Abstract
Platelet-endothelial cell adhesion molecule-1 (PECAM-1) has role in atherosclerotic plaque development as well as in thrombosis leading to myocardial infarction (MI). Present study was aimed to analyse the association of PECAM-1 Leu125Val gene polymorphism with MI in Indian population. Subjects included healthy individuals as control (N = 116) and MI patients (N = 100) divided into two groups; MI patients at presentation of the acute event (MI-Group-1, N = 46) and patients with recent event of MI stabilized with treatment 4.5 days from their symptoms (MI-Group-2, N = 54). The difference in the distribution of Leu125Val genotype frequencies of controls and patients did not reach statistical significance. However Leu allele frequency (0.57) was more associated with MI patients as compared to control (0.504). sPECAM-1 levels were significantly elevated in patients at acute event of MI (MI-Group-1) by 44.1% (P = 0.009) as compared to controls and by 95.2% (P = 0.001) as compared to stabilized MI patients (MI-Group-2).
Keywords: Myocardial infarction, Gene polymorphism, Platelet endothelial cell adhesion molecule-1
Introduction
Platelet endothelial cell adhesion (PECAM-1) is of the immunoglobulin (Ig)-superfamily with wide variety of functions in platelet activation, inflammation, cell survival and the immune response and involved in transendothelial migration of monocytes (TEM) [1–4]. Thus it has a role in atherosclerotic plaque formation. It has also been demonstrated to have a role in thrombus formation subsequent to plaque rupture leading to myocardial infarction (MI) [1, 5]. PECAM-1 is a 130-kDa trans-membrane glycoprotein. It has 6 Ig-like extracellular domains (encoded by exon 3–8) a short trans-membrane domain (encoded by exon 9) and a short cytoplasmic tail (encoded by exon 10–16) [6, 7]. The importance of the first domain of PECAM-1 (encoded by exon 3) is underscored since PECAM-1 forms homophilic binding via its 1st or 1st plus 2nd extracellular Ig-like domains or heterophilic binding with other molecules to mediate cell–cell adhesion [3, 8]. A Mutation in PECAM-1 gene in Exon-3 has been identified that involves a base pair substitution (C→G) at position +373 causing Leucine (Leu) to Valine (Val) substitution at amino acid position 125 (rs668) [9]. Since the interaction or activation of PECAM-1 is mainly via homophilic binding with its 1st extracellular Ig-like domains [3, 8], this polymorphism might affect the homophilic binding capability and influence individual susceptibility to coronary artery disease (CAD).
Association between Leu125Val PECAM-1 polymorphism and CAD have been analysed in Caucasians [10–13], Japanese [14], Chinese [15, 16] and Asian Indian residents of Singapore [17]. Although studies discussed so far seem to suggest conflicting data, those investigating the association of PECAM-1 polymorphism and risk of atherogenesis always show increased risk with 125Val [10, 15–17] and association of PECAM-1 polymorphism and risk of MI show increased risk with 125Leu [14]. This is thought to be due to different effects of polymorphisms in PECAM-1. One variant (125Val) is thought to alter PECAM-1-mediated transendothelial migration of monocytes leading to increased risk of atherosclerosis while the other variant (125Leu) is thought to modulate platelet activation leading to increased risk of MI [18].
Overall incidence of CAD as well as MI is alarmingly high in our population [19]. To the best of our knowledge there are no reports on association of Leu125Val polymorphism with MI in Indian population. In the present study we evaluate if PECAM-1 gene Leu125Val polymorphism is associated with and increases the risk of MI in Indian population.
Materials and Methods
Study Population
Study population consisted of patients with MI (N = 100) and healthy individuals as controls (N = 116). Subjects with MI in patient group were recruited from Sir H. N. Hospital and Research Centre and Rajawadi Municipal Hospital from June 2005 till March 2008. Individuals recruited under control groups were volunteers visiting Sir H. N. Medical Research Society. Within both control and patient groups, subjects were of Indian origin such as Maharashtrians, Gujarathi, and Marwari from western zone of India in decreasing order in number.
MI patients were sub-grouped under two categories as per the time of their blood collection. Patients under Group-1 (MI-Group-1, N = 46) were with prolonged chest pain more than 30 min and ST elevation more than 0.1 mV on at least two adjacent leads and admitted in the emergency department of cardiac unit with clinical diagnosis of acute myocardial infarction (AMI). These patients were with first episode of chest pain and had neither past history of such clinical symptoms nor were on any known medications for the same. Their blood sample was collected at presentation before administering of any thrombolytic therapy for routine analysis such as electrolytes, cardiac enzymes, complete blood count and prothrombin time. Remaining serum was stored at −80°C and EDTA blood sample at 4°C for further analysis. Patients under Group-2 (MI-Group-2, N = 54) were with recent event of MI on treatment and their blood sample was collected on the day of the coronary angiography before coronary intervention which was on an average 4.5 days from the stabilization of their symptoms. On angiography patients demonstrated 50% or more stenosis in at least one major coronary artery. Exclusion criteria were: diabetes valvular heart disease, known cardiomyopathy, malignancy, renal or liver diseases, current use of anti-inflammatory (except Aspirin or statin) or immunosuppressive drugs.
The controls were healthy individuals with systolic blood pressure/diastolic blood pressure (SBP/DBP) = 135/85 mmHg or less, with no risk factors of CAD or clinical symptoms of any other organic disease. Their blood sample was collected after over night 12 h fast. Those subjects with fasting glucose levels >110 mg/dl, serum transaminases, Blood Urea Nitrogen (BUN), and Creatinine levels beyond normal range or abnormal ECGs were excluded from the control group. As per the selection criteria in each group, subjects were recruited in study only after obtaining their informed consent. Information regarding their demographic status, clinical history, family history and medications were noted down in detail. The ethical committee of Sir H. N. Hospital and Research Centre and Rajawadi Municipal Hospital approved the study protocol.
DNA Extraction
Genomic DNA extraction was carried out from peripheral blood leukocytes using the salting out method of Miller et al. [20].
Genotyping of PECAM-1 +373 C/G (Leu125Val) Gene Polymorphism
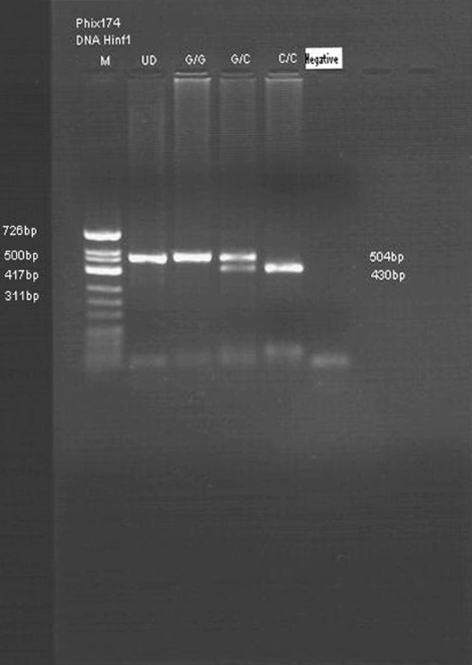
It was carried out by polymerase chain reaction (PCR)-based restriction digestion method as described by Fang et al. [17] with modifications. A 504 base pair (bp) portion of the PECAM-1 gene covering the single nucleotide polymorphism C+373G (Leu125Val) at exon-3 was amplified using Forward primer (5′-CTATCAGCCTGGCCCTGTAG-3′) and Reverse primer (5′-TATTCACGCCACTGTGTGCT-3′). The reaction tube contained Taq buffer (1X), dNTP (200 μM), 1.5 mmol MgCl2, 25 pmol of each primer, 1.0 unit of Taq polymerase and 100 ng of genomic DNA in 50 μl of reaction mixture. PCR was carried out in thermal cycler “T Gradient” from “Biometra” (Genetix Biotech Asia Pvt. Ltd.). The cycling conditions were initial denaturation at 95°C for 5 min which was followed by 30 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 45 s and extension at 72°C for 2.0 min followed by final extension at 72°C for 10 min. The PCR products were then digested with restriction enzyme Pvull at 37°C for 16 h. The digested products were electrophoresed in a 2.0% agarose gel along with a molecular marker, PhiX174 DNA/HinfI Marker. The gels were stained with Ethidium-bromide and visualized by U.V. light. Restriction enzymes PvuII recognized wild type sequence. After digestion with PvuII the wild-type (C/C or Leu125Leu) genotype generated a shorter (restricted) fragment of 430 bp. Homozygous mutants (G/G or Val125Val) were defined by presence of unrestricted longer fragments (504 bp). The heterozygotes (C/G or Leu125Val) were identified by presence of two bands, each corresponding to the 504 and 430 bp fragments (Fig. 1).
Fig. 1.
Digested products of amplified PECAM-1 depicting Leu125Val polymorphism. Lane M—molecular weight marker PhiX-174 DNA Hinf I, Lane UD—undigested product, Lane GG—band at 504 bp-Val125Val genotype, Lane CG band at 504 and 430 bp (Leu125Val) genotype and Lane CC band at 430 bp (Leu125Leu), Lane negative–negative control
Estimation of Soluble (s) Levels of PECAM-1
An aliquot of serum was stored at −80°C for estimation of soluble levels of PECAM-1, by commercially available enzyme-linked immunosorbent assay (ELISA) kit with monoclonal antibody against, according to the manufacturer’s instructions of Bender MedSystem. The minimum detectable level of sPECAM-1 was 0.06 ng/ml, while the intra assay coefficient of variation was 2.5% and inter assay coefficient of variation was 7.4%, respectively.
Statistical Analysis
Results are expressed as frequency and percentages, Mean ± SD for parametric variables and Median with inter quartile ranges for non-parametric variables such as sPECAM-1. For parametric variables analysis of significance of difference between two groups was performed by student’s unpaired t test. Non-parametric test such as Mann–Whitney U test was applied for comparing significance between two medians. Correlations of sPECAM-1 with other variables were evaluated by Spearman’s rank correlation test. The Chi square statistics with Yates correction was used to determine whether allele or genotype frequencies were significantly different between patients and the control groups. P value < 0.05 was considered statistically significant. Analyses were performed using statistical software SPSS (version 16.0, Chicago, IL).
Results
Table 1 represents the demographic data of controls as well as of all patients. The controls and MI patients were age matched. The patient group was characterized by decreased High Density Lipoprotein (HDL)-cholesterol. The percentage of subjects with smoking habits and alcohol consumption were more in patient groups as compared to controls.
Table 1.
Demographic data of control and patients
| Controls | MI-Group-1 | MI-Group-2 | |
|---|---|---|---|
| No | 116 | 46 | 54 |
| Male/female | 68/48 | 33/13 | 39/15 |
| Age (years) | 51.61 ± 6.1 | 50.6 ± 10.86NS | 51.5 ± 9.96NS |
| SBP (mmHg) | 117.4 ± 10.64 | 131.18 ± 27.13*** | 129.9 ± 16.8*** |
| DBP (mmHg) | 78.7 ± 6.53 | 81.75 ± 25.2NS | 84.8 ± 9.13*** |
| Total cholesterol (TC) (mM) | 5.22 ± 0.96 | 4.68 ± 1.12** | 4.7 ± 1.12** |
| HDL-cholesterol (HDLC) (mM) | 1.19 ± 0.24 | 1.03 ± 0.25** | 1.02 ± 0.18*** |
| TC/HDLC | 4.42 ± 0.80 | 4.48 ± .77NS | 4.59 ± .83NS |
| Triglyceride (mM) | 1.54 ± 0.94 | 1.56 ± 0.78NS | 1.65 ± 0.72NS |
| LDL-cholesterol (mM) | 3.35 ± 0.74 | 2.89 ± 0.81** | 2.85 ± 0.97** |
| Smoking | 13 (8.9%) | 15 (32.6%) | 18 (33.3%) |
| Alcohol consumption | 9 (7.7%) | 10 (21.7%) | 06 (11.1%) |
| Diabetes | 0 | 11 (23.9%) | 1 (1.85%) |
| Hypertension | 0 | 10 (21.7%) | 13 (24.1%) |
NS non significant, * P < 0.05, ** P < 0.01, *** P < 0.0001
PECAM-1 (Leu125Val) Genotype Distribution of Patient Groups as Compared to Controls
Genotype and allele frequencies were tested for Hardy–Weinberg equilibrium and the data met the assumptions of the Hardy–Weinberg Theory. The genotyping data of patients of MI-Group-1 and MI-Group-2 were combined for analyzing under MI group. The genotype frequencies of Leu125Leu, Leu125Val and Val125Val of MI group were 35.0, 44.0 and 21.0% and of controls were 23.3, 54.3 and 22.4% respectively. The difference in the distribution of these frequencies between the two groups did not reach statistical significance (χ2 = 3.77, degree of freedom [df] = 2, non-significant [NS]). However there was increased prevalence of 125Leu allele frequency (0.57) with MI group as compared to that of control (0.504) (Table 2).
Table 2.
PECAM-1 Leu125Val genotype and allele frequency distribution
| Genotype frequencies | Allele frequencies | ||||
|---|---|---|---|---|---|
| Controls (N = 116) | MI Group (MI-Group 1 and MI-Group-2) (N = 100) | Controls (N = 116) | MI Group (MI-Group 1 and MI-Group-2) (N = 100) | ||
| Leu125Leu | 27 (23.3%) | 35 (35.0%) | 125Leu | 0.504 | 0.57 |
| Leu125Val | 63 (54.3%) | 44 (44.0%) | 125Val | 0.496 | 0.43 |
| Val125Val | 26 (22.4%) | 21 (21.0%) | |||
| χ2 = 3.77, df = 2 NS | χ2 = 1.86, df = 1 NS | ||||
NS non significant, df degree of freedom
Within control group the Leu125Val genotype distribution between gender, presence and absence of smoking habits and alcohol consumption as well as in combined patient groups in addition, between presence and absence of risk factors such as hypertension and diabetes mellitus did not reach statistical significance (Data not shown). The genotype distribution of combined patient groups as compared to respective controls of male, female, smoking habits and alcohol consumption did not demonstrate significance difference either (Data not shown).
Analysis of sPECAM-1
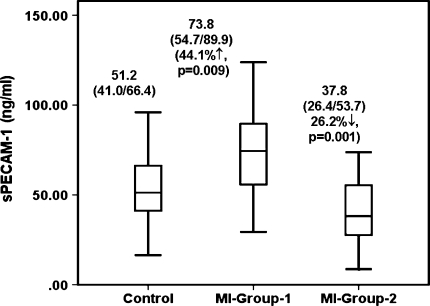
Figure 2 depicts the sPECAM-1 levels of each group. The median of sPECAM-1 levels of MI-Group-1 (73.8 [54.7/89.9] ng/ml) was significantly (P = 0.009) elevated by 44.1% as compared to controls while median of sPECAM-1 of MI-Group-2 (37.8 [26.4/53.7] ng/ml) was significantly (P = 0.001) reduced by 26.2% as compared to controls. Thus as compared to treated MI patients (MI-Group-2) levels of sPECAM-1 of MI patients at acute event (MI-Group-1) were significantly (P = 0.001) elevated by 95.2%. As compared to respective male, female, smoking habits and alcohol consumption the trend of sPECAM-1 levels of MI patients at acute event and of treated MI patients as compared to controls remained same as seen overall. Similar analysis between treated MI patients and MI patients at acute event demonstrated same trend as seen overall (Data not shown).
Fig. 2.
Soluble levels of PECAM-1
sPECAM-1 level was analysed within control group between gender, presence and absence of smoking habits and alcohol consumption. Within both patient groups in addition sPECAM-1 levels were also analysed between presence and absence of hypertension and in MI Group-1 between presence and absence of diabetes mellitus. The significant difference was found only within MI group at acute event (MI-Group-1) wherein females demonstrated significantly (P = 0.006) higher sPECAM-1 levels (89.6 [81/127] ng/ml) as compared to males (64.0 [35.0/73.4] ng/ml) by 40%. With respect to other risk factors in any group there was no significant difference in the sPECAM-1 levels (Data not shown).
Correlation analysis of sPECAM-1 within controls, MI patients at acute event (MI-Group-1) and treated patients (MI-Group-2), with parameters of demographic data, lipid profile did not demonstrate statistical significance (Data not shown).
Analysis of sPECAM-1 Levels as per Leu125Val Genotype
There was no significant difference in the sPECAM-1 level as per genotype within controls and treated MI group. However within MI-Group-1 at acute event patients with Val125Val genotype were associated with elevated levels of sPECAM-1 as compared to patients with Leu125Leu genotype by 44.1% (P = 0.05, NS) and as compared to patients with Leu125Val genotype by 37.3% (P = 0.028) (Table 3).
Table 3.
Soluble levels of PECAM-1 (ng/ml) according to genotype
| Leu125Leu | Leu125Val | Val125Val | |
|---|---|---|---|
| Control (N = 116) | 50.67 (39.6/62.2) (N = 27) |
52.4 (40.8/67.6) (N = 63) |
57.6 (40.6/76) (N = 26) |
| MI-Group-1 (N = 46) | 66.4 (42.3/111.67) (N = 12) |
69.7 (46.2/80.9) (N = 25) |
95.7 (87.4/170.6) (N = 9) •44.1% ↑, NS ••37.3% ↑ (P < 0.05) |
| MI-Group-2 (N = 54) | 38.8 (33.8/52.3) (N = 23) |
35.0 (24.7/49.3) (N = 19) |
48.5 (38.6/63.6) (N = 12) |
• With respect to Leu125Leu genotype, •• with respect to Leu125Val genotype
NS non significant
Discussion
PECAM-1 mediates transendothelial migration of circulating leukocytes during atherosclerotic plaque development and is also involved in platelet activation and thus thrombus formation. A polymorphism in PECAM-1 gene C+373G (Leu125Val) in Exon-3 encoding first extracellular Ig-like domain that mediates the homophilic binding of PECAM-1 has been documented. In the present study association of this polymorphism with MI in Indian population was analysed. There was no significant association observed of Leu125Val polymorphism with MI when compared against controls. However 125Leu allele frequency (0.57) was more associated with MI group as compared to controls (0.504). Soluble levels of PECAM-1 were significantly elevated in MI patients at acute event (MI-Group-1) as compared to treated MI patients (MI-Group-2).
Association of Leu125Val polymorphism in CAD have been analysed in various other population as well. Wenzel et al. [10] have reported allele frequencies of the Leu125/Val125 in German population as 0.49/0.51 in controls and 0.35/0.65 in CAD patients reporting increased Val125 allele in CAD patients (P < 0.01). Among Asian population strong association with CAD of Leu125Val polymorphism has been noted down by Song et al. [15] and Wei et al. [16] in Chinese population. Against these reports Gardemann et al. [11] in German population and Elrayees et al. [12] in British population did not find Leu125Val gene polymorphism of PECAM-1 as an independent risk factor of CAD. A study by Listi et al. [13] in population from Sicily (Italy), reported no significant association of this polymorphism with MI while Sasaoka et al. [14] in Japanese population have reported increased 125Leu allele frequency (0.52 vs. 0.447, P = 0.048) in MI patients as compared to controls.
There is an interesting study by Fang and co-workers [17] wherein association of Leu125Val polymorphism of PECAM-1 gene with CAD in Asian Indians of common origin i.e. of south Indian settled in Singapore for over 3 generations has been reported. They found that Val125Val polymorphism of PECAM-1 gene was significantly (P = 0.009) associated with CAD with frequency of Val125Val genotype being 16% as compared to 10% in the controls. One important point to be noted down is that in their study none of the patient had MI. Thus in their study there was strong association of 125Val with atherogenesis with prevalence of 125Val allele frequency being 0.464 in CAD against 0.336 in controls (P = 0.008). 125Val variant is suggested to alter PECAM-1-mediated transendothelial migration of monocytes leading to increased risk of atherosclerosis [18].
In the present study the patients population comprised of subjects who suffered from MI. We did not observe significant association of PECAM-1 Leu125Val gene polymorphism with MI. Reason for not finding an association in our study could be ethnic heterogeneity of our population (patients and controls) from other populations demonstrating association. However 125Leu variant suggested to modulate platelet activation leading to increased risk of MI [18] was more associated with MI patients (0.57) as compared to controls (0.504). Similar finding is also reported by Sasaoka et al. [14] in MI.
In our study there was significant increase in sPECAM-1 levels in MI patients at acute event (MI-Group-1) as compared to treated patients (MI-Group-2). The increase in the sPECAM-1 levels at acute event of MI is in consistent with its role in atherosclerosis and thrombosis. Similar finding has been reported by Serebruany et al. [21] and Soeki et al. [22]. The decreased levels of sPECAM-1 observed in treated MI patients in the present study may be accounted by the intense medical treatment received by these patients and that their blood sample was collected on an average 4.5 days from the stabilization of their symptoms. Thus in the present study sPECAM-1 was associated with MI group but only at acute event. In the same group (MI-Group-1) sPECAM-1 levels were elevated in females as compared to male suggesting presence of risk factor otherwise in a low risk group. It could be further screened for its role as a candidate biomarker for thrombosis.
The influence of PECAM-1 genotype on soluble levels of PECAM-1 has been reported in the literature. Wei et al. [16] have reported elevated levels of sPECAM-1 associated with subjects with Val125Val genotype in Chinese population of Singapore while Fang et al. [17] have reported no effect of the genotype on the soluble levels in Asian Indians (South Indians) of Singapore. While analyzing the influence of PECAM-1 genotype on soluble levels of PECAM-1 within each group in the present study, there were increased levels associated with patients of Val125Val genotype of MI-Group-1 as compared to other two genotypes. This suggests minor contribution of Val125Val polymorph towards increased expression at acute event, which may have led to increased soluble levels in these patients.
Limitation of Study
There was lack of equal number of MI patients under each subgroup to that of controls. However combined MI patients of MI-Group-1 (N = 46) and MI-Group-2 (N = 54) were N = 100 nearer to that of controls (N = 116) allowing for analysis of genotyping data. As peripheral levels of PECAM-1 may get affected of the patient’s clinical status, soluble levels of sPECAM-1 were analysed as per MI-Group-1 (at acute event) and MI-Group-2 (stabilized with treatment 4.5 days from their symptoms) against controls and comparison was also carried out between these two groups. There was trend of increased sPECAM-1 levels associated with Val125Val genotype however significant increase levels associated with Val125Val genotype of MI-Group-1 patients at acute event as compared to other two genotypes of the same group is required to be confirmed in larger population study.
Conclusion
Leu125Val gene polymorphism in the present study did not demonstrate significant association with MI however there was trend of increased 125Leu allele frequency with MI. sPECAM-1 levels were elevated in the blood of the MI patients at acute event as compared to the stabilized MI patients with treatment suggesting its role in thrombosis.
References
- 1.Maruya E, Saji H, Seki S, Fujii Y, Kato K, Kai S, et al. Evidence that CD31, CD49b, and CD62L are immunodominant minor histocompatibility antigens in HLA identical sibling bone marrow transplants. Blood. 1998;92:2169–2176. [PubMed] [Google Scholar]
- 2.Newman PJ, Berndt MC, Gorski J, White GC, II, Lyman S, Paddock C, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 3.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, et al. Involvement of platelet endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 5.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan HC, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes Commun. 1995;3:45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- 7.Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- 8.Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- 9.Behar E, Chao NJ, Hiraki DD, Krishnaswamy S, Brown BW, Zehnder JL, et al. Polymorphism of adhesion molecule CD31 and its role in acute graft-versus-host disease. N Engl J Med. 1996;334:286–291. doi: 10.1056/NEJM199602013340502. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel K, Baumann G, Felix SB. The homozygous combination of Leu125Val and Ser563Asn polymorphisms in the PECAM1 (CD31) gene is associated with early severe coronary heart disease. Hum Mutat. 1999;14:545. doi: 10.1002/(SICI)1098-1004(199912)14:6<545::AID-HUMU20>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Gardemann A, Knapp A, Katz N, Tillmanns H, Haberbosch W. No evidence for the CD31 C/G gene polymorphism as an independent risk factor of coronary heart disease. Thromb Haemost. 2000;83:629. [PubMed] [Google Scholar]
- 12.Elrayess MA, Webb KE, Flavell DM, Syvänne M, Taskinen MR, Frick MH, et al. A novel functional polymorphism in the PECAM-1 gene (53G>A) is associated with progression of atherosclerosis in the LOCAT and REGRESS studies. Atherosclerosis. 2003;168:131–138. doi: 10.1016/S0021-9150(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 13.Listì F, Candore G, Lio D, Cavallone L, Colonna-Romano G, Caruso M, et al. Association between platelet endothelial cellular adhesion molecule 1 (PECAM-1/CD31) polymorphisms and acute myocardial infarction: a study in patients from Sicily. Eur J Immunogenet. 2004;31:175–178. doi: 10.1111/j.1365-2370.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 14.Sasaoka T, Kimura A, Hohta SA, Fukuda N, Kurosawa T, Izumi T. Polymorphisms in the platelet-endothelial cell adhesion molecule-1 (PECAM-1) gene, Asn563Ser and Gly670Arg, associated with myocardial infarction in the Japanese. Ann NY Acad Sci. 2001;947: 259–69; discussion 269–70. [DOI] [PubMed]
- 15.Song FC, Chen AH, Tang XM, Zhang WX, Qian XX, Li JQ, et al. Association of platelet endothelial cell adhesion molecule-1 gene polymorphism with coronary heart disease. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:156–158. [PubMed] [Google Scholar]
- 16.Wei H, Fang L, Chowdhury SH, Gong N, Xiong Z, Song J, et al. Platelet-endothelial cell adhesion molecule-1 gene polymorphism and its soluble level are associated with severe coronary artery stenosis in Chinese Singaporean. Clin Biochem. 2004;37:1091–1097. doi: 10.1016/j.clinbiochem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Fang L, Wei H, Chowdhury SH, Gong N, Song J, Heng CK, et al. Association of Leu125Val polymorphism of platelet endothelial cell adhesion molecule-1 (PECAM-1) gene and soluble level of PECAM-1 with coronary artery disease in Asian Indians. Indian J Med Res. 2005;121:92–99. [PubMed] [Google Scholar]
- 18.Elrayess MA, Talmud PJ. Platelet endothelial cell adhesion molecule (PECAM-1) and coronary heart disease. Indian J Med Res. 2005;121:77–79. [PubMed] [Google Scholar]
- 19.Enas EA, Senthilkumar A. Coronary artery disease in Asian Indians: an update and review. Internet J Cardiol. 2002;1(2). http://www.ispub.com/journal/the_internet_journal_of_cardiology/volume_1_number_2_10/article/coronary_artery_disease_in_asian_indians_an_update_and_review.html.
- 20.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serebruany VL, Murugesan SR, Pothula A, Semaan H, Gurbel PA. Soluble PECAM-1, but not P-selectin, nor osteonectin identify acute myocardial infarction in patients presenting with chest pain. Cardiology. 1999;91:50–55. doi: 10.1159/000006876. [DOI] [PubMed] [Google Scholar]
- 22.Soeki T, Tamura Y, Shinohara H, Sakabe K, Onose Y, Fukuda N. Increased soluble platelet/endothelial cell adhesion molecule-1 in the early stages of acute coronary syndromes. Int J Cardiol. 2003;90:261–268. doi: 10.1016/S0167-5273(02)00564-8. [DOI] [PubMed] [Google Scholar]