Abstract
Andean Indians have used coca leaves (Erythroxylon coca and related species) for centuries to enhance physical performance. The benefits and disadvantages of using coca leaf have been a subject of many political debates. The aim of this study was to investigate the effects of chewing coca leaves on biochemical and physiological parameters. Cutaneous microdialysis catheters were used to estimate systemic biochemical changes. We subjected 10 healthy adult males (local residents) in Cajamarca (Peru, altitude 2700 m) to a standardised exercise routine on a stationary cycle ergometer. The blood pressure, oxygen saturation (digital), pulse, VO2 max and ECG (Holter monitor) were recorded before the exercise. Cutaneous microdialysis catheters were introduced in the forearm. The subjects were given to chew 8 g of coca leaves with a small amount of lime. They were then placed on the cycle ergometer for 20 min. Blood pressure, oxygen saturation, pulse, ECG and VO2 max were recorded. Pyruvate, glucose, lactate, glycerol and glutamate levels were estimated. Oxygen saturation, blood pressure, and pulse rate did not show any significant changes between the two groups. Glucose levels showed hyperglycaemic response. Glycerol, Lactate and Pyruvate increased. Glutamate remained unchanged. Similar changes were not seen in the controls. These results suggest that coca leaves have blocked the glycolytic pathway of glucose oxidation resulting in accumulation of glucose and pyruvate. The energy requirement for exercise is being met with beta-oxidation of fatty acids. The glycerol released was also getting accumulated since its pathway for oxidation was blocked. These experimental findings suggest that chewing coca leaves is beneficial during exercise and that the effects are felt over a prolonged period of sustained physical activity.
Keywords: Coca leaves, Biochemical effects, Cutaneous microdialysis
Introduction
Andean Indians have used coca leaves for centuries to enhance physical performance. The modern methods of obtaining cocaine were not known to the Andean culture. The benefits and disadvantages of using coca leaf had been a subject of many political debates. Spielvogel et al. [1] and Favier et al. [2] have reported the physiological benefits of coca leaves. The latter concluded that the beneficial effects of coca chewing on exercise tolerance were not related to either improved maximal exercise capacity or increased work efficiency. The beneficial effect on fatty acids was alluded to in their report. Hanna 1971 [3], concluded that heart rates and oxygen intake were not significantly different between those who chew coca and those who do not. Carter and Mann [4] have demonstrated, through a study of over 3,000 leaf users, that mine workers, the largest consumers, chew an average of 13 oz a week, i.e. extracts an average of 3.9 net grams of alkaloids per week. Therefore, the theoretical maximum dose is half a gram, in a period of 24 h (always assuming 100% efficiency in mouth extraction).
A recent investigation conducted by Instituto Boliviano de Biologica de Altura (Gregorio Lanza) [5] disclosed that after chewing about 30 g of leaves, blood cocaine contents can be traced to around 98 ng using High Pressure Liquid Chromatography. That is 0.000000098 g! Paracelsus [6] commented, “Every element in nature has its own poison and its antidote as well. There is a need to revert to the natural sources for remedy.” We would like to make a distinction between chewing coca leaves by the Indians of Andes and using cocaine as a recreational drug by the western cultures.
The aim of this study was to investigate the effects of chewing coca leaves on the biochemical and physiological parameters. Based on our earlier experience with the biochemical changes at high altitude [HA] [7], we decided to study the biochemical parameters as markers of adaptation to HA. Cutaneous microdialysis is now known to reflect systemic biochemical changes [8, 9]. We chose to use this technique as it is relatively non-invasive and it is possible to monitor the changes continuously over a long period of time.
Materials and Methods
The Ethics Committee, Andean Institute of Andean Biology, Lima, Peru, approved the study protocol.
We subjected 10 healthy adult males (local residents) in Cajamarca (Peru, altitude 2700 m) to a standardised exercise routine on a stationary cycle ergometer. The blood pressure, oxygen saturation (digital), pulse, VO2 max and ECG (Holter monitor) were recorded before the exercise. Blood samples were drawn to estimate the hormone levels (testosterone and progesterone). Only four of the subjects who chewed coca leaves agreed to give two blood samples. All the controls were happy to provide the blood samples.
Cutaneous microdialysis catheters (Mfg. CMA Microdialysis AB, Sweden) were introduced in the forearm and attached to the pump. At the end of 20 min the perfusate sample was collected. The subject was given to chew 8 g of coca leaves. They were then placed on the exercise machine for 20 min. The rate of revolution was maintained between 80 and 100 cycles/min. At the end of 20 min, the perfusate of the microdialysis was collected. Blood pressure, oxygen saturation, pulse, ECG and VO2 max were recorded. The subjects were allowed to rest for 20 min. All the above parameters were again recorded and the perfusate was collected. The subjects were again reintroduced to the exercise program. This cycle was repeated eight times. The subjects were chewing the coca leaves during the entire period of the experiment.
Four adult male subjects who were also residents of Cajamarca were used as controls. The same exercise routine was followed, but these did not use coca leaves. The VO2 max in sub-maximal exercise was measured in cycle ergometer by the Fox test. The values were calculated using the Fox equation for men (VO2 max = 6.3 – 0.0193 × FC). The values were corrected by the factor for the age.
Pyruvate, glucose, lactate, glycerol and glutamate levels were estimated in the microdialysis samples using the standard analyser provided by the company. The values of three subjects who had used coca leaves were excluded, as the volume of the perfusate was insufficient due to improper placement of the catheter.
Statistical Analysis
The changes in serum analyte measurements during the experiments were computed from start to finish of each test period for each subject. These were calculated as a net change and also as a percentage change as follows:
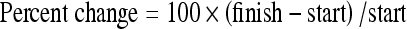 |
Positive values indicate an increase in value. Differences in baselines between test and control groups were tested using the Mann–Whitney U-test. Mean differences in change between exposed and control groups were tested using Mann–Whitney U-test at a significance level of 0.05. All calculations were carried out using statistical software (SPSS for Windows).
Results
Oxygen saturation, blood pressure, and pulse rate did not show any significant changes between the two groups. The ECG recording (Holter monitor) did not show any changes during the entire period. The VO2 max (ml kg−1 min−1 was 53.02 ± 3.06 in the coca chewers (Table 1). Among the non-chewers, it was 66.59 ± 6.33 (Table 2). The differences were not statistically significant. The aerobic capacity (VO2 max) was classified as either excellent or average according to the classification of American Heart Association 1972 [11].
Table 1.
VO2 max in coca-leaf chewing group
| No. | Age | VO2 max (l/min) | VO2 max (ml × kg−1 × min−1) | Condition |
|---|---|---|---|---|
| 1 | 50 | 2.98 | 47.3 | Excellent |
| 2 | 30 | 3.5 | 64.81 | Excellent |
| 3 | 56 | 2.53 | 32.03 | Median |
| 4 | 33 | 3.16 | 57.45 | Excellent |
| 5 | 38 | 3.75 | 50.68 | Excellent |
| 6 | 34 | 3.45 | 52.27 | Excellent |
| 7 | 45 | 2.96 | 65.77 | Excellent |
| 8 | 38 | 3.18 | 55.79 | Excellent |
| 9 | 31 | 3.79 | 47.97 | Good |
| 10 | 53 | 2.58 | 56.09 | Excellent |
| X | 40.8 | 3.188 | 53.016 | |
| SD | 9.5312352 | 0.4400707 | 9.6788902 | |
| SE | 3.01621367 | 0.13926288 | 3.06293994 |
Table 2.
VO2 max in control group
| No. | Age | VO2 max (l/min) | VO2 max (ml × kg−1 × min−1) | Condition |
|---|---|---|---|---|
| 1 | 17 | 4.18 | 83.6 | Excellent |
| 2 | 22 | 4.14 | 65.71 | Excellent |
| 3 | 23 | 3.39 | 52.97 | Excellent |
| 4 | 25 | 4.23 | 64.09 | Excellent |
| X | 21.75 | 3.985 | 66.5925 | |
| SD | 3.40342964 | 0.39837169 | 12.6737113 | |
| SE | 1.70171482 | 0.19918584 | 6.33685565 |
The changes in the biochemical parameters are shown in Tables 3 and 4. The glucose levels showed a hyperglycaemic response to coca chewing even after the exercise was completed.
Table 3.
Absolute changes in analytes
| No. | Baseline (SD) | Mean change: Period 1 (SD) | Mean change: Period 2 (SD) | |
|---|---|---|---|---|
| Glucose | ||||
| Cases | 8 | 2.7 (1.2) | 0.75 (1.66) | −0.38 (0.88) |
| Controls | 4 | 2.0 (0.3) | 0.70 (0.59) | −0.65 (0.73) |
| Glutamate | ||||
| Cases | 8 | 18.9 (12.6) | 7.1 (5.1) | 3.3 (4.6) |
| Controls | 3 | 25.0 (11.8) | 12.0 (10.5) | 17.0 (9.9)* |
| Glycerol | ||||
| Cases | 6 | 55.8 (30.0) | 42.2 (70.6) | 51.0 (63.0) |
| Controls | 4 | 52.3 (26.8) | 25.3 (20.9) | −22.7 (21.2) |
| Lactate | ||||
| Cases | 8 | 0.77 (0.50) | 1.21 (1.37) | 0.47 (0.74) |
| Controls | 3 | 0.80 (0.50) | 0.57 (0.90) | −0.73 (1.01)* |
| Pyruvate | ||||
| Cases | 8 | 29.3 (14.6) | 68.1 (85.6) | 59.3 (106.5) |
| Controls | 3 | 47.7 (29.1) | 16.0 (24.3) | −9.0 (16.1) |
* P < 0.05 (Mann–Whitney U-test)
Table 4.
Percentage changes in analytes
| No. | Mean % change: Period 1 (SD) | Mean % change: Period 2 (SD) | |
|---|---|---|---|
| Glucose | |||
| Cases | 8 | 38.1 (73.1) | −7.5 (15.1) |
| Controls | 4 | 30.6 (27.0) | −13.4 (22.1) |
| Glutamate | |||
| Cases | 8 | 78.1 (107.4) | 153.4 (332.3) |
| Controls | 3 | 30.1 (11.9) | 296.1 (288.3) |
| Glycerol | |||
| Cases | 6 | 59.6 (97.0) | 70.8 (79.8) |
| Controls | 4 | 43.4 (29.6) | −32.9 (22.7)* |
| Lactate | |||
| Cases | 8 | 92.2 (127.2) | 11.6 (18.4) |
| Controls | 3 | 50.9 (62.0) | −21.1 (23.3)* |
| Pyruvate | |||
| Cases | 8 | 96.4 (124.6) | 56.4 (107.4) |
| Controls | 3 | 54.3 (83.8) | −7.5 (11.6) |
* P < 0.05 (Mann–Whitney U-test)
The glycerol values showed accumulation at the end of the second episode of exercise. This was seen in two of the control subjects as well. The pyruvate was seen accumulating in almost all the coca-chewing volunteers. Two controlled subjects also showed pyruvate accumulation, but to a lesser degree. The lactate was getting progressively accumulated in volunteers chewing coca. There was also an accumulation of lactate in the controls, but the magnitude was not as high as in those who were chewing coca leaves. The glutamate values were more or less unaffected among the coca chewers. It showed a significant increase in the controls.
Discussion
The purpose of this pilot study was to determine if there were any subtle biochemical changes, which were influenced by chewing the coca leaves. Standard methods of assessing a person’s response to continued exercise such as blood pressure, pulse rate, VO2 max and ECG changes did not show any significant changes between the two groups.
The experimental findings suggested that chewing coca leaves induces biochemical changes that enhance physical performance at high altitude. These changes appeared sustained and were detectable at the later stages of the experiment.
During normal short-term exercise carbohydrates are the primary source of energy. In this situation the respiratory quotient (RQ) remains near 1. The glucose, glycerol and pyruvate did not accumulate as they were utilized as soon as they were formed. However, lactate accumulated as its utilization by the liver in re-conversion to glucose and glycogen lagged behind. This was probably taking place during the resting period.
Under endurance of long-term exercise the carbohydrate reserves being small the energy source is switched over to fatty acids and fats. The RQ under this condition is below 1. The fats are hydrolysed into fatty acids and glycerol. The glycerol enters the glycolytic pathway and metabolised to glycerol pyruvate pathway. The fatty acids undergo beta-oxidation entering Krebs cycle as acetyl-coenzyme A.
It seems that coca leaves have blocked the glycolytic pathway of glucose oxidation at the Pyruvate Dehydrogenase level resulting in accumulation of glucose and pyruvate. The energy requirement for exercise is being met with beta-oxidation of fatty acids. The glycerol released was also getting accumulated since its pathway for oxidation was blocked.
These experimental findings suggest that chewing coca leaves gives a beneficial effect during a performance of exercise and that the beneficial effects are felt over a prolonged period of sustained physical activity. Perhaps this gives the users energy to function at a sustained level over long periods of time.
Lipid fuel sources are energy substrates during prolonged exercise of moderate intensity. Plasma and muscle triglycerides and free fatty acids make a significant contribution to lipid metabolism. Grant [11]. The individual contribution from each of these towards skeletal muscle mechanism is discussed in detail by Turcotte [12].
Aerobic Glycolysis is a major producer of usable energy during high endurance physical activity. The aerobic Lipolysis provides energy through Krebs cycle during sustained low levels of activity. The switch over from Glycolysis to Lipolysis is an adaptation to prolonged low levels of activity and efficient use of energy sources. It is possible that coca leaves have adaptogens, which are capable of influencing the switch depending on the level of activity [13, 14].
The duration of the experiment was for a period of two and half hours. Perhaps if this experiment had been continued for a much longer period the affects could have been more clearly visible.
It is also possible that the beneficial effects of chewing coca leaves are related to the flavonoids found in the coca leaves and not because of release of the cocaine. The amount of cocaine that is released in the process of these customary chewing coca leaves is extremely small and unlikely to be off any physiological benefit. Further research into the effects of flavonoids is being looked into. It is also planned to perform the field experiments on a much larger population over a longer period of time to evaluate this problem further.
References
- 1.Spielvogel H, Caceres E, Koubi H, Sempore B, Sauvain M, Favier R. Effects of coca chewing on metabolic and hormonal changes during graded incremental exercises to maximum. J Appl Physiol. 1996;80:643–649. doi: 10.1152/jappl.1996.80.2.643. [DOI] [PubMed] [Google Scholar]
- 2.Favier R, Caceres E, Koubi H, Sempore B, Sauvain M, Spielvogel H. Effects of coca chewing on hormonal and metabolic responses during prolonged sub maximal exercises. J Appl Physiol. 1996;80:650–655. doi: 10.1152/jappl.1996.80.2.650. [DOI] [PubMed] [Google Scholar]
- 3.Hanna JM. The effects of coca chewing on exercise in the Quechua of Peru. Hum Biol. 1971;42:1–11. [PubMed] [Google Scholar]
- 4.Carter, Mann. Chapter II, Coca leaves: scientific aspects. In: Gumuciol JH, editor, Cocaine the legend. La Paz, Bolivia: Hisbol; 1995.
- 5.Lanza G. Coca prohibited, Chapter I. LaPaz, Bolivia: Hisbol; 1995.
- 6.Paracelsus. Theosophy 1938 March;26(5):197–204.
- 7.Nagabhushana S, Venkatesh T, Casikar V. The olfactory system regulates acute mountain sickness. J Stress Physiol Biochem. 2009;5:4–15. [Google Scholar]
- 8.Anderson CD. Cutaneous microdialysis: is it worth the sweat? J Invest Dermatol. 2006;126(6):1207–1209. doi: 10.1038/sj.jid.5700221. [DOI] [PubMed] [Google Scholar]
- 9.Muller M. Microdialysis has wide applicability for in vivo measurements. BMJ. 2002;324:588–591. doi: 10.1136/bmj.324.7337.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pechar G, McArdle W, Katch F, Magel J, DeLuca J. Specificity of cardiorespiratory adaptation to bicycle and treadmill training. J Appl Physiol. 1974;36(6):753–756. doi: 10.1152/jappl.1974.36.6.753. [DOI] [PubMed] [Google Scholar]
- 11.Grant R. Energy systems used during exercise: online article. Articlesbase 2008; posted online Dec 3rd.
- 12.Turcotte LP. Role of fats in exercise: types and quality. Clin Sports Med. 1999;18(3):485–498. doi: 10.1016/S0278-5919(05)70163-0. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Grover SK, Shyam R, Divekar HM, Gupta AK, Srivastava KK. Enhanced thermo genesis in rats by a composite Indian Herbal preparation—I and its mechanism of action. J Altern Complement Med. 2007;5(3):245–251. doi: 10.1089/acm.1999.5.245. [DOI] [PubMed] [Google Scholar]
- 14.Graver SK, Divekar HM, Kumar R, Pashwa ML, Bharadwaj SK, Gupta AK, Srivastava KK. Experimental evaluation of a composite Indian herbal preparation—II (CIHIPII) as an adaptogen and its mechanism of action. Pharm Biol. 1995;33:148–154. doi: 10.3109/13880209509055216. [DOI] [Google Scholar]


