C, et al. Aircraft and road traffic noise and children's cognition and health: a cross-national study. Lancet. 2005;365:1942–9. doi: 10.1016/S0140-6736(05)66660-3. [DOI] [PubMed] [Google Scholar]
- 2.Guski R. Zum Anspruch auf Ruhe beim Wohnen. Zeitschrift für Lärmbekämpfung. 1991;38:61–5. [Google Scholar]
- 3.Meier U. Das Schlafverhalten der deutschen Bevölkerung - eine repräsentative Studie. Somnologie. 2004;8:87–94. [Google Scholar]
- 4.Basner M, Samel A. Effects of nocturnal aircraft noise on sleep structure. Somnologie. 2005;9:84–95. [Google Scholar]
- 5.Griefahn B, Marks A, Robens S. Noise emitted from road, rail and air traffic and their effects on sleep. J Sound Vib. 2006;295:129–40. [Google Scholar]
- 6.Basner M, Van den Berg M, Griefahn B. Aircraft noise effects on sleep: Mechanisms, mitigation and research needs. Noise Health. 2010;12:95–109. doi: 10.4103/1463-1741.63210. [DOI] [PubMed] [Google Scholar]
- 7.Basner M. Nocturnal aircraft noise increases objectively assessed daytime sleepiness. Somnologie. 2008;12:110–7. [Google Scholar]
- 8.Griefahn B, Bröde P, Marks A, Basner M. Autonomic arousals related to traffic noise during sleep. Sleep. 2008;31:569–77. doi: 10.1093/sleep/31.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11:135–42. doi: 10.1016/j.smrv.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Ohrstrom E, Hadzibajramovic E, Holmes M, Svensson H. Effects of road traffic noise on sleep: Studies on children and adults. J Env Psychol. 2006;26:116–26. [Google Scholar]
- 11.Babisch W. Dose-effect curve and risk estimation: Federal Environmental Agency (Umweltbundesamt) Berlin, Germany: 2006. Transportation noise and cardiovascular risk. Review and synthesis of epidemiological studies. WaBoLu-Heft 01/06. [Google Scholar]
- 12.Jarup L, Babisch W, Houthuijs D, et al. Hypertension and exposure to noise near airports: the HYENA study. Environ Health Perspect. 2008;116:329–33. doi: 10.1289/ehp.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miedema HM. Relationship between exposure to multiple noise sources and noise annoyance. J Acoust Soc Am. 2004;116:949–57. doi: 10.1121/1.1766305. [DOI] [PubMed] [Google Scholar]
- 14.Oswald I, Taylor AM, Treisman M. Discriminative responses to stimulation during human sleep. Brain. 1960;83:440–53. doi: 10.1093/brain/83.3.440. [DOI] [PubMed] [Google Scholar]
- 15.Perrin F, Garcia-Larrea L, Mauguiere F, Bastuji H. A differential brain response to the subject's own name persists during sleep. ClinNeurophysiol. 1999;110:2153–64. doi: 10.1016/s1388-2457(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 16.Advisory Group for Aerospace Research and Development (AGARD) AGARDOGRAPH No. 308. Human Performance Assessment Methods. Neuilly-Sur-Seine: 1989. [Google Scholar]
- 17.Dinges DF, Powell JW. Microcomputer analysis of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;6:652–5. [Google Scholar]
- 18.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–49. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 19.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: Public Health Service, U.S. Government, Printing Office; 1968. [Google Scholar]
- 21.Bonnet M, Carley DW, Carskadon MA, et al. EEG arousals: Scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 22.Van Dongen HP, Olofsen E, Dinges DF, Maislin G. Mixed-model regression analysis and dealing with interindividual differences. Methods Enzymol. 2004;384:139–71. doi: 10.1016/S0076-6879(04)84010-2. [DOI] [PubMed] [Google Scholar]
- 23.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 24.Ollerhead JB, Jones CJ, Cadoux RE, et al. Report of a field study of aircraft noise and sleep disturbance. London, United Kingdom: Department of Transport; [Google Scholar]
- 25.Passchier-Vermeer W, Vos H, Steenbekkers J HM, Van der Ploeg FD, Groothuis-Oudshoorn K. Report 2002.027. Netherlands: TNO; 2002. Sleep disturbance and aircraft noise exposure - exposure effect relationships. [Google Scholar]
- 26.Basner M, Isermann U, Samel A. Aircraft noise effects on sleep: Application of the results of a large polysomnographic field study. J Acoust Soc Am. 2006;119:2772–84. doi: 10.1121/1.2184247. [DOI] [PubMed] [Google Scholar]
- 27.Brink M, Basner M, Schierz C, et al. Determining physiological reaction probabilities to noise events during sleep. Somnologie. 2009;13:236–43. [Google Scholar]
- 28.Vernet M. Effect of train noise on sleep for people living in houses bordering the railway line. J Sound Vib. 1979;66:483–92. [Google Scholar]
- 29.Muzet A, Weber LD, Di Nisi J, Ehrhart J. Comparison de la reactivite cardiovasculaire au bruit au cours de la veille et du somneil. Strasbourg: Centre d'etude bioclimatique du CNRS; 1985. Convention No. 82243. [Google Scholar]
- 30.Marks A, Griefahn B, Basner M. Event-related awakenings caused by nocturnal transportation noise. Noise Contr Eng J. 2008;56:52–62. [Google Scholar]
- 31.Griefahn B, Basner M, Bröde P, Robens S. Development of a sleep disturbance index (SDI) for the assessment of noise-induced sleep disturbances. Somnologie. 2008;12:150–7. [Google Scholar]
- 32.Basner M, Müller U, Elmenhorst EM, Kluge G, Griefahn B. Aircraft noise effects on sleep: a systematic comparison of EEG awakenings and automatically detected cardiac activations. Physiol Meas. 2008;29:1089–103. doi: 10.1088/0967-3334/29/9/007. [DOI] [PubMed] [Google Scholar]
- 33.Hofman W, Kumar A, Eberhardt JL. Vallet M. Comparative evaluation of sleep disturbance due to noises from airplanes, trains and trucks. Noise & Man ′93; 6th International Congress; Bron: 1993. pp. 559–62. INRETS 2. [Google Scholar]
- 34.Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 35.Pearsons K, Barber D, Tabachnick BG, Fidell S. Predicting noise-induced sleep disturbance. J Acoust Soc Am. 1995;97:331–8. [Google Scholar]
- 36.Basner M, Buess H, Elmenhorst D, et al. Report FB2004-07/E, Deutsches Zentrum für Luft- und Raumfahrt (DLR) Cologne, Germany: 2004. Effects of nocturnal aircraft noise (Volume 1): Executive summary. [Google Scholar]
- 37.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 38.Cote KA, Epps TM, Campbell KB. The role of the spindle in human information processing of high-intensity stimuli during sleep. J Sleep Res. 2000;9:19–26. doi: 10.1046/j.1365-2869.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- 39.Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010 Oct;20:R626–7. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]