Abstract
Study Objective:
The adaptive value of the endogenous circadian clock arises from its ability to synchronize (i.e., entrain) to external light-dark (LD) cycles at an appropriate phase. Studies have suggested that advanced circadian phase alignment might result from shortening of the period length of the clock. Here we explore mechanisms that contribute to an early activity phase in CAST/EiJ (CAST) mice.
Methods:
We investigated circadian rhythms of wheel-running activity in C57BL/6J (B6), CAST and 2 strains of B6.CAST congenic mice, which carry CAST segments introgressed in a B6 genome.
Results:
When entrained, all CAST mice initiate daily activity several hours earlier than normal mice. This difference could not be explained by alterations in the endogenous period, as activity onset did not correlate with period length. However, the photic phase-shifting responses in these mice were phase-lagged by 3 hours relative to their activity. Attenuated light masking responses were also found in CAST mice, which allow for activity normally inhibited by light. A previously identified quantitative trait locus (QTL), Era1, which contributes to the early activity trait, was confirmed and refined here using two B6.CAST congenic strains. Surprisingly, these B6.CAST mice exhibited longer rather than shorter endogenous periods, further demonstrating that the advanced phase in these mice is not due to alterations in period.
Conclusions:
CAST mice have an advanced activity phase similar to human advanced sleep phase syndrome. This advanced phase is not due to its shorter period length or smaller light-induced phase shifts, but appears to be related to both light masking and altered coupling of the circadian pacemaker with various outputs. Lastly, a QTL influencing this trait was confirmed and narrowed using congenic mice as a first step toward gene identification.
Citation:
Jiang P; Striz M; Wisor JP; O'Hara BF. Behavioral and genetic dissection of a mouse model for advanced sleep phase syndrome. SLEEP 2011;34(1):39-48.
Keywords: Phase angle, phase response curve, mouse genetics, quantitative trait loci, Era1
SLEEP IS REGULATED BY A HOMEOSTATIC PROCESS AND A CIRCADIAN PROCESS. THE LATTER IS THOUGHT TO BE ADAPTIVE BY RESTRICTING SLEEP to a favorable time of day (i.e., phase). However, in humans, the timing of sleep and activity exhibit considerable variation. In extreme cases, such as advanced or delayed sleep phase syndrome (ASPS or DSPS), sleep phases are several hours early or late, generating conflicts with social demands as well as negative consequences to physical health.1–4 These disorders are thought to reflect misalignment between endogenous circadian rhythms and external timing cues.
In mammals, circadian rhythms are generated by an endogenous clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus, which free-runs under constant conditions with a period of ∼24 h and can be synchronized, or entrained, to the external light-dark (LD) cycle. Light entrains the clock by inducing phase delays during early subjective night and phase advances during late subjective night, as can be described in the phase response curve (PRC). These light-induced responses ensure that the clock adjusts to seasonal changes in the timing of dawn and dusk, but also correct for the deviation of the internal circadian period from 24 h.5 If the circadian period is longer or shorter than 24 h, the clock must be advanced or delayed, respectively, on a daily basis to maintain entrainment.
The PRC also dictates the phase angle (i.e., phase relation) between the clock and the LD cycle to which the animal is entrained.6 With shorter circadian periods, entrainment occurs at an earlier phase angle, as synchronization will only be achieved with greater magnitude phase delays. Such phase delays are only achieved when the clock is exposed to light during a larger portion of the early subjective night. This model of circadian phase determination has been supported by studies in both animal models and humans.7–9 The extreme morningness in one patient with familial ASPS (fASPS), whose endogenous period was assessed, was also found to be associated with a shorter circadian period.10 Despite these successes, alterations in circadian period may not be sufficient to explain all (or even most) circadian phase variations. A recent study in a more general population suggested that human “morning larks” and “evening owls” may be attributed to other properties of the clock.11
Forward genetic approaches have been taken to elucidate molecular mechanisms of circadian phase determination. The hamster tau mutation, which confers an extremely short period and thus a pronounced early phase, was mapped to the Casein kinase 1-epsilon (Csnk1e) gene, the product of which phosphorylates the core-clock PERIOD proteins.12 In two kindreds of fASPS, extreme morningness was linked in different pedigrees to mutations in human Period 2 (Per2) or Casein kinase 1-delta (Csnk1d) genes, both causing shortened periods.13,14 These and other circadian genetic studies focused mostly on the molecular determinants of circadian period, but did not provide insights into the phase alignment of overt rhythms relative to cycles of entraining signals such as light.
We have previously taken an alternative forward genetic approach, quantitative trait loci (QTL) analysis, which exploits natural genetic variation that accumulated over many years of evolution, to search for genetic determinants of circadian phase in a backcross population originated from two genetically divergent mouse strains, CAST/EiJ (CAST) and C57BL/6J (B6).15 Mouse “early runner” behavior similar to human ASPS and a QTL on chromosome 18, named Era1, which contributes to this phenotype, were identified. In this article, we show that several circadian endophenotypes, circadian clock properties that may contribute to the early runner phenotype, are found in the parental CAST strain and the B6.CAST.18 congenic mice,16,17 in which a portion of chromosome 18 has been transferred from the CAST strain onto the B6 background. Our results suggest that other factors, aside from endogenous period, influence circadian phase determination in CAST mice. CAST is a unique animal model for the study of ASPS, circadian entrainment, phase relationships, and other poorly understood aspects of the circadian system.
MATERIALS AND METHODS
Animals
Six-week old male and female C57BL/6J (B6) and CAST/EiJ (CAST) mice were purchased from the Jackson Laboratory, Bar Harbor, ME. CAST/EiJ is an inbred strain derived from the subspecies Mus musculus castaneus. 54 (B6 × CAST) × CAST backcross mice were bred by crossing female B6 mice to male CAST mice and subsequently backcrossing the female F1 progeny to male CAST mice. Two strains of B6.CAST congenic mice (also known as Genome-Tagged Mice, GTM17) were obtained from the University of California, Los Angeles. They carry CAST segments of proximal and middle portions of chromosome 18 introgressed on an otherwise 99% B6 background (B6.CAST.18P and B6.CAST.18M, respectively). These two congenic strains were chosen because the CAST segments in these strains overlap with the mapped Era1 QTL region (Figure 7A). Female and male B6.CAST congenic mice were mated in our animal facility at the University of Kentucky. All animal procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
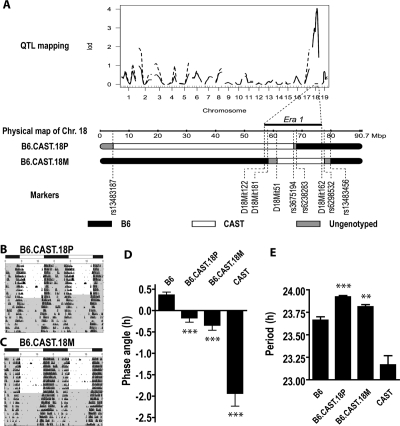
Figure 7.
Congenic lines demonstrate an effect of the chromosome 18 genotype on wheel running phenotype. (A) Portions of chromosome 18 have been transferred from the CAST to the B6 background in congenic strains. The early runner phenotype was previously linked to a locus between marker D18Mit122 and D18Mit162 on chromosome 18 (Era1) by QTL analysis (upper panel reproduced from ref.15). Era1 is here aligned with the physical map of chromosome 18 and the genetic markers that have been genotyped as homozygous B6 (black in schematic representations of chromosome 18) or homozygous CAST (white in schematic representation of chromosome 18) in B6.CAST.18P and B6.CAST.18M mice. Marker and genotype information were adopted from Davis et al17 with modification of the B6.CAST.18M genotypes according to our more detailed genotyping results. The current proximal flanking marker is D18Mit181 and the proximal internal marker is D18Mit51. Wheel running data from one male B6.CAST.18P mouse (B) and one male B6.CAST.18M mouse (C) were double-plotted in percentile actogram format over 20 days (10 days in LD12:12 using 83 lux green light and 10 days in constant dark). Time spent in darkness is indicated by gray shading. (D) Phase angle of entrainment is altered in both congenic strains. Group mean ± SEM values of phase angle of entrainment are derived from DD data. ANOVA, F = 28.69, P < 0.001. ***P < 0.001 vs. B6, Student t. (E) Free running period length is (surprisingly) longer in congenic mice relative to B6. Group mean ± SEM values are shown. ANOVA, F = 34.47, P < 0.001. **P < 0.01; ***P < 0.001 vs. B6, Student t for unpaired measures. B6, B6.CAST.18P and B6.CAST.18M mice also differed significantly from CAST, P < 0.001, Student t for unpaired measures.
Wheel-Running Activity Recording
Mouse wheel-running activity was monitored in light-tight chambers (Phenome Technologies, Lincolnshire, IL). Male mice ≥ 8 weeks old were individually housed at 23 ± 1°C with unrestricted access to a running wheel, the rotations of which were detected by a mechanical switch connected to a desktop computer. Food and water were provided ad libitum. Data were recorded and analyzed using Clocklab software (Coulbourn Instruments, Whitehall, PA). Green LED light (526 nm max, 50% bandwidth ± 20 nm, 11.8 μW/cm2, approximately 83 lux as measured inside of mouse cages, 100 lux as measured at the bottom of the light chamber) and white fluorescent light (22.3 μW/cm2, approximately 160 lux as measured inside the mouse cage) were provided 24.5 cm above the cage floor. Cages were changed every 7 days. Data from days on which cage changes occurred were excluded from analysis.
Activity Onset, Phase Angle of Entrainment, and Circadian Period
Mice, including CAST (n = 10), B6 (n = 8), (B6 × CAST) × CAST (n = 54), B6.CAST.18P (n = 9), and B6.CAST.18M (n = 8), were first housed in a 12-h light/12-h dark (LD12:12) cycle of green light for ≥ 21 days prior to all experimental manipulations of lighting conditions. Daily wheel running activity onset was first defined by Clocklab software as the time of day that best approximates a pattern of 6 h of inactivity (i.e., lower than the 20th percentile activity level) followed by a 6-h period of high activity. The computer-defined activity onsets were then adjusted by eye for any obvious mis-scored values, and averaged over the last 10 LD cycles.
Mice of the CAST, B6, B6.CAST.18P and B6.CAST.18M strains were then subjected to ≥ 14 days of constant dark (DD) housing for measurement of the phase angle of entrainment and circadian period. Phase angle of entrainment was defined by extrapolating, to the last day of LD12:12 housing, a line fitted through activity onsets during the first 14 days of DD housing. Free running period in DD was determined by chi-square periodogram analysis of data from days 1-14 in DD, using Clocklab software.
Photic Phase Response Curve and Tau Response Curve
Subsequent to the measurement of phase angle of entrainment and circadian period in DD, CAST mice and B6 mice were exposed to a single 30-min green light pulse once every 2 weeks. All mice were subjected to the light pulse simultaneously, and as a result the circadian time of the pulse was random for each animal. Individual mice were subjected to anywhere from 3 to 9 light pulses and thus were in constant darkness punctuated by 30-min green light pulses for up to 193 days. Because the number and circadian times of light pulses administered to each animal were random, it was not possible to analyze the data in a repeated measures design. The phase shifts induced by these light pulses were treated as independent measures. To determine the magnitude of phase shifts in response to light pulses, 2 fitted lines were drawn through activity onsets (defined as described above) during the 10 circadian cycles preceding light exposure, and cycles 4-13 subsequent to light exposure, respectively. The phase shifts were calculated similarly as described by Spoelstra et al.20 Briefly, Δϕ = ϕ2−ϕ1, where ϕ1 = (((tLP − tOns1) mod τ1) × 24/τ1 + 12) mod 24, and ϕ2 = (((tLP − tOns2) mod τ2) × 24/τ2 + 12) mod 24, both expressed in circadian hours. tLP is the time when the light pulse is given, while tOns and τ are the time of activity onset and free running period before (tOns1, τ1) and after (tOns2, τ2) light exposure, determined by the 2 fitted lines described above. A 2-harmonic Fourier function21 with a fixed period of 24 circadian hours was fitted to the phase response curves for CAST and B6 strains, using the curve fitting tool of the Matlab software package (MathWorks, Inc. Natick, MA.). The magnitude of changes in circadian period in response to light pulses was determined simply by Δτ = τ2 − τ1, where the values of τ1 and τ2 were determined as described above.
Masking and Activity Density Before Dark
Negative masking effects of white and green light were assessed by exposing CAST (n = 5), B6.CAST.18P (n = 5), B6.CAST.18M (n = 5), and B6 (n = 4) mice to light during a 60-min interval beginning 30 min after dark onset in an LD12:12 cycle of green light. Each mouse was exposed to one 60-min white light exposure and one 60-min green light exposure, both of which were preceded by 6 uninterrupted LD cycles. The magnitude of negative masking by light exposure was quantified as the “percentage of activity remaining” during the 60-min light exposure at ZT12.5-13.5. This variable was measured by dividing the number of wheel revolutions during light exposure by the number of wheel revolutions during the analogous time interval averaged over the previous 2 nights and multiplying the resulting number by 100%.
The same 5 CAST mice were also used to study the effect of lighting intensity and/or spectra on activity density before dark. Mice were first housed in LD12:12 cycles with white light at ∼160 lux for 3 weeks, and then in the same LD cycles with green light at ∼83 lux for another 3 weeks. Activity density before dark under white and green light housing was measured by averaging total wheel revolutions occurring between the wheel running activity onset and dark onset over the last 10 cycles in LD12:12, and then dividing this number by the total minutes during which the animal was active before dark onset.
Recording of Sleep and Wake Behavior
A subset of (B6 × CAST) × CAST backcross mice (n = 14) was also tested on an automated piezoelectric system, which has been shown to have > 90% sensitivity relative to electroencephalographically defined sleep.18,19 Sleep and wake behavior were continuously recorded over more than 10 LD12:12 cycles.
Time of awakening for the major wake bout each day was defined as the time between ZT4 and ZT15 when the percent of wake first reached 75% of the night time average for a sustained period.
Statistics
In comparisons of the parental CAST and B6 strains (wheel running activity onset, phase angle of entrainment, maximum phase delay and phase advance magnitudes, tau response magnitude), Student t for independent measures was applied. For within-strain comparisons of green and white light effects (wheel running activity onset, activity density, magnitude of negative masking), Student t for paired measures was applied. In comparisons of both parental strains and the two chromosome 18 congenic lines, (free running period in DD, phase angle of entrainment), one-way ANOVA for independent measures was applied, and was followed by Student t for independent measures when significant. Statistics were performed with SAS software, version 9.2.
RESULTS
Early Runner Phenotype in CAST Mice
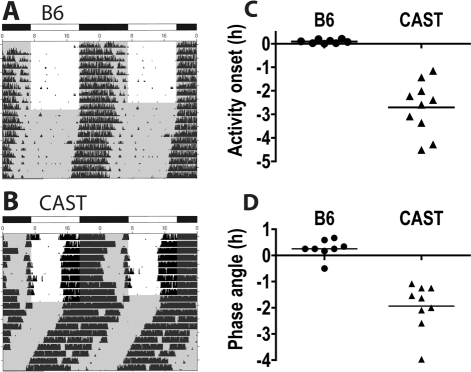
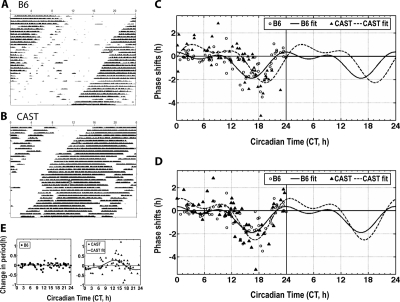
When CAST and B6 mice were housed in LD12:12, using monochromatic green light, the timing of wheel running relative to the LD cycle differed strikingly between the two strains (Figure 1C). Wheel running onset was 2.70 ± 0.35 h (n = 10) before dark onset in CAST mice, and nearly coincided with dark onset in B6 mice (0.086 ± 0.030 h after dark onset, n = 8; P < 0.001 vs. CAST). This finding was also observed independently by our collaborators using similar lighting conditions.22 As light exposure can also acutely affect activity in mice (i.e., masking), the true phase angle of entrainment for wheel-running activity rhythm needs to be assessed by extrapolation of a line drawn through the onsets of activity in constant dark (DD) subsequent to LD12:12. Upon release into DD, B6 and CAST mice also differed significantly in the phase angle of entrainment (Figure 1D; P < 0.001). Only one B6 male exhibited a negative phase angle of entrainment (0.5 h before dark in this animal); this value was > 3 SD from the mean of B6. When the data from this mouse were excluded from analysis, the phase angle of entrainment for the remaining B6 population (n = 7) was even more delayed (0.360 ± 0.073 h after dark onset). Meanwhile, phase angle of entrainment in CAST mice was 1.93 ± 0.30 h before lights-off, and was strongly correlated with the timing of wheel running onset in LD12:12 across animals within the CAST strain (Pearson r = 0.8732, P = 0.0021), indicating that the early activity onset in CAST mice under LD12:12 conditions was a valid measure of entrained phase. These data demonstrate that the early runner phenotype, characterized by an advanced activity onset in LD and an advanced phase angle of entrainment, can be detected in inbred CAST mice under green light.
Figure 1.
CAST/EiJ (CAST) mice and C57BL/6J (B6) mice differ in phase angle of entrainment. Wheel running data from one male B6 mouse (A) and one male CAST mouse (B) were plotted in percentile actogram format. The height of the vertical tick is proportional to the number of wheel revolutions in that interval, relative to the 24-h average. Data for 9 days in LD12:12 and 11 days in constant dark are shown. Time spent in darkness is indicated by gray shading. Individual values (circles for B6 and triangles for CAST) and group means (horizontal bars) are shown for activity onset derived from LD12:12 data (C) and phase angle of entrainment derived from DD data (D). Note: (1) Only one B6 mouse had a phase angle earlier than dark onset. This value is 3 SD from the group mean and was discarded as an outlier. (2) One CAST mouse died before release into DD, thus only 9 CAST values are plotted in panel D.
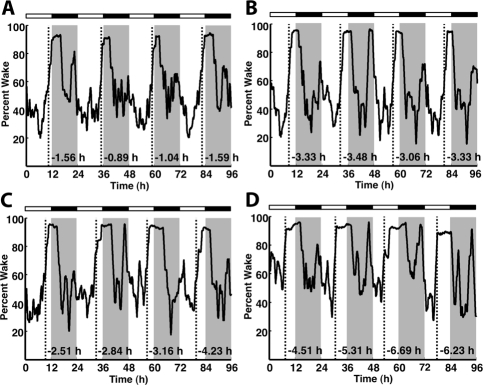
We also tested the wheel running activity of 54 (B6 × CAST) × CAST backcross mice under LD 12:12 cycles using these green lighting conditions. Compared to our previous study using more intense white lighting conditions, a higher percentage of (B6 × CAST) × CAST backcross mice exhibited the early runner phenotype under green light (data not shown), indicating that green light may have increased the penetrance of alleles that contribute to the early runner phenotype. Studies have shown that phase preference in mammals may be altered by the availability of a running wheel.23,24 To test this possibility, a subset of (B6 × CAST) × CAST backcross mice (n = 14) were also phenotyped using a piezoelectric system which scores the percentage of time spent asleep.18,19 Without access to a running wheel, the early activity onset under entrained conditions persisted as an earlier daily awakening time (Figure 2). These data clearly demonstrate that the early runner phenotype represents entrainment at an advanced phase for activity/rest, as well as sleep/wake, regardless of the availability of a running wheel.
Figure 2.
Early runner mice exhibit early daily awakening when tested on a piezoelectric system in the absence of a wheel. Data for 4 consecutive LD 12:12 cycles recorded from 4 (B6 × CAST) × CAST backcross mice are shown. Timing of light/dark is indicated both by gray shading and the white/black bar at the top of each panel. Dashed lines show the computer-defined onset for the major wake period within each LD cycle. Sleep-wake patterns and wake onset assessed by the piezoelectric system closely matched wheel-running patterns and onsets, as shown by these 4 examples: (A) A mouse with previous wheel running onset approximately 1 h before dark; (B) and (C) Mice with previous wheel running onsets 3 h prior to dark; (D) Mouse with wheel running onset 5 h before dark. In general, all mice examined had similar activity onsets whether assessed by wheel running, or, several weeks later, by piezoelectric defined sleep and wake.
Lack of Correlation Between Period and Phase Within the CAST Strain
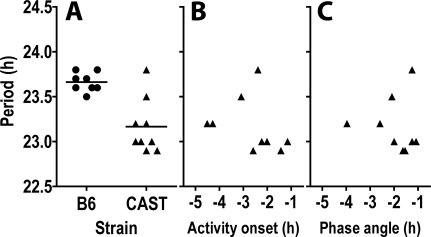
Circadian period can influence the phase angle of entrainment. To test whether the early activity onset in CAST mice can be attributed to a shorter circadian period, we transferred mice to constant dark to assess their endogenous period (Figure 3A). The circadian period in CAST mice (23.17 ± 0.10 h) was indeed shorter than that of B6 (23.66 ± 0.04 h, P = 0.001). However, CAST mice with similar circadian period exhibited wide variations in activity onset under LD as well as in phase angle of entrainment. There was no significant correlation between circadian period and either phase of activity onset under LD12:12 (Pearson r = −0.3078, P = 0.42, Figure 3B) or phase angle of entrainment (Pearson r = −0.06375, P = 0.87, Figure 3C) within the CAST population. In addition, CAST mice whose period length fell in the range of B6 mice initiated their daily activity 2-3 h earlier than B6 mice. Thus, factors other than endogenous period must contribute to the early activity onset in CAST mice under entrained conditions.
Figure 3.
Circadian period is shorter in CAST than in B6 mice and does not correlate with activity onset in LD12:12 or phase angle of entrainment within the CAST strain. (A) Individual values (circles for B6 and triangles for CAST) and group means (horizontal bars) are shown for circadian period in DD. Scatterplots of circadian period against activity onset derived from LD12:12 data (B) or phase angle of entrainment derived from DD data (C) show no correlation (P > 0.40) within the CAST strain.
A Phase-Lagged PRC in CAST Mice
Because the circadian period and the PRC together generally determine the phase at which animals will be entrained to the LD cycle, we hypothesize that CAST mice differ from B6 in their PRC, which then contributes to the early activity onset in CAST mice. To test this hypothesis, CAST and B6 mice were housed under DD conditions, and were randomly given a 30-min green light pulse every 2 weeks. Both CAST and B6 mice exhibited photic phase shifts in response to 30-min green light pulses (Figure 4A, B, C). However, there were significant strain differences in the timing of circadian responses to light. Unlike B6 mice, light exposure close to activity onset in CAST mice did not phase delay the activity pattern, while light that fell several hours after activity onset induced significant phase delays. We fitted the phase response data from each strain using 2-harmonic Fourier curves (Figure 4C). The circadian time (CT, with CT12 defined as activity onset in DD for nocturnal animals) of maximum phase delay was CT16.41 in B6 mice and 3.05 h later at CT19.46 in CAST mice. The circadian time of maximum phase advance was CT23.92 in B6 mice and 3.46 h later at CT3.38 in CAST mice. Although the timing of maximal photic phase responses was shifted in CAST relative to B6 mice, the magnitude of maximal phase shifts (i.e., those occurring in response to light pulses delivered within ± 1 h of the maximum delay and advance points on the PRC) did not differ between the strains. Maximum phase delays were −2.45 ± 0.50 h in CAST (n = 7) and −1.31 ± 0.31 h in B6 mice (n = 5; P = 0.083 vs. CAST). Maximum phase advances were 0.98 ± 0.43 h in CAST (n = 4) and 0.17 ± 0.16 h in B6 mice (n = 6; P = 0.153 vs. CAST).
Figure 4.
Phase response and tau response curves differ between B6 and CAST mice. Phase and tau (i.e., circadian period) responses to green light were measured in B6 (A) and CAST (B) mice exposed to 30-min green light pulses at random circadian phases at 14-day intervals when animals were housed under DD. One light pulse for B6 and two light pulses for CAST are shown as gray dots. (C) A 2-harmonic Fourier fitted phase response curve was generated from n = 65 phase shifts measured in B6 mice (circles and solid line) and n = 59 phase shifts measured in CAST mice (triangles and dashed line). The fitted curves were re-plotted to the right for clarity. (D) Adjusting the definition of the circadian phase of light exposure for each CAST mouse according to its activity onset in LD alters the timing of the phase response curve in CAST mice (triangles and dashed line). (E) The tau response curve demonstrates that light exposure does not influence circadian period in B6 (left) but has a slowing effect in CAST mice, as shown by the 2-harmonic Fourier fitted line (right).
Noting that the difference between the strains in the timing of peak delays and advances in the PRC was very similar in magnitude to the difference between the strains in the timing of wheel running onset in an LD12:12 cycle, we reasoned that the photic PRC might have a distinct phase relationship to wheel running in the two strains and be otherwise identical. To account for this possibility, we defined circadian time according to the phase of wheel running reltive to the daily onset of darkness under LD12:12, (e.g., activity onset of an animal in DD is defined as CT10 if this animal initiates activity at ZT10 in LD12:12), and plotted the photic PRC accordingly (Figure 4D). When the data were transformed in this manner, the PRCs were nearly identical in the two stains. The maximum phase delay was at CT17.12 in CAST mice, < 1 h later than in B6 mice. The maximum phase advance was at CT0.79 in CAST mice, < 1 h later than in B6 mice. The magnitude of maximal phase delays and advances did not differ between the strains. These data demonstrate that the photic PRC differs between CAST and B6 mice primarily in its phase relationship to wheel running, and that the traditional definition of activity onset as CT12 in nocturnal rodents is probably inappropriate in CAST mice.
In addition to causing an acute phase shift, light exposure can also alter circadian period in the longer term. To document this effect of photic stimulation, we measured a photic tau response curve, in which the change in circadian period after a light pulse is plotted against the circadian time at which the light pulse is delivered (Figure 4E). Circadian period was selectively increased by light exposure only in CAST (P = 0.01) and only when light was administered during the first few hours after the onset of wheel running. The mean increase in circadian period was 0.25 ± 0.08 h (n = 12) in CAST mice exposed to light during CT12-15 (the circadian phase at which light exposure overlaps with wheel running in an LD12:12 cycle, using the traditional definition of CT12 = activity onset in DD for CAST mice). There was no consistent effect of light pulses on circadian period in B6 mice whether they were exposed to light at CT12-15 (0.056 ± 0.058 h, n = 5) or at any other phase of the circadian cycle (Figure 4E).
Blunted Light-Induced Masking Responses in CAST Mice
Normally, light exposure acutely suppresses wheel-running activity in mice. The fact that CAST mice exhibit wheel running during the light phase of the LD12:12 cycle indicates that masking is at least attenuated to some degree in these mice. To quantify the suppression of wheel running by light, mice of both strains were exposed to 1-h light pulses beginning at 30 min into the dark portion of the LD12:12 cycle (Figure 5). B6 mice exhibited a near total (> 95%) suppression of wheel running during light exposure whether the light was green at ∼83 lux (P < 0.001, light exposure vs. baseline) or white at ∼160 lux (P < 0.001, light exposure vs. baseline). By contrast, CAST mice exhibited no significant suppression of wheel running by green light but a modest 30% suppression of wheel running by white light (P < 0.05, light exposure vs. baseline). These data demonstrate that the negative masking effect of light is strongly attenuated in CAST mice.
Figure 5.
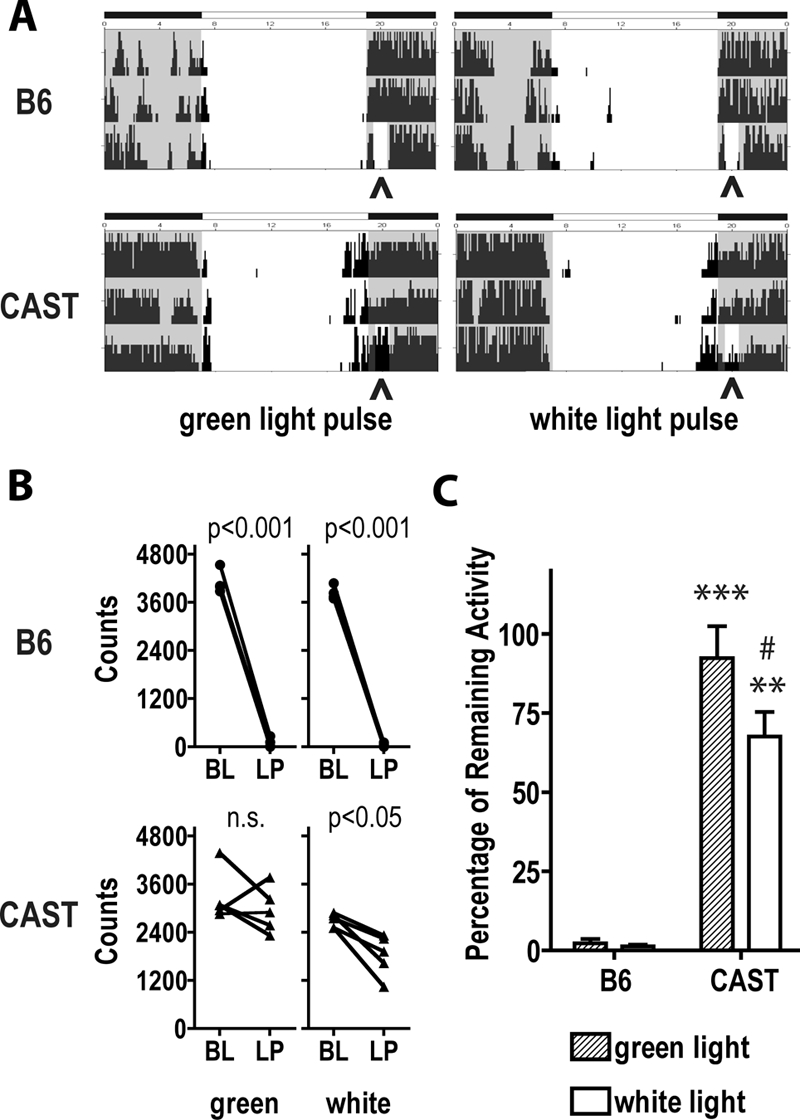
Negative masking effects of light are strain and light dependent. (A) Seventy-two hours of wheel running data from B6 (top) and CAST (bottom) mice demonstrate the effects of green (left) and white (right) light exposure on wheel running activity. Animals were entrained under LD12:12 conditions using green light. The timing of the light pulse (ZT12.5-ZT13.5 on the third night shown) is illustrated by gray shading and carets at the base of each actogram. (B) The amount of wheel running during light pulse (LP) at ZT12.5-ZT13.5 and during the control dark condition is plotted individually for each B6 (upper panel) and CAST (lower panel) mouse. Baseline (BL) activity in the control dark condition is calculated as the mean wheel-running activity at the same Zeitgeber time (ZT) 2 days preceding the light pulse. P values from paired Student t-test are labeled at the top of each panel. n.s., not significant. (C) Group mean ± SEM values for wheel running during light exposure at ZT12.5-ZT13.5 are plotted as a percentage of baseline activity. ***P < 0.001, **P < 0.01, B6 vs. CAST, Student t-test. #, P < 0.05, green vs. white light within CAST mice, Student t for paired measures.
As the sensitivity of light-induced masking responses in CAST mice is affected by the spectra and/or intensity of lighting conditions, we reasoned that lighting spectra and/or intensity may also modulate the stability of wheel-running activity before dark in CAST mice. To evaluate this effect, we housed CAST mice under LD conditions first using white light at ∼160 lux and then green light at ∼83 lux. Activity onset was delayed by approximately three-quarters of an hour when CAST mice were housed in white light relative to green light (Figure 6B; P < 0.05). The intensity of wheel running at the daily activity onset was also influenced by the cage lighting: activity density (the number of wheel revolutions occurring per unit time between daily wheel running onset and dark onset) was greater in CAST mice under green light than under white light (Figure 6C; P < 0.05). Thus, the early runner phenotype in CAST mice is modulated by the intensity and/or wavelength of environmental lighting.
Figure 6.
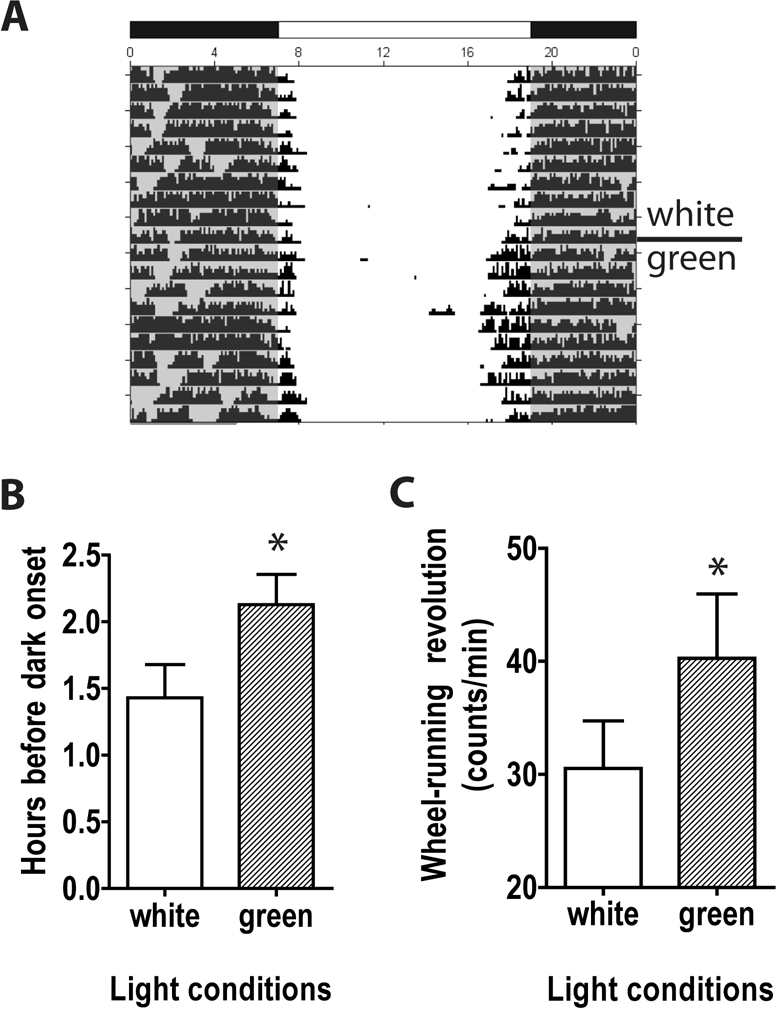
Activity density before dark in CAST mice housed in LD12:12 is dependent on the type of light exposure. (A) Wheel-running data are shown from one male CAST mouse housed in white light (days 1-10) or green light (days 11-20). Mean ± SEM are plotted for mathematically defined and visually adjusted activity onsets (B) and activity intensity defined by number of wheel running revolutions during the interval between activity onset and dark onset (C) for CAST mice housed in white (white bars) and green light (grey bars). *P < 0.05 green vs. white light, Student t for paired measures.
Wheel Running Phenotypes in Chromosome 18 Congenic Strains
In a (B6 × CAST) × CAST backcross population, we previously identified a QTL, named Era1, that contributes to 10% of the phenotypic variance in activity onset under LD conditions.15 To validate this QTL, wheel running phenotypes were measured in two congenic mouse strains in which proximal (B6.CAST.18P) and middle (B6.CAST.18M) segments of chromosome 18 have been transferred from the CAST to the B6 background by selective breeding (Figure 7A). Mice of both congenic strains exhibited entrainment to a LD12:12 cycle with the bulk of wheel running activity restricted to the dark portion of the cycle (Figure 7B, C). The phase angle of entrainment, as assessed by extrapolation of a line drawn through the daily onset of wheel running activity in constant dark subsequent to entrainment, was intermediate between the parental strains for both congenic lines (Figure 7D). The phase angle of entrainment was advanced by an average of 0.53 h in B6.CAST.18P relative to B6 mice (P < 0.001) and by an average of 0.70 h in B6.CAST.18M relative to B6 mice (P < 0.001). The phase angle of entrainment was significantly delayed in both congenic strains relative to CAST mice (P < 0.001). These data demonstrate that a modest but significant portion of the advanced phase angle of entrainment in CAST mice can be attributed to a locus (or loci) on the mid-portion of chromosome 18. Moreover, the Era1 QTL is now mapped to a genomic region where the CAST segments in B6.CAST.18P and B6.CAST.18M overlap.
Negative masking of wheel running activity was also measured in B6.CAST.18P and B6.CAST.18M congenic lines during the dark portion of the LD cycle, as it was in the parental strains. These congenic lines appeared to have intact negative masking, as they exhibited > 95% suppression of wheel-running activity when exposed to either a ∼83 lux green light pulse or a ∼160 lux white light at ZT12.5-13.5, identical to B6 mice.
When animals of the B6.CAST.18P and B6.CAST.18M strains were housed in constant dark, robust circadian rhythmicity was maintained (Figure 7B, C). Circadian period was unexpectedly longer in the congenic strains than in both parental strains (Figure 7E). Circadian period was longer by 0.26 h in B6.CAST.18P relative to B6 mice (P < 0.001) and by 0.15 h in B6.CAST.18M relative to B6 mice (P < 0.01). Circadian period was also significantly longer in both strains than in the CAST parental strain (P < 0.001). These data demonstrate that the shorter circadian period in CAST relative to B6 mice is not conferred by genetic material on proximal or mid-chromosome 18, and that the earlier phase angle of entrainment in the two congenic strains is not caused by a shorter circadian period.
DISCUSSION
Studies in rodents12,25–28 and humans13,14 have demonstrated that the circadian clock is profoundly affected by genetic variability. A good deal of information has been obtained about the molecular underpinnings of the circadian clock from these genetic studies,29–31 but information on the molecular mechanisms underlying phase control under entrained conditions is still lacking. The current report reveals that the early runner phenotype co-varies with a number of circadian endophenotypes in CAST mice, including strain differences in circadian period, altered timing of the PRC relative to circadian wheel running rhythms, and attenuated light masking of wheel running. Only a subset of these circadian endophenotypes is replicated in B6.CAST.18 congenic lines, and their phase angle of entrainment is intermediate between the parental strains. The congenic lines were not expected to fully replicate the early runner phenotype, as our previous QTL study on (B6 × CAST) × CAST backcross progeny indicated that the Era1 QTL on chromosome 18 is responsible for approximately 10% of the phenotypic variance (at least under the white lighting conditions used in that study). Nonetheless, an advanced phase angle of entrainment relative to B6 mice was indeed apparent in B6.CAST.18P and B6.CAST.18M strains and is thus conferred by CAST alleles on the mid-portions of chromosome 18.
CAST and B6 mice differ in circadian period, as shown here and previously.15 The shorter circadian period in CAST might contribute to the early phase of activity onset in these mice, as shorter circadian period dictates an earlier activity onset in entrained conditions, absent any other differences among experimental subjects.6 However, in neither the genetically diverse B6 × CAST backcross15 or intercross22 populations, nor the genetically uniform CAST population studied here, did circadian period correlate significantly with phase angle of entrainment, as is often assumed. Furthermore, the coincidence of early phase angle of entrainment and longer circadian period in B6.CAST.18P and B6.CAST.18M mice relative to B6 was not expected from this theoretical standpoint. It is possible that circadian period is not lengthened intrinsically in B6.CAST.18P and B6.CAST.18M mice, but rather it is increased as a consequence of light exposure during the entrainment process. Indeed, in the CAST strain (Figure 4E) and other rodent species,32 increases in circadian period (tau) can occur in response to light exposure, as demonstrated by the “tau response curve.”32 We hypothesize that the lengthened circadian period in B6.CAST.18P and B6.CAST.18M mice relative to both parental strains may be due to the combination of (1) an intrinsic circadian period similar to that of B6 mice, conferred by loci distinct from the Era1 QTL, and (2) slowing of the clock due to repeated daily exposure to light at around the time of wheel running onset in LD, conferred in part by the Era1 locus.
The photic PRCs for CAST and B6 strains were virtually identical when the abscissa values were transformed to account for individual differences in timing of wheel running relative to light exposure in the LD12:12 cycle. It is clear from this observation that circadian resetting in response to photic stimulation is intact in CAST mice, and that the light-sensitive oscillator in the SCN is functional. In fact, the magnitude of maximal phase delays was somewhat greater in CAST than B6 mice, but did not quite reach significance, perhaps due to the limited number of phase delays collected within the 2-h peak phase delay window of the PRC. Rather than the photic phase-shifting response being deficient in CAST mice, the timing of wheel running as an output of the circadian clock is coupled to the light-sensitive oscillator at a distinct phase in CAST when compared to B6 mice. In support of this model, the SCN oscillator itself, as defined by rhythms of Period gene expression, is not detectably phase advanced in early runner (B6 × CAST) × CAST mice relative to controls.15 Thus, alterations in activity rhythms may be a result of downstream mechanisms on the output side of the clock,33 as appear likely in these CAST mice. The mammalian circadian system is believed to be organized in a hierarchy: the master clock located in the SCN controls the oscillations of downstream clocks, which in turn regulate rhythmic physiological processes including activity/rest and sleep/wake cycles. Studies also suggest the presence of SCN-independent circadian oscillations that can be induced by timed non-photic stimulation such as restricted food access.34 Additionally, under a forced desynchrony protocol, separate compartments within the SCN can be shown to oscillate independently.35 It is thus possible that the mechanisms linking the SCN oscillator to downstream oscillators, SCN-independent oscillators to SCN-oscillators, or independent oscillators within the SCN, are modulated by loci that are polymorphic between CAST and B6 mice, including Era1. It is also possible that output pathways such as those utilizing cardiotrophin-like cytokine, which normally inhibit activity late in the day,36 are deficient or altered in CAST mice.
The attenuated light masking of wheel running observed in CAST mice relative to B6 mice is likely to influence, in part, the early runner phenotype. Indeed, an early activity onset under normal LD conditions would not be observed at all if CAST mice were to exhibit negative masking to the same degree as B6 mice. Both attenuation of masking and the advanced activity onset in LD were more robust in CAST mice when the light exposure was restricted to approximately 83 lux of green light. It is possible that the less-extreme early activity onset in CAST mice entrained under ∼160 lux of white light resulted from modest negative masking, as activity density before lights-off was reduced under white light compared to green light. Alternatively, this parallel may suggest that the spectral sensitivity, and thus the relevant photoreceptors for phase determination and masking, are similar if not identical. Both melanopsin containing ganglion cells and classical photoreceptors are capable of performing non-image forming retinal irradiance detection,37,38 and consequently, it cannot be determined whether any specific phototransduction pathway is deficient in CAST mice based on the current results. It is worth noting, however, that B6.CAST congenic lines exhibited intact negative masking, consistent with the fact that the advanced phase angle of entrainment in B6.CAST.18P and B6.CAST.18M mice did not lead to earlier activity onset under LD12:12 conditions, and that no known photopigment genes map to the portion of chromosome 18 shared by the CAST segments in B6.CAST.18P and B6.CAST.18M strains.
Collectively, these data begin to explain the early runner phenotype phenomenologically, if not mechanistically. For entrainment to occur, CAST mice and B6 mice alike require a phase-delaying effect of light to counteract the phase advancing effect of a circadian period less than 24 h. The advance of wheel running onset in CAST mice relative to B6 mice reflects, in part, the difference in the timing of light responses relative to wheel running in the two strains. Further, the attenuation of negative masking in CAST mice allows the early runner phenotype to be detected in LD12:12. Relationships between entrained phase and other circadian properties have been documented in many studies. In humans, subjects with similar endogenous period length can still exhibit large variations in their time-of-day preference, as their clock differs in oscillation amplitude and sensitivity to phase-shifting agents.11 Despite an extremely long period, Clock mutant mice entrain to an LD cycle at a phase that coincides with dark onset, as in wild-type animals, because of a low-amplitude circadian oscillation and consequently high-magnitude photic phase-shifting responses produced by the Clock mutation.39 The shorter circadian period and slightly lower amplitude PRC in BALB/cJ mice leads to a phase angle of entrainment that is similar to that of the CAST mice observed in the current study, although they exhibit very little wheel running in the hours before dark onset in an LD12:12 cycle due to masking,40 even when similar green light conditions are used.41 Our current study differs from those reports by suggesting a novel mechanism of circadian phase determination, which may involve altered coupling between different oscillators of the mammalian circadian system, or between the SCN and major output pathways. In addition, light-induced masking in CAST mice was impaired in concert with the advanced phase angle of entrainment to maintain robust activity in the preferred circadian phase. This suggests the possibility of positive selection for altered chronotypes among wild populations of Mus musculus castaneus from which CAST mice are derived. It is also conceivable that human chronotype variation was and is under similar selection pressures, but as in mice, this will have to await the identification of more specific gene alleles that influence chronotype. Thus far, among common allelic variants already described in humans, Per3 alleles are perhaps the best candidates.42–44
The phenotypic data from B6.CAST.18P and B6.CAST.18M provide confirmation that the Era1 QTL on chromosome 18 identified in our previous work influences the unusual circadian traits in CAST mice. Circadian QTL analysis has the potential to identify novel circadian loci. The refined Era1 locus is a 10 Mb region, containing 73 annotated and several predicted genes. Among the candidate loci are Casein kinase 1-alpha 1 (Csnk1a1) and Gastrin-releasing peptide (Grp). The former is functionally similar to two other casein kinases known to influence circadian rhythms: casein kinase 1δ13 and casein kinase 1ε.12 We have sequenced the entire coding region, several intron-exon boundaries, and several hundred base pairs upstream from the transcription start site of Csnk1a1 in both CAST and B6 genotypes. A small number of single nucleotide polymorphisms exist between the strains, but none appear to have functional consequences (data not shown). GRP has been proposed as the signal communicating across functionally heterogeneous SCN sub-regions.45 We have not, to date, sequenced any portion of the Grp locus in the two strains. In a QTL study of (B6 × BALB) F2 hybrid mice, 5 QTLs and 2 epistatic interactions, different from those loci that influence circadian period, were found to influence the phase angle of entrainment.41 This observation further strengthens the QTL approach for the genetic dissection of these poorly understood circadian traits. More generally, CAST and the congenic mice derived from them are likely to be a valuable resource for studies of the molecular mechanisms of phase relationships, and their regulation by light. The CAST strain may also provide a mouse model for human ASPS and other circadian disorders related to sleep and activity phase. Indeed, the complex and multifactorial nature of the unusual circadian phenotypes seen in CAST mice is similar to the majority of cases of ASPS in humans, as opposed to the very rare point mutations causing familial ASPS.13,14
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Drs. Kazu Shimomura and Joe Takahashi for the sharing of data, advice, and helpful comments on the manuscript. This work was supported by a grant from the DoD, AFOSR, FA9550-05-1-0464 (BFO), NIH grant R01 MH067752 (BFO), and internal funding by the University of Kentucky. JW was supported in part by the American Sleep Medicine Foundation fellowship #24-YI-03. We also thank Dr. Richard Davis and others at UCLA who provided the B6.CAST congenic mice (designated GTM strains).
REFERENCES
- 1.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Isr J Psychiatry Relat Sci. 2002;39:11–8. [PubMed] [Google Scholar]
- 3.Baker SK, Zee PC. Circadian disorders of the sleep-wake cycle. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 606–14. [Google Scholar]
- 4.Zisapel N. Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs. 2001;15:311–28. doi: 10.2165/00023210-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–74. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- 6.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 7.Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 8.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–9. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 9.Aschoff JA. Freerunning and entrained circadian rhythms. In: Aschoff JA, editor. Handbook of behavioral neurobiology. ed. New York: Plenum Press; 1981. pp. 81–93. [Google Scholar]
- 10.Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–5. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 11.Brown SA, Kunz D, Dumas A, et al. Molecular insights into human daily behavior. Proc Natl Acad Sci U S A. 2008;105:1602–7. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowrey PL, Shimomura K, Antoch MP, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–91. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 14.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 15.Wisor JP, Striz M, DeVoss J, Murphy GM, Jr, Edgar DM, O'Hara BF. A novel quantitative trait locus on mouse chromosome 18, “era1,” modifies the entrainment of circadian rhythms. Sleep. 2007;30:1255–63. doi: 10.1093/sleep/30.10.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iakoubova OA, Olsson CL, Dains KM, et al. Genome-tagged mice (GTM): two sets of genome-wide congenic strains. Genomics. 2001;74:89–104. doi: 10.1006/geno.2000.6497. [DOI] [PubMed] [Google Scholar]
- 17.Davis RC, Jin A, Rosales M, et al. A genome-wide set of congenic mouse strains derived from CAST/Ei on a C57BL/6 background. Genomics. 2007;90:306–13. doi: 10.1016/j.ygeno.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Donohue K, Medonza D, Crane E, O'Hara B. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. BioMedical Engineering OnLine. 2008;7:14. doi: 10.1186/1475-925X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores AE, Flores JE, Deshpande H, et al. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54:225–33. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- 20.Spoelstra K, Albrecht U, van der Horst GTJ, Brauer V, Daan S. Phase Responses to light pulses in mice lacking functional per or cry genes. J Biol Rhythms. 2004;19:518–29. doi: 10.1177/0748730404268122. [DOI] [PubMed] [Google Scholar]
- 21.Comas M, Beersma DGM, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–72. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- 22.Jiang P, Striz M, Shimomura K, Takahashi JS, Wisor JP, O'Hara BF. A mouse model for familial advanced sleep phase syndrome. Sleep. 2008;31:A363–4. [Google Scholar]
- 23.Kas MJH, Edgar DM. A nonphotic stimulus inverts the diurnal-nocturnal phase preference in Octodon degus. J. Neurosci. 1999;19:328–33. doi: 10.1523/JNEUROSCI.19-01-00328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mistlberger RE, Holmes MM. Behavioral feedback regulation of circadian rhythm phase angle in light-dark entrained mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R813–21. doi: 10.1152/ajpregu.2000.279.3.R813. [DOI] [PubMed] [Google Scholar]
- 25.Zheng B, Albrecht U, Kaasik K, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–94. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 26.Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol. 2000;20:6269–75. doi: 10.1128/mcb.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 28.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowrey PL, Takahashi JS. Mammalian circadin biology: Elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–15. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 31.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 32.Daan S. The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms. 2000;15:195–207. doi: 10.1177/074873040001500301. [DOI] [PubMed] [Google Scholar]
- 33.Eskin A. Identification and physiology of circadian pacemakers. Introduction. Fed Proc. 1979;38:2570–2. [PubMed] [Google Scholar]
- 34.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–92. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 35.de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat Neurosci. 2006;9:212. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- 37.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 38.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vitaterna MH, Ko CH, Chang A-M, et al. The mouse clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103:9327–32. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 1990;10:3685–94. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimomura K, Low-Zeddies SS, King DP, et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–80. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 42.Ebisawa T, Uchiyama M, Kajimura N, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–6. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 44.Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14:151–60. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Antle MC, Kriegsfeld LJ, Silver R. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J. Neurosci. 2005;25:2447–54. doi: 10.1523/JNEUROSCI.4696-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]