Abstract
Study Objectives:
Slow wave EEG activity in NREM sleep decreases by more than 60% between ages 10 and 20 years. Slow wave EEG activity also declines across NREM periods (NREMPs) within a night, and this decline is thought to represent the dynamics of sleep homeostasis. We used longitudinal data to determine whether these homeostatic dynamics change across adolescence.
Design:
All-night sleep EEG was recorded semiannually for 6 years.
Setting:
EEG was recorded with ambulatory recorders in the subjects' homes.
Participants:
Sixty-seven subjects in 2 cohorts, one starting at age 9 and one starting at age 12 years.
Measurements and Results:
For NREM delta (1-4 Hz) and theta (4-8 Hz) EEG, we tested whether the proportion of spectral energy contained in the first NREMP changes with age. We also tested for age changes in the parameters of the process S exponential decline. For both delta and theta, the proportion of energy in the first NREMP declined significantly across ages 9 to 18 years. Process S parameters SWA0 and TWA0, respectively, represent slow wave (delta) activity and theta wave activity at the beginning of the night. SWA0 and TWA0 declined significantly (P < 0.0001) across ages 9 to 18.
Conclusions:
These declines indicate that the intensity of the homeostatic or restorative processes at the beginning of sleep diminished across adolescence. We propose that this change in sleep regulation is caused by the synaptic pruning that occurs during adolescent brain maturation.
Citation:
Campbell IG; Darchia N; Higgins LM; Dykan IV; Davis NM; de Bie E; Feinberg I. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. SLEEP 2011;34(1):83-91.
Keywords: Development, maturation, FFT, longitudinal
SLEEP AND EEG CHANGES ARE PART OF THE BIOLOGICAL AND BEHAVIORAL MATURATION OF ADOLESCENCE. LEFT TO THEIR OWN DEVICES, ADOLESCENTS in developed countries shift their sleep patterns in a manner that produces later bedtimes and later wake times.1 The adolescent changes in the sleep electroencephalogram (EEG) are even more dramatic than sleep schedule changes. Cross-sectional studies demonstrated that visually scored slow wave sleep and computer quantified slow wave EEG activity decline steeply across adolescence.2–7 Longitudinal measurements can provide a more precise assessment of adolescent sleep EEG changes. In our longitudinal study, all-night NREM delta (1-4 Hz) EEG power was unchanged between ages 9 and 11 and then decreased by more than 65% between ages 11 and 17 years.8 NREM theta (4-8 Hz) EEG power showed a different maturational pattern. It declined significantly between ages 9 and 11 years and still declined by more than 60% between ages 11 and 17 years.
The delta EEG of slow wave sleep reflects a process by which the brain reverses the effects of waking.3 In the recovery sleep following sleep deprivation, visually scored slow wave sleep duration increases9 as does slow wave EEG power.10 Conversely, daytime napping decreases slow wave EEG power in subsequent NREM sleep.11–14 Slow wave EEG power declines across the night, and this decline indicates the progression of a recuperative or homeostatic process.3,15 On average, power is greatest in the first NREM period (NREMP1) and declines across consecutive NREMPs as the recuperative need is met. The delta decline across NREMPs differs greatly between individuals and also differs from night to night within an individual. However, the average trend in grouped data can provide useful information about sleep homeostasis. The model fit to the exponential delta decline, process S, has been an important stimulus for sleep research over the past 25 years.15,16
Several forms of the process S equations have been published.16,17 A simple description of the exponential decline in slow wave activity across the night is the form:
The terms of the equation are as follows:
SWAt = slow wave activity at time t
SWA0 = slow wave activity at time 0
t = time (min) from sleep onset
Tau = the time constant (min) of the exponential decline
SWA∞ = the slow wave activity at the asymptote of the decline
Supplemental Figure S1 shows an exponential decline curve of this type and demonstrates how 25% changes in Tau, SWA0, and SWA∞ would affect the shape of the curve. An exponential decline equation can be fit to the across-night decline of both delta and theta EEG within NREM sleep. In this report, we label the delta parameters SWA0, Tauδ, and SWA∞ and the theta parameters TWA0, Tauθ, and TWA∞.
Previous articles fitting an exponential curve to the slow wave activity decline across NREMPs have standardized the slow wave activity (power) in each NREMP as a percent of average all-night slow wave power. This standardization adjusts for individual differences in absolute EEG power. More importantly, the standardization allows us to investigate the maturational changes in the decline in slow wave EEG across the night independently of the huge maturational change in all-night slow wave EEG power. Although delta power decreases substantially across adolescence, it is not clear whether there are changes in the regulation of standardized slow wave activity across the night (process S). Jenni and Carskadon7 and Jenni et al.18 investigated this question with cross-sectional data. They found that 2 groups of subjects (n = 8 each) with mean ages 11.9 and 14.2 years differed in the accumulation of sleep pressure during waking, but that the groups' declines in slow wave activity across sleep did not differ significantly in any process S parameter.
Here we use longitudinal data from 2 age cohorts covering the age range 9 to 18 years to determine whether slow wave sleep regulation changes across adolescence. We examine the across the night dynamics of both delta (1-4 Hz) and theta (4-8 Hz) EEG, 2 frequencies that respond homeostatically to prior waking duration and decline exponentially across NREMPs. We test whether the proportion of NREM delta (or theta) spectral energy in NREMP1 changes with age and whether there are age-related changes in the parameters of the process S exponential decline.
METHODS
The UC Davis Sleep Lab is carrying out a multiyear longitudinal study of adolescent changes in sleep and the EEG. This article uses data from the first 6 years of that study. The methods we use have been described in detail in 2 previous publications.19,20
Subjects
Seventy subjects in 2 cohorts were enrolled at the start of the study. We started recording EEG from subjects in the C9 cohort (n = 32) at approximately age 9 years and from subjects in the C12 cohort (n = 38) at approximately age 12 years. Three subjects failed to complete the first 3 years of the study. Only the remaining 67 subjects (C9 N = 30, 15 female; C12 N = 37, 19 female) are included in the analyses. Fifty-six subjects completed all 6 years of the study. The UC Davis IRB approved all procedures. Subjects received monetary compensation for their participation.
Experiment Design
At approximately 6 month intervals, all-night EEG was recorded on 4 consecutive nights at the subjects' homes under the subjects' habitual sleep schedule and in their typical sleep environment. Laboratory personnel did not monitor the subjects during the night. On the first 2 nights, typically Wednesday and Thursday, subjects kept their habitual weekday sleep schedules. The third and fourth nights (typically Friday and Saturday) were extended nights with the subjects keeping their habitual weekday bedtime but sleeping as long as possible (up to 12 h). Night 4 data were not used in this article because the extended sleep on night 3 would have affected the night 4 EEG.
Subjects' sleep schedules changed across the 6years of this study. As described in Campbell et al.,21 their bedtimes became later, but their rise times did not change significantly. Although we allowed their sleep schedules to change with age, we required subjects to maintain their current habitual weekday sleep schedules for 5 days prior to the first night of recording. Wrist actigraphy (Mini Mitter Actiwatch A16) confirmed adherence to this schedule. Napping was prohibited. Subjects who napped or deviated from their habitual schedule were rescheduled for recording on a subsequent week.
EEG Recording
EEG electrodes were applied at Fz, C3, C4, Cz, O1, and either O2 or Pz with A1 and A2 mastoid electrodes. Signals were recorded versus a reference. The signal for EEG electrode versus mastoid was obtained by subtraction (e.g., C3 vs. ref minus A1 vs. ref). EOG was recorded as right and left outer canthi versus forehead. Grass H2O ambulatory EEG recorders (200 Hz digitization rate) were used for the 1st through 9th recordings. During the 10th recording, Grass Instruments retired the H2O recorder and replaced it with the Aura ambulatory EEG recorder (400 Hz digitization rate). The Aura recorders were used for all subjects for the 11th and 12th recordings and for about 75% of the subjects for the 10th recording. Supplemental Figure S2 shows frequency response curves for the 2 types of ambulatory EEG recorders. The filter characteristics were very similar across 1 to 8 Hz. Thus, we obtained comparable data for delta and theta EEG from both recorders.
Sleep Stage Scoring and EEG Analysis
Digitized EEG signals were displayed on a computer monitor (using Pass Plus, Delta Software), and each 20-sec epoch was visually scored as wake, stage 1, NREM sleep, REM sleep, or movement using Rechtschaffen and Kales22 criteria, modified by collapsing stages 2, 3, and 4 into one NREM sleep stage. Artifacts were scored in addition to and independently of sleep stage. All records were scored by 2 raters, and a senior lab scientist resolved discrepancies. Sleep cycles were defined according to Feinberg and Floyd23 criteria. Children frequently have exceptionally long duration of cycle 1 because of “skipped” first REM periods.5,7,24 We separated these long first NREM periods into 2 cycles when all-night plots of delta EEG power showed 2 clear delta peaks separated by a trough ≥ 10 min. Of the 713 nights used in the cycle analysis, 154 had skipped first REM periods.
Based on the number of artifacts, we chose one central EEG lead for analysis, either C3 or C4 versus contralateral mastoid. Data from the primary lead were analyzed with spectral analysis (Pass Plus, Delta Software) using Fast Fourier transform on 5.12 second Welch tapered windows25 with 2.62-sec overlap, producing 8 windows per 20-sec epoch. Spectral energy in each frequency band was summed as the power in each 20-sec epoch.
For each NREMP, energy for the delta (1-4 Hz) and theta (4-8 Hz) bands was summed for all artifact-free NREM sleep epochs. Power was calculated as energy divided by the seconds of artifact-free NREM sleep. Power was standardized for each night by expressing the power in each NREMP as a percent of average power in NREMPs 1 through 5 (averaged as NREMP1-5 energy divided by total seconds of NREMP1-5 sleep duration). Only nights containing 5 complete NREMPs were used. We defined a complete NREMP5 as having ≥ 15 min of NREM followed by any amount of REM sleep.
Statistics
Data used for statistical analysis
The statistical analysis of trends across NREMPs requires a time point at which to locate the power measure for the NREMP. For this time point we (as have others before us7) used the NREMP midpoint. The timing of the NREMP midpoints changes between recordings, complicating data averaging from multiple nights. Therefore, we chose a single night (priority order: night 2, night 3, night 1) from each subject at each of the 12 semiannual recording periods. Occasionally (54 of 767 subject recording sessions) a missing data point arose when a subject lacked 5 complete cycles on any of the 3 nights or neither C3-A2 nor C4-A1 was usable. For each semiannual recording period for both cohorts, Table 1 shows the number of subject-nights used in the analysis and the mean subject age. All statistical analyses were conducted on data from the 2 cohorts combined. Because of the number of analyses, we used an α of 0.01.
Table 1.
Mean age and standard deviation (SD) at the 12 semiannual recordings for both the C9 and C12 cohorts.
| Cohort C9 |
Cohort C12 |
|||||
|---|---|---|---|---|---|---|
| Recording | N | Mean age (years) | SD | N | Mean age (years) | SD |
| 1 | 29 | 9.32 | 0.20 | 37 | 12.29 | 0.19 |
| 2 | 29 | 9.82 | 0.21 | 35 | 12.80 | 0.20 |
| 3 | 29 | 10.35 | 0.23 | 33 | 13.33 | 0.20 |
| 4 | 25 | 10.88 | 0.21 | 36 | 13.86 | 0.22 |
| 5 | 28 | 11.40 | 0.24 | 32 | 14.37 | 0.24 |
| 6 | 27 | 11.93 | 0.25 | 37 | 14.87 | 0.22 |
| 7 | 28 | 12.41 | 0.27 | 33 | 15.33 | 0.20 |
| 8 | 26 | 12.94 | 0.25 | 32 | 15.88 | 0.24 |
| 9 | 26 | 13.53 | 0.29 | 31 | 16.43 | 0.21 |
| 10 | 26 | 13.98 | 0.30 | 27 | 16.95 | 0.20 |
| 11 | 24 | 14.48 | 0.32 | 30 | 17.46 | 0.23 |
| 12 | 22 | 14.96 | 0.32 | 31 | 17.95 | 0.26 |
The number (N) of nights analyzed at each recording period are also shown. Data analyzed were from 713 nights with 5 complete NREM periods from 67 subjects.
Analysis of age effects on average power
Mixed-effect analysis is particularly suited for analyzing longitudinal data.26,27 This analysis is somewhat similar to regression but allows for repeated measurements from a subject over time because it takes into account the inherent correlation of repeated measures. Longitudinal data violate the independent observation assumption of typical regression statistics. Mixed-effect analysis can also accommodate missing data which are virtually inevitable in extended longitudinal studies. We used linear mixed-effect analysis to determine whether the average power (averaged from all NREM sleep in NREMPs 1-5) in 1-4 Hz EEG during NREM sleep changed with age and whether the age change differed in the 2 cohorts. We conducted a similar analysis for average power in 4-8 Hz EEG during NREM sleep.
Analysis of age effects on the proportion of spectral energy in NREMP1
We also used linear mixed-effect analysis to determine whether the proportion of NREM delta energy occurring in NREMP1 changed with age. We performed a separate linear mixed-effect analysis to determine whether the proportion of NREM theta energy in NREMP1 changed with age.
Analysis of age effects on process S parameters
We used an advanced nonlinear mixed-effect analysis (SAS proc NLMIXED) to determine if there were maturational changes in the parameters of the exponential decline in delta power (and separately theta power) across consecutive NREMPs of the night. In this analysis, standardized power in each NREMP was the dependent variable, and midpoint of the NREMP and the subjects' ages at which each recording occurred were independent variables. This analysis generated estimates (and standard errors) for the process S parameters SWA0, Tauδ, and SWA∞ and determined whether these parameters changed significantly with age. In this advanced analysis that simultaneously evaluated both the exponential decline across the night and the age effects on this decline, age changes were evaluated using a linear model with an intercept at age 9 years. A separate analysis for theta power determined estimates of TWA0, Tauθ, and TWA∞ and whether they changed significantly with age. In yet another separate analysis, we included a cohort factor in order to evaluate whether the 2 cohorts differed in the parameters of the exponential decline and the age effects on these parameters.
Mixed-effect analysis refers to a combination of fixed effects and random effects such that the fixed effects account for the main effects of the study factors and the random effects account for the variance between subjects. The advanced nonlinear analysis was conducted with SWA0 and Tauδ as random effects, i.e., accounting for the between subject variance in these 2 parameters. The analysis also accounted for the covariance between SWA0 and Tauδ because the standardization of the data creates an interaction such that subjects with a higher SWA0 tend to have a lower Tauδ. Similar random effects and interactions in TWA0 and Tauθ were used for the analysis of theta trends. Previous analyses of the exponential decline in EEG power across NREMPs have not explicitly used methods that recognize that the consecutive NREMP data are repeated measures on the same subject, nor have these analyses recognized that the parameters of the exponential decline are necessarily correlated when the data are standardized. Because the present study appears to be the first to analyze the process S equation parameters with mixed-effect analysis, the SAS NLMixed program for the analysis of age changes in the process S parameters for delta EEG is included as supplemental information.
Time course of maturational changes in process S parameters
We tested if a linear model for the age change in process S parameters was the best model by comparing the BIC fit statistic from the linear model to the BIC fit statistic from models with centered age squared, centered age cubed, square root of centered age, natural log of centered age, and exponential centered age. We further evaluated the pattern of the age change in the process S parameters by determining the parameters at each recording period and plotting these values versus the mean age at each recording period. Nonlinear mixed-effect analysis with SWA0 (or TWA0) as the random effect estimated the process S parameters at each semiannual recording period (i.e., without an age factor) for each cohort.
Data Presentation
Graphically presenting the data for 5 cycles for all subjects or for all 12 recording periods would produce an overwhelming number of figures. Figure 3 compares mean data from the first recording from the C9 cohort with the twelfth recording from the C12 cohort. In Figures 1, 2, and 4, values at each recording are plotted versus mean subject age at that recording. This presentation may give the incorrect impression that age was treated as a categorical variable. However, in all statistical analyses, age was a continuous variable.
Figure 3.
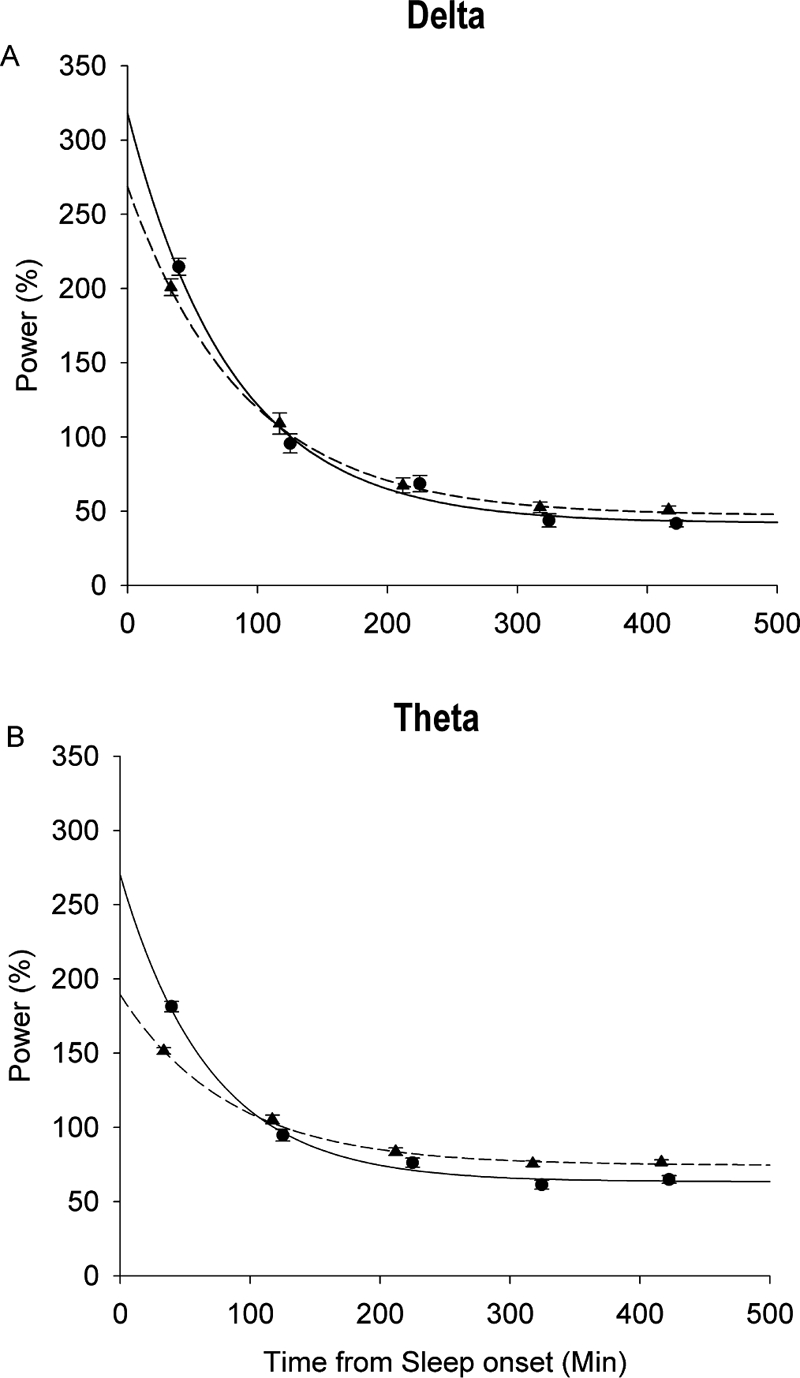
Mean (± SE) standardized power in each NREMP is plotted against time from sleep onset for the 1st recording from the C9 cohort (mean age 9.3 years; circles, solid lines) and 12th recording from the C12 cohort (mean age 17.9 years; triangles, dashed lines). Data at each NREMP are standardized as a percent of average power in NREMPs 1-5. Data are plotted at the average NREMP midpoint. Lines are exponential curves fit by nonlinear mixed-effect analysis. For delta power (A), the across-NREMP power decline began at lower starting point in the older subjects. This age-related change is even more pronounced for the across-NREMP theta power decline (B).
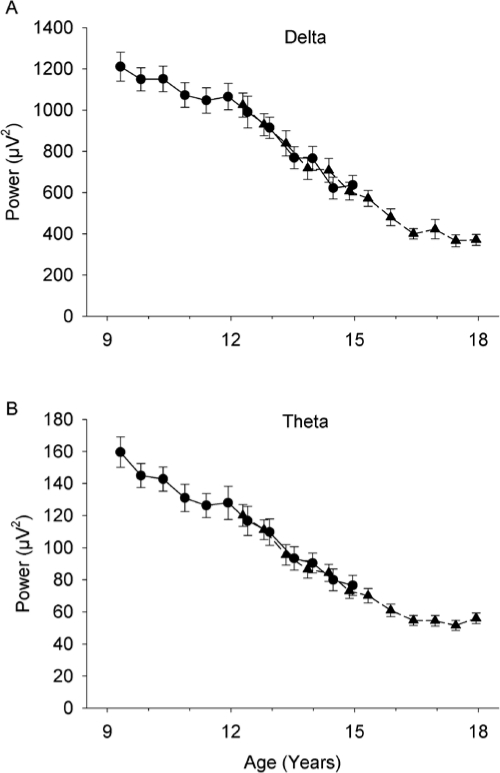
Figure 1.
Age related decline in all night (cycles 1-5) average NREM delta (1-4 Hz) power and theta (4-8 Hz) power. Mean (± SE) power at each semiannual recording is plotted against mean age for both the C9 (circles, solid lines) and C12 (triangles, dashed lines) cohorts. Both delta (A) and theta (B) power declined massively and significantly.
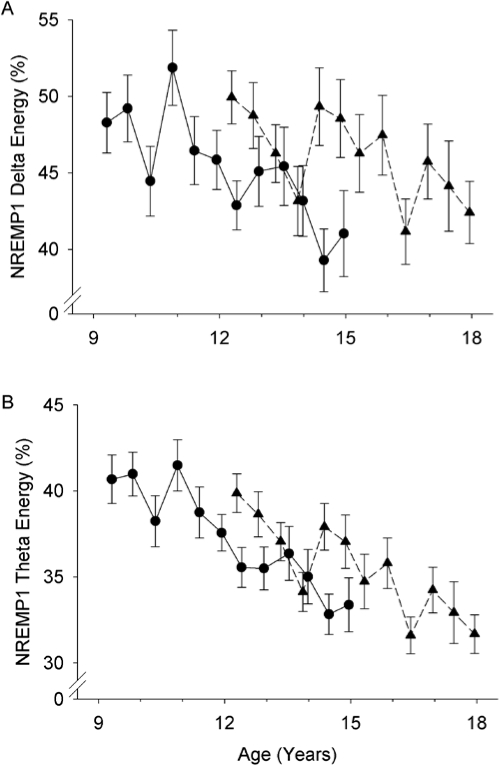
Figure 2.
Age-related decline in NREMP1 energy as a percent of total energy in NREMPs 1-5. Format is similar to Figure 1. A. Percent delta energy in NREMP1 declined across adolescence. B. Percent theta energy in NREMP1 also declined across adolescence. Cohort differences were not significant.
Figure 4.
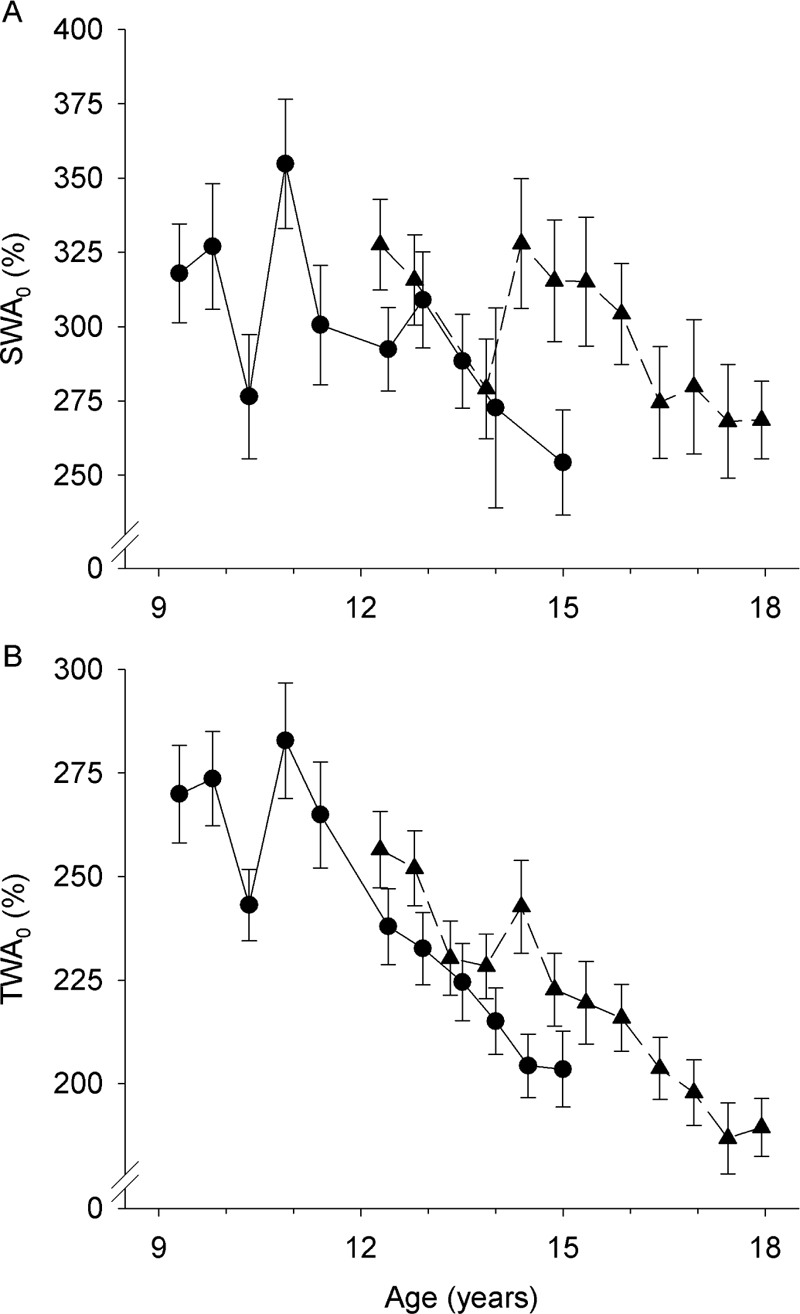
Nonlinear mixed-effect analysis estimates (± SE) of SWA0 (A) and TWA0 (B) for C9 (circles, solid lines) and C12 (triangles, dashed lines). Both process S parameters show a general decrease with age, but the effect is variable. Differences between the C9 and C12 cohorts in the 3 year period when the cohorts overlap are not statistically significant but further demonstrate the variability.
RESULTS
Age Effects on All-Night Power in Delta and Theta Bands during NREM Sleep
EEG power in the delta (1-4 Hz) frequency band during NREM sleep declined steeply and significantly (F1,645 = 405, P < 0.0001) across adolescence (Figure 1A). Between ages 9 and 18 years, average delta power declined 69%. NREM theta (4-8 Hz) EEG power also declined steeply and significantly (F1,645 = 351, P < 0.0001) between ages 9 and 18 years (Figure 1B). For both delta and theta EEG, average power for the 2 cohorts was similar between ages 12 to 15 years where the cohort ages overlap, and the age related decline did not differ (delta P = 0.09, theta P = 0.11) between cohorts. All further analyses were conducted on standardized data. This standardization allowed us to examine the age related changes in delta distribution across the night independently of the decline in absolute power across age.
Age effects on the proportion of spectral energy in NREMP1
The proportion of NREM delta energy in NREMP1 decreased significantly (F1,645 = 18.8, P < 0.0001) across adolescence (Figure 2). From approximately 50% at age 9 years, the percent of total delta energy in NREMP1 decreased by about 1 (0.95 estimate ± 0.22 standard error) percentage point per year. The age cohorts did not differ in the intercept (F1,65 = 1.1, P = 0.30) or the rate of decline (F1,644 = 0.30, P = 0.59). The decrease in NREMP1 total energy resulted from both a NREMP1 shortening (F1,645 = 9.4, P = 0.0023) and a NREMP1 standardized power decline (F1,645 = 16.2, P < 0.0001). Also, the proportion of NREM theta energy in NREMP1 decreased significantly (F1,645 = 80.0, P < 0.0001) by approximately 1.2 percentage points per year across adolescence. For theta there was also no significant cohort effect in the intercept (F1,65 = 0.9, P = 0.34) or the decline rate (F1,644 = 0.18, P = 0.67).
Age effects on process S parameters
Average standardized delta and theta power declined exponentially across the 5 NREMPs of the night (Figure 3). As noted in the introduction, this decline has been described by an exponential equation with the parameters SWA0, Tau, and SWA∞. Age effects on these parameters were tested with nonlinear mixed-effect analysis. The results, including P-values, are shown in Table 2. For standardized delta power, SWA0 declined significantly by an average rate of about 7 percentage points per year. SWA∞ increased with age, but this increase did not reach the 0.01 level of significance. Tauδ did not change significantly with age. Compared to the age change in the delta power decline, the age change in the theta power decline was larger and statistically more robust. TWA0 declined significantly by about 10 percentage points per year. The asymptote, TWA∞, increased significantly by about 1½ percentage points per year. Tauθ increased with age but this increase did not reach the 0.01 level of significance. These age-related changes in the exponential decline in power are shown in Figures 3A (delta) and 3B (theta), which compare the across-NREMP exponential decline in standardized power between the first recording from the C9 cohort and the twelfth recording from the C12 cohort.
Table 2.
Results of nonlinear mixed effect analysis of the age effects on across night exponential decline in standardized delta (1-4 Hz) power and theta (4-8 Hz) power.
| Curve Fit |
Test of Age Effects |
||||
|---|---|---|---|---|---|
| Delta | Estimated Age 9 Value | Standard Error | Change per year | t65 | P |
| SWA0 (%) | 329 | 9.2 | −7.4 | −5.95 | < 0.0001 |
| Tau (min) | 94 | 4.6 | 1.0 | 1.4 | 0.16 |
| SWA∞ (%) | 32 | 2.2 | 0.9 | 2.3 | 0.024 |
| Theta | |||||
| TWA0 (%) | 276 | 4.9 | −9.7 | −13.2 | < 0.0001 |
| Tau (min) | 75 | 3.1 | 1.1 | 2.1 | 0.044 |
| TWA∞ (%) | 59 | 1.0 | 1.6 | 8.9 | < 0.0001 |
The table shows the estimated values at age 9 for the parameters of the exponential decline and how these parameters changed with age between 9 and 18 years. For both delta (SWA0) and theta (TWA0), standardized power at the start of the night declined significantly with age. Bold type indicates a significant (α = 0.01) age effect.
Time course of maturational changes in process S parameters
In the above evaluation of maturational changes in process S parameters, we used a simple linear model for age with age “centered” by subtracting 9 years from all ages. Examining the fit statistic, BIC, when other age models (e.g., squared or natural log) were used, showed that none of these other models provided a better fit than the linear model (Table 3). In order to examine more closely the time course of the process S changes, we fitted an exponential curve to the data at each recording period. Figure 4 shows the time course of the age change in SWA0, and TWA0, the parameters most significantly affected by age. SWA0 showed an overall decline from age 9 to age 18 years. However, the parameter is quite variable, changing greatly from one recording to the next and having a large between-subject variance as shown by the large standard errors. Despite the large cohort difference apparent at some recordings at which the ages overlap, e.g. 15 years, the age change in SWA0 did not show a significant (t65 = −0.55, P = 0.59) cohort difference. As can be seen in Figures 2 and 4, the maturational time course of SWA0 was quite similar to the maturational time course of the proportion of delta energy in NREMP1.
Table 3.
Goodness of fit (BIC, smaller is better fit) for different models of age change in process S parameters and significance of age change in the parameters.
| Model | BIC Fit | SWA0 | Tauδ | SWA∞ |
|---|---|---|---|---|
| Age-8.8 | 34661 | < 0.0001 | 0.16 | 0.024 |
| (Age-8.8)2 | 34679 | < 0.0001 | 0.31 | 0.0077 |
| (Age-8.8)3 | 34684 | < 0.0001 | 0.58 | 0.0028 |
| (Age-8.8)½ | 34672 | < 0.0001 | 0.10 | 0.11 |
| (Age-8.8)−1 | 34714 | 0.54 | 0.58 | 0.53 |
| ln(Age-8.8) | 34692 | 0.0008 | 0.16 | 0.43 |
| e(age-8.8) | 34703 | < 0.0001 | 0.70 | 0.0014 |
The linear model of age change (centered by subtracting 8.8 years) provided the best fit. The age change in SWA0 was significant for all models except inverse of age. Bold type indicates a significant (α = 0.01) age effect. To avoid square roots and logs of negative numbers, age was centered by subtracting 8.8, the youngest age at which a subject was recorded, rather than 9.0.
Because light exposure affects slow wave EEG dynamics across the night,28 the semiannual recording design of our experiment raises the possibility of a seasonal contribution to the variability between recordings. We tested for this possibility by adding a seasonal factor to the analysis of age effects on the process-S parameters. SWA0 did not vary by season whether we compared spring and summer to fall and winter (P = 0.70), April through August to October through February (P = 0.91), May through July to November through February (P = 0.36), or school year to summer vacation (P = 0.21). In all of these analyses the relation of SWA0 to age remained significant at P < 0.0001.
DISCUSSION
We used longitudinal data from two age cohorts to evaluate adolescent maturational changes in the regulation of delta and theta EEG across NREMPs. The proportion of delta in the first NREMP declined across ages 9 to 18 years, and the peak normalized delta power at the start of the night (SWA0) declined significantly with age. Even stronger age changes were found for normalized theta EEG power. In the following discussion, we interpret these age changes in the two main homeostatic frequency bands as reflecting maturational changes in homeostatic sleep regulation. We propose that these changes are manifestations of the pervasive adolescent brain reorganization driven by synaptic pruning, originally hypothesized in 1982.29
Both visually scored slow wave sleep3,30 and computer measured slow wave EEG activity2,5–7 decline steeply across adolescence. Our longitudinal data from age 9 to age 18 years show a strong age related decline in both NREM delta and NREM theta power. Our previous nonlinear analysis found that the adolescent decline in delta power occurred later than the theta power decline.8 In the current analysis we use a linear model to simply document the age-related declines in power. The huge (69% decline) reduction in delta power across a 9-year age range emphasizes the importance of standardizing the data to remove the strong age effect on power in order to determine if the distribution of power across the night changes with age.
Had the adolescent decline in delta power been equally distributed across the night, the standardized delta decline across NREMPs would not have changed. In that case, the first NREMP in a 9-year-old would have provided proportionally the same amount of recuperation (delta energy) as that in an 18-year-old. The current data show that this is not the case. The proportion of delta energy in NREMP1 is higher at younger ages and declines across adolescence. Thus, not only does all night slow wave EEG activity decline across adolescence, but the regulation of slow wave EEG within the night also changes. The decline in the proportion of NREMP1 delta energy results from both a shortening of NREMP1 and a decline in the standardized delta power in NREMP1. Feinberg3 recognized the importance of NREMP1 delta by noting that the age differences in the across-night trends in slow wave sleep duration, NREMP duration, and REMP duration entirely depend on the difference in the first NREMP.
The maturational change in homeostatic regulation of sleep within the night is also reflected in the age-related decline in SWA0, the process S parameter that represents the rate of delta production at the start of the night. SWA0 is closely related to the proportion of delta in NREMP1, as demonstrated by the similarity of the trends in Figures 2 and 4. The age-related decline in SWA0 further indicates that, at age 9 compared to age 18, the recuperative processes reflected by the 1-4 Hz EEG are more concentrated in the first part of the night. Despite the fact that Tauδ (the time constant or shape of the decline) does not change, the SWA0 decrease indicates a reduction in the rate of across-NREMP decline of normalized delta because the delta decline is initiated at a lower level and falls to a similar or higher asymptote.
In a recent study on the effects of age on delta EEG homeostasis, Jenni et al.18 reported that process S, homeostatic sleep pressure, builds up more quickly across the day in younger children (mean age 11.9 years) than in adolescents (mean age 14.2 years). Furthermore, children showed a smaller delta EEG response to sleep deprivation than did adolescents. The authors concluded that, compared to adolescents, younger children are closer to their maximum delta capacity after a normal day. Integrating our current findings with their data suggests the following model of the effects of adolescent brain maturation on slow wave sleep homeostasis. Homeostatic sleep need accumulates rapidly in pre-adolescents who then begin the night's sleep at a high initial rate of recuperation. As children progress through adolescence, homeostatic need accumulates more slowly, and they begin their recuperation at a lower rate, as indicated by the decline in the proportion of delta in NREMP1 and in the decline in SWA0.
Sleep deprivation is the classic stimulus for increasing homeostatic need. Interestingly, sleep deprivation produces a similar effect as does youth, i.e., an increase in SWA0 without a change in Tau.31 We are not proposing that preadolescent children are sleep deprived. Unlike sleep deprived adults, these children remain alert throughout the day. The children's high level of waking brain activity, reflected in their higher rate of cerebral metabolism,32,33 would cause homeostatic sleep need to accumulate to high levels by the time the children go to sleep.
We have long interpreted the high levels of delta EEG and the high rates of brain metabolism in pre-adolescents as the result of high levels of synaptic density.29 High synaptic density would directly elevate delta power by increasing the size of the neuronal populations whose membrane potentials oscillate in synchrony. In addition, changes in synaptic density affect brain activity during waking and, thereby, indirectly contribute to the sleep homeostasis patterns reflected in the decline in delta power across the night. Declining synaptic density would decrease the neuronal flux of waking brain activity and thereby decrease the need for sleep-dependent recuperation by providing less “substrate” for the recuperative process. This reduced substrate would lower the initial rate of recuperation, decreasing SWA0. It is consistent with this model that synaptic density, waking cerebral metabolic rate and delta wave amplitude decline in parallel across adolescence.34 Using Tononi and Cirelli's35 synaptic homeostasis model of sleep to interpret the current data, younger adolescents with higher synaptic density accumulate synaptic weight more rapidly. This higher synaptic weight at the end of a normal day would position the younger subjects higher on the recuperation curve (as does sleep deprivation) and produce a greater proportion of delta energy in NREMP1 and a higher SWA0.
As we have noted previously, our longitudinal data show a significant reduction in sleep duration across adolescence.21 This reduction raises the possibility that the SWA0 decline is related to reduced sleep duration rather than age. However, sleep deprivation increases SWA0.31 Declining sleep duration across adolescence would likely act to increase rather than decrease SWA0. A post hoc analysis that added a sleep duration factor to our analysis of age effects on the process S parameters confirmed that the decline in SWA0 was not related to the change in sleep duration. The change in SWA0 with age remained significant (P < 0.0001) when changes in sleep duration were statistically controlled.
Theta, like delta, responds homeostatically to sleep deprivation.10 The preceding discussion of age-related changes in the homeostatic regulation of delta applies equally to theta. Compared to delta, the decline in the proportion of theta energy in NREMP1 was slightly larger, and the yearly decline in TWA0 exceeded the yearly decline in SWA0. We previously reported that the maturational reduction in theta power begins earlier than the reduction in delta power.8 All-night delta power begins to decrease after age 11 years, whereas theta power begins to decrease well before age 10 years. We hypothesize that the earlier and more reliable changes in theta regulation are due to earlier synaptic pruning in theta circuits and might parallel the earlier MRI measured thinning of allocortical (3-layer) structures.36
The decline of standardized delta across the night documented here was not detected in previous cross-sectional studies.6,18,37 As shown in Figure 4, the parameters of this model have substantial variability between subjects and from one semiannual recording to the next. This is in stark contrast to the adolescent decline in all-night delta and theta power (Figure 1), where the data from the two cohorts are highly similar in the three years of overlap. Only the increased power of a larger N and a longitudinal design using 12 repeated measurements allowed us to detect the age-related decline in SWA0. We discuss below the inherent variability of the across night decline in delta EEG. This variability impairs our ability to describe the time course of the adolescent decline in SWA0. Although the linear model of age change produced the best fit, one would need a larger subject pool using a consistent EEG derivation to firmly determine whether the decline proceeds linearly or is more complex. Despite the variability of the process S parameters, the declines of SWA0 and TWA0 across adolescence were highly significant (P < 0.0001). We are not as certain with regard to age changes in asymptotes and the time constants of the declines across the night. These process S parameters were either significant for theta and not for delta or approached but did not reach a significance level of 0.01. Although this study with 713 nights from 67 subjects included much more data than any previous analysis of adolescent age changes in slow wave EEG regulation, a still larger sample might be needed to determine whether the process S asymptote and time constant change across adolescence.
Although mixed-effect analysis accommodates repeated measurements from individual subjects, the resultant curve represents the average trend across NREMPs. Modeling average data obscures important properties of the across NREMP decline that are apparent in the individual subjects' data.24,38 In our adolescent data, delta power rarely declines in a smooth exponential manner across consecutive NREM periods in individual subject nights. Indeed, Preud'homme et al.39 recently demonstrated that a declining exponential is not an appropriate function for a single night of human sleep. In our recordings from children and adolescents, delta power is almost always highest in the first NREM period, but the delta power in the second NREM period does, on rare occasions, exceed that in the first. A more frequent deviation from exponential decline is greater delta power in the third than in the second NREM period. The recuperative processes of sleep may be interrupted by internal and external factors that disrupt sleep and lower the delta power within a NREM period causing deviations from the exponential decline. Such interruptions might produce a delta debt that is compensated for later in the night. Thus, Dijk and Beersma40 demonstrated that disruption of slow wave sleep without waking the subject produces an increase in slow wave activity in subsequent sleep. In group data, the timing of any external and internal disruptions would average out to produce a smooth exponential decline. However, one of us (IF) has raised the possibility that the original hypothesis of an exponential decline based on a metabolic model is incorrect.38 The pattern of delta decline across NREMPs in individuals may ultimately prove more consistent with the irregularity of a pulsatile endocrine model.38
In summary, our longitudinal study used 713 nights of data from 67 subjects covering the age range 9 to 18 years. The maturational decline in the proportion of delta energy in the first NREMP and the maturational decline in SWA0 firmly establish that homeostatic sleep regulation changes across adolescence.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by United States Public Health Service grant R01 MH62521. Rahman Azari, PhD of the UC Davis Department of Statistics provided valuable statistical assistance. Adair McPherson, PhD provided proofreading and editing help with the multiple revisions of this manuscript. We thank the subjects who participated in the study and their parents for their cooperation. Work was performed at University of California, Davis.
Plots of the exponential decline equation SWAt = (300 − 30) * e-t/100 + 30 (solid lines) compared to plots (dashed lines) of the equation with 25% changes in the parameters Tau, SWA0, or SWA∞. A. Tau is increased from 100 min to 125 min. B. SWA0 is decreased from 300% to 225%. C. SWA∞ is increased from 30% to 37.5%.
Frequency response curves for the H2O (solid circles) and Aura (open circles) amplifiers. Mean (+/− s.e., n = 15 channels) output amplitude as a percent of input amplitude is plotted against frequency on a log scale. Filtering on the H2O and Aura is very similar across 1-8 Hz, corresponding to delta and theta EEG frequencies. The greatest disparity is the 2% difference at 1 Hz. Low frequency filters for both the Aura and H2O are single pole filters with a −3 dB point at 0.5 Hz and a 6 dB/octave slope. High frequency filtering on the Aura and H2O eliminates possible aliasing in the delta and theta frequency range. The Aura has a three pole high frequency filter with a −3 dB point at 100 Hz and an 18 dB/octave slope. Based on the frequency response curve, the −3 dB point for the H2O high frequency filter is at approximately 55 Hz.
SUPPLEMENTAL STATISTICAL INFORMATION
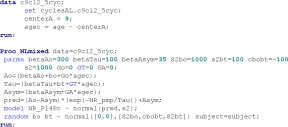
We used SAS proc NLMixed to evaluate age changes in process S parameters. In the following SAS program the dependent variable is standardized NREM delta power (NR_P14Pc). Independent variables are NREM period midpoint (NR_pmp) and subject age in years centered by subtracting 9. For details on the procedure see SAS v.9.1 example 51.1.
 |
REFERENCES
- 1.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–87. [PubMed] [Google Scholar]
- 2.Coble PA, Reynolds CF, III, Kupfer DJ, Houck P. Electroencephalographic sleep of healthy children. Part II: findings using automated delta and REM sleep measurement methods. Sleep. 1987;10:551–62. [PubMed] [Google Scholar]
- 3.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg I, Carlson VR. Sleep variables as a function of age in man. Arch Gen Psychiatry. 1968;18:239–50. [Google Scholar]
- 5.Feinberg I, March JD, Flach K, Maloney T, Chern W-J, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (delta) electroencephalogram of human sleep. Brain Dysfunc. 1990;3:183–92. [Google Scholar]
- 6.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. J Sleep Res. 2001;10:165–72. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 7.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 8.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger RJ, Oswald I. Effects of sleep deprivation on behaviour, subsequent sleep, and dreaming. J Ment Sci. 1962;108:457–65. doi: 10.1192/bjp.108.455.457. [DOI] [PubMed] [Google Scholar]
- 10.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep-deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–93. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg I, March JD, Floyd TC, Jimison R, Bossom-Demitrack L, Katz PH. Homeostatic changes during post-nap sleep maintain baseline levels of delta EEG. Electroencephalogr Clin Neurophysiol. 1985;61:134–7. doi: 10.1016/0013-4694(85)91051-x. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg I, Maloney T, March JD. Precise conservation of NREM Period 1 (NREMP1) delta across naps and nocturnal sleep: implications for REM latency and NREM/REM alternation. Sleep. 1992;15:400–3. doi: 10.1093/sleep/15.5.400. [DOI] [PubMed] [Google Scholar]
- 13.Werth E, Dijk D-J, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–R10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- 14.Campbell IG, Feinberg I. Homeostatic sleep response to naps is similar in normal elderly and young adults. Neurobiol Aging. 2005;26:135–44. doi: 10.1016/j.neurobiolaging.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 16.Daan S, Beersma DGM, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R78. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 17.Achermann P, Dijk D-J, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 18.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 19.Campbell IG, Darchia N, Khaw WY, Higgins LM, Feinberg I. Sleep EEG evidence of sex differences in adolescent brain maturation. Sleep. 2005;28:637–43. doi: 10.1093/sleep/28.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–R9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell IG, Higgins LM, Trinidad JM, Richardson P, Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep. 2007;30:1677–87. doi: 10.1093/sleep/30.12.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Washington, DC: Public Health Services, U.S. Government Printing Office; 1968. [Google Scholar]
- 23.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg I, March JD. Cyclic delta peaks during sleep: result of a pulsatile endocrine process? Arch Gen Psychiatry. 1988;45:1141–2. doi: 10.1001/archpsyc.1988.01800360089015. [DOI] [PubMed] [Google Scholar]
- 25.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical recipes: the art of scientific computing, 3rd ed. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 26.Twisk JWR. Applied longitudinal data analysis for epidemiology. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 27.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;23:323–55. [Google Scholar]
- 28.Cajochen C, Dijk DJ, Borbely AA. Dynamics of EEG slow-wave activity and core body temperature in human sleep after exposure to bright light. Sleep. 1992;15:337–43. [PubMed] [Google Scholar]
- 29.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982/1983;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 30.Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: findings using standard measurement methods. Sleep. 1984;7:289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- 31.Dijk D-J, Brunner DP, Borbély AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–R61. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 32.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy C, Sokoloff L. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children; normal values for cerebral blood flow and cerebral metabolic rate during childhood. J Clin Invest. 1957;36:1130–7. doi: 10.1172/JCI103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg I, Thode HC, Jr., Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 35.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darchia N, Campbell IG, Tan X, Feinberg I. Kinetics of NREM delta EEG power density across NREM periods depend on age and on delta-band designation. Sleep. 2007;30:71–9. doi: 10.1093/sleep/30.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feinberg I, March JD. Observations on delta homeostasis, the one-stimulus model of NREM-REM alternation and the neurobiologic implications of experimental dream studies. Behav Brain Res. 1995;69:97–108. doi: 10.1016/0166-4328(95)00010-q. [DOI] [PubMed] [Google Scholar]
- 39.Preud'homme XA, Lanquart JP, Krystal AD, Bogaerts P, Linkowski P. Modeling slow-wave activity dynamics: does an exponentially dampened periodic function really fit a single night of normal human sleep? Clin Neurophysiol. 2008;119:2753–61. doi: 10.1016/j.clinph.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Dijk DJ, Beersma DGM. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72:312–20. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plots of the exponential decline equation SWAt = (300 − 30) * e-t/100 + 30 (solid lines) compared to plots (dashed lines) of the equation with 25% changes in the parameters Tau, SWA0, or SWA∞. A. Tau is increased from 100 min to 125 min. B. SWA0 is decreased from 300% to 225%. C. SWA∞ is increased from 30% to 37.5%.
Frequency response curves for the H2O (solid circles) and Aura (open circles) amplifiers. Mean (+/− s.e., n = 15 channels) output amplitude as a percent of input amplitude is plotted against frequency on a log scale. Filtering on the H2O and Aura is very similar across 1-8 Hz, corresponding to delta and theta EEG frequencies. The greatest disparity is the 2% difference at 1 Hz. Low frequency filters for both the Aura and H2O are single pole filters with a −3 dB point at 0.5 Hz and a 6 dB/octave slope. High frequency filtering on the Aura and H2O eliminates possible aliasing in the delta and theta frequency range. The Aura has a three pole high frequency filter with a −3 dB point at 100 Hz and an 18 dB/octave slope. Based on the frequency response curve, the −3 dB point for the H2O high frequency filter is at approximately 55 Hz.