Abstract
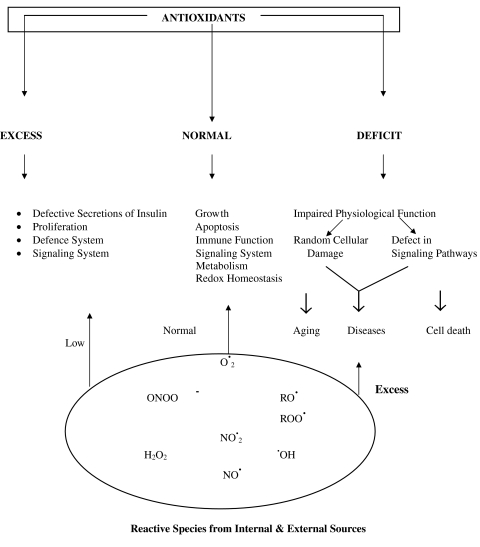
The antioxidants are essential molecules in human system but are not miracle molecules. They are neither performance enhancers nor can prevent or cure diseases when taken in excess. Their supplemental value is debateable. In fact, many high quality clinical trials on antioxidant supplement have shown no effect or adverse outcomes ranging from morbidity to all cause mortality. Several Chochrane Meta-analysis and Markov Model techniques, which are presently best available statistical models to derive conclusive answers for comparing large number of trials, support these claims. Nevertheless none of these statistical techniques are flawless. Hence, more efforts are needed to develop perfect statistical model to analyze the pooled data and further double blind, placebo controlled interventional clinical trials, which are gold standard, should be implicitly conducted to get explicit answers. Superoxide dismutase (SOD), glutathione peroxidase and catalase are termed as primary antioxidants as these scavenge superoxide anion and hydrogen peroxide. All these three enzymes are inducible enzymes, thereby inherently meaning that body increases or decreases their activity as per requirement. Hence there is no need to attempt to manipulate their activity nor have such efforts been clinically useful. SOD administration has been tried in some conditions especially in cancer and myocardial infarction but has largely failed, probably because SOD is a large molecule and can not cross cell membrane. The dietary antioxidants, including nutrient antioxidants are chain breaking antioxidants and in tandem with enzyme antioxidants temper the reactive oxygen species (ROS) and reactive nitrogen species (RNS) within physiological limits. Since body is able to regulate its own requirements of enzyme antioxidants, the diet must provide adequate quantity of non-enzymic antioxidants to meet the normal requirements and provide protection in exigent condition. So far, there is no evidence that human tissues ever experience the torrent of reactive species and that in chronic conditions with mildly enhanced generation of reactive species, the body can meet them squarely if antioxidants defense system in tissues is biochemically optimized. We are not yet certain about optimal levels of antioxidants in tissues. Two ways have been used to assess them: first by dietary intake and second by measuring plasma levels. Lately determination of plasma/serum level of antioxidants is considered better index for diagnostic and prognostic purposes. The recommended levels for vitamin A, E and C and beta carotene are 2.2–2.8 μmol/l; 27.5–30 μmol/l; 40–50 μmol/l and 0.4–0.5 μmol/l, respectively. The requirement and recommended blood levels of other dietary antioxidants are not established. The resolved issues are (1) essential to scavenge excess of radical species (2) participants in redox homeostasis (3) selective antioxidants activity against radical species (4) there is no universal antioxidant and 5) therapeutic value in case of deficiency. The overarching issues are (1) therapeutic value as adjuvant therapy in management of diseases (2) supplemental value in developing population (3) selective interactivity of antioxidant in different tissues and on different substrates (4) quantitative contribution in redox balance (5) mechanisms of adverse action on excess supplementation (6) advantages and disadvantages of prooxidant behavior of antioxidants (7) behavior in cohorts with polymorphic differences (8) interaction and intervention in radiotherapy, diabetes and diabetic complications and cardiovascular diseases (9) preventive behavior in neurological disorders (10) benefits of non-nutrient dietary antioxidants (11) markers to assess optimized antioxidants status (12) assessment of benefits of supplementation in alcoholics and heavy smokers. The unresolved and intriguing issues are (1) many compounds such as vitamin A and many others possessing both antioxidant and non-antioxidant properties contribute to both the activities in vivo or exclusively only to non-antioxidant activity and (2) since human tissues do not experience the surge of FR, whether there is any need to develop stronger synthetic antioxidants. Theoretically such antioxidants may do more harm than good.
Keywords: Antioxidants , ROS, RNS, Oxidative stress, Apoptosis, Redox homeostasis
Introduction
Antioxidants (AO) have gone through a gradual transition from “Miracle Molecules” to “Marvelous Molecules” to “Physiological Molecules”[1–20]. No doubt they are vital cogs in numerous metabolic reactions and are co players in redox homeostasis [21–25] but then are many others cellular molecules of equal importance. Further,many AO perform equally or even more important of non-antioxidant duties [20, 26, 27].The examples are role of vitamin A in vision [28], vitamin C in collagen synthesis [29–33] and vitamin E in smooth muscle cell proliferation endothelial dysfunction platelet aggregation by a protein kinase C dependent mechanism and tumor suppression [26, 34–36].
With distinctive demonstration of the involvement of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in human physiology [8, 10, 24, 37–45], the concept of “Toxic molecules” for free radicals FR is now outdated. In fact, there is a strong doubt if there is any acute phase FR generation is involved in etiopathogenesis of human diseases; that raised oxidative stress (OS) in majority of the disease is consequence and not the cause [6–8, 12, 18, 46–48] that raised OS is not present in all the patients [3, 5, 14, 49–51] and that a specific level of OS is necessary for vital activities [43, 52, 53]. Attempts to manipulate OS below this level may result in serious consequences. These observations restrict the role AO. Arguably excess quantity of antioxidant in tissues may lead to deleterious consequences. Many high quality recent studies are available in support [28, 54–58]. However, many issues are still unresolved or controversial. Subsequent pages highlight these issues and suggest the remedies to handle them.
Reactive Oxygen Species and Reactive Nitrogen Species Transition from Villain to Votary
In the recent times, no concept in Modern Medicine has perhaps evoked more tumultuous and turbulent storm as FR for their scorching behavior and AO for their exciting counter action to save human life from disease and disaster though both the concepts have undergone serious transition lately. FR were initially presumed to be purely toxic metabolites with attributes to initiate or promote any disease, the list growing to over hundred in a short time [6]. AO on the other hand, were considered God-sent molecules, which could resolutely neutralize their effects [59]. FR caused further scare when it was observed that they created many reactive toxic non-radical species in track in the body before being finally brought down to ground state energy level molecule [60]. These FR and their non-radical reactive species were derived from oxygen and nitrogen and were collectively designated as ROS and RNS. Since FR were accused as causal factors in a large number of diseases, these were sometimes referred as “Free Radical Diseases”. Some of the important diseases and health issues in this category are cancer [61–67], cardiovascular diseases [68–73], atherosclerosis [74–81], neurological disorders [82–86], renal disorders [87–90], liver disorders [91–93], hypertension [50, 94, 95], rheumatoid arthritis [96, 97], adult respiratory distress syndrome, auto-immune deficiency diseases [98, 99], inflammation, degenerative disorders associated with aging [100–103], diabetes mellitus[104–107], diabetic complications [108–111], cataract [112, 113] and obesity [114–119].
Concomitantly, as expected, AO received increasing approbation for their commendable performance to: (i) scavenge FR (ii) eliminate reactive non-radical species (iii) encage the ill effects of ROS and RNS (iv) provide protection against radical damage and (v) provide an advance protective umbrella against any possible attack by ROS and RNS. Thus various therapies and strategies have been suggested [120–127] (Table 1). Simultaneously countless reports are there in literature for supplement of various antioxidants. Little over a decade ago the cover of Time magazine read “The real power of vitamins; new researches show they may help fight cancer, heart disease and the ravages of aging”. The article inside referred these attributes to antioxidant actions of some vitamins [15, 16]. Though there could be no denial to the benefits of these nutrients AO, ironically the health and therapeutic power of AO was overly magnified by pharmaceutical industry for self-gain rather than compassion for human health. Resultantly their reputation plummeted and we see an exponential growth of literature on “FR Toxins” and “AO Elixirs” though largely unwieldy, in last thirty years or so. Then reputation of AO began to slump with adverse outcomes in clinical trials [2, 28, 52, 53, 56, 128–135]. The visible signs of a transition began and the concepts on FR and began to go through radical modification. Before we proceed to discuss about the transitions in these areas it would be prudent to ponder over the genesis of shock-waves created by FR and fascination for AO. We perceive several reasons. First, FR are known in chemistry for over a century for their volcanic reactivity and were used in several processes including the polymerization in plastic materials; second they are fragmented atoms or molecules which can fragment other atoms and molecules, third they can propagate chain reactions thereby damaging large number of other molecules; fourth they can torment the material to reshape and deshape due to their high energy content; and fifth many FR have capacity to destroy and evaporate the living or non-living matter due to their high energy, high reactivity and chain invoking capacity [136]. Then, setting the cat among pigeons came the sordid episode of the atomic fission in Hiroshima and Nagasaki where the explosion of atomic bomb led to death and disappearance of thousands of lives within minutes due to generation of FR. By the end of 1950, the explosive and tyrannical strength of FR in organic chemistry was well established. So, when the researches of Girshman [137] and Harman [138, 139] demonstrated that FR are produced in human tissues; and that they act as dangerous chemical species in downstream of aging processes, it sent creeks, shrieks and horror in scientific community in particular and world community in general. All got fraught with dangers of FR. In 1954 Rebacca Girshnam and her associates [137] proposed a synergy between radiation toxicity and oxygen toxicity in human system; and that FR played as interlocutor between these toxicities and decline of physiological processes in aging. Rocking the boat further, close on the heels of these discoveries, came the researches of Denham Harman [138, 139] which convinced him the similarity between radiation effects and FR on cellular system and proposed that irradiation accelerated aging of an organism; that the decline of physiological functions mimicked aging process; and that accumulation of damaged products was the major cause of toxicity, He forcefully argued that since all these ill effects were basically due to oxidizing potential of FR generated by irradiation, the AO could prevent many of these effects by quenching them though he was not clear about the type of AO nor their quantity and quality. Thus, the seed of “Pejorative Role of FR and Benedictive Role of AO” was sown, which grew too rapidly (Fig. 1) but clumily. Nonetheless, none of the above suppositions for FR and AO sustained long and a conceptual change began to occur with the following discoveries: (a) in many diseases raised oxidative stress (OS), due to enhanced generation of ROS and ROS, is a consequence and not the cause (b) only in some diseases, it is a cause but not a single player. (c) the simple theme that in some diseases, the mechanistic processes impinge on a torrent of oxidants, which can be countered by a single or cocktail of AO started gradually getting discredited. (d) AO are not the sensors of oxidation process in any place or in all the places or the universal effectors of all genes. (e) imagination of an universal antioxidant is neither acceptable nor conceivable. (f) ROS and RNS decisively serve several useful purposes in low to moderate concentration in metabolic reactions and for modulation of many physiological processes; and that AO must not be overloaded in the body to suppress the ROS and RNS level below physiologically desired level. (g) several AO are essential for life but excess may lead to increased morbidity and mortality. (h) AO are cell specific and radical species specific (i) many chemical species may be imbued with AO property but in human body they may not exert the AO action. (j) the antioxidant action may not be proportional in vivo to that observed in vitro;. and (k) many AO also perform several non-antioxidant activities highly beneficial to cell [5, 6, 8, 11–18, 39, 43, 140]. In summary, by the turn of the century, the undue fear for OS injury in human health started waning and the appreciation for their contribution in cellular physiology began growing [10, 37–41, 44, 45, 140]. Obviously, an opposite view became progressively louder for AO [52, 53, 56, 133, 141–144].
Table 1.
Selective behavior of dietary antioxidants on DNA from healthy human lymphocytes
| Quercetin caffeic acid | Protective effect |
|---|---|
| Epigallocatechir | Damaging effects |
| Epigallocatechin gallate | |
| Catechin | No effect |
| Epicatechin | |
| Catechin gallate | |
| Epicatechin gallate | |
| Vitamin C | |
| Alpha iscopherol |
Fig. 1.
Effects of antioxidants on human health
Smoke After Fire
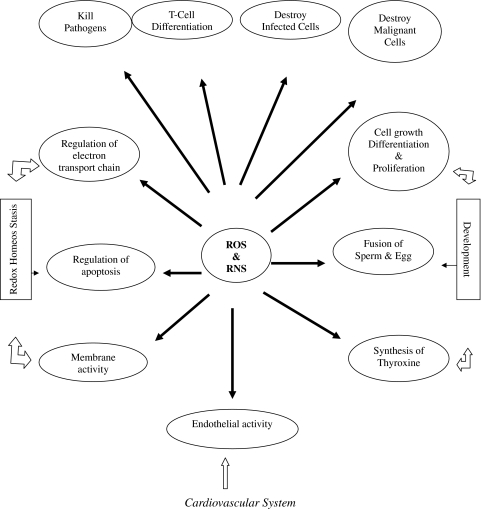
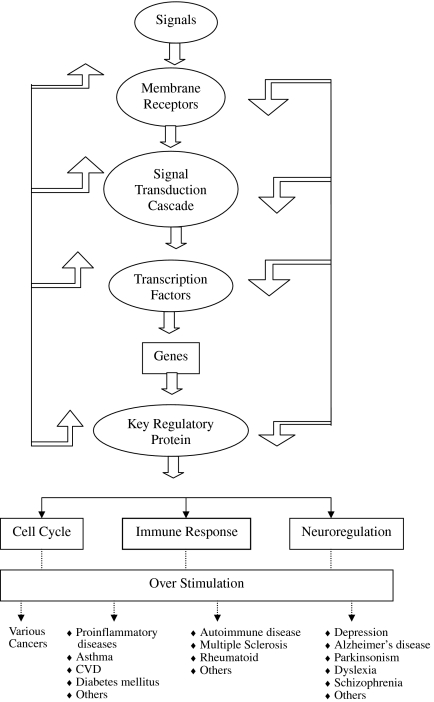
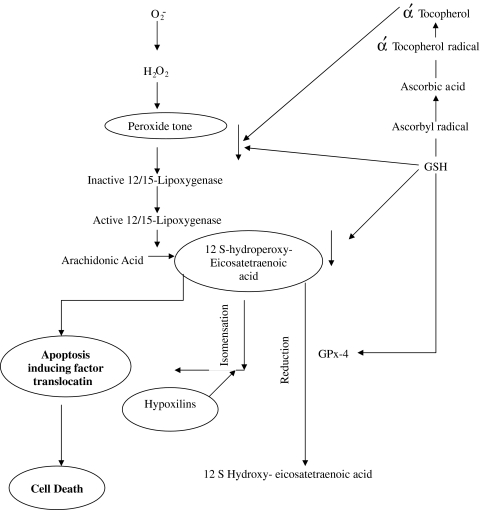
ROS and RNS easily react in vivo with many cellular species and show especial inclination for lipids, proteins, carbohydrates and nucleic acids, especially DNA. Depending on the conditions, they damage or destroy these molecules [7, 9, 143, 144]. They can easily deshape or mutate DNA which clearly gives inkling that they can cause carcinogenesis. Enhanced production of FR in vivo, which is theoretically possible in many situations, can occur and can cause disruption of cellular activities resulting in disease and death [62, 68, 76, 145]. The ex vivo and animal studies further substantiate that ROS and RNS mediated oxidations of dense nutrients and nucleic acids are crucial events of many unfavorable actions. However, putative evidence that AO even if adequately available can always prevent these undesirable actions is not yet available nor the reduction of OS is always accompanied with alleviation of the disease. Further, although in animal and cell culture studies it has been demonstrated that OS has causal relationship in several pathological conditions such as insulin resistance and chronic disease; and that AO can abolish or prevent these conditions [146] but clinical studies so far have been unsupportive [130–133]. Why AO, showing effective potential in the management of diseases or conditions in animals and cell culture, have failed to demonstrate the similar effects in clinical trials is still intriguing. The one plausible explanation is that experimental studies do not mimic clinical conditions or do not represent the exact conditions prevailing in humans. In recent years, there are numerous reports suggesting the failure of various AO to heal those pathologies where causal role of ROS and RNS has been implicated, and there are yet another series of researches reporting antioxidant therapy causing serious complications and side effects, which is in contradiction to expected effects [39, 41]. As such, the emerging recent view is that excess generation of FR may not be causing the disease but vice versa i.e. the disease process may be aggravating the radical generation resulting in the raised OS, thus a phenomenon akin to “Smoke After Fire”. Therefore, the active damaging role of FR in various diseases is now being repeatedly questioned. Some researches have gone to the extent to suggest that raised OS or other oxidant markers may just be the indicator of the end-stage tissue injury and nothing beyond. On the contrary, there is overwhelming evidence that ROS and RNS are involved in large number of physiological processes such as endothelial function, development and redox homeostasis (Fig. 2) intracellular signaling. Figure 3 gives an overview of redox regulation of signal transduction pathway. Apoptosis is subtle phenomenon of programmed cell death involving several mechanisms. Recently Loscalzo [21] has proposed one mechanism in which peroxide tone duly regulated by antioxidants and reactive oxygen species, induces cell death (Fig. 4). Very recently, the succinct study of Owusu-Ansah and Baneerjee [37] conclude “The developmentally regulated, moderately high ROS level in progenitor population sensitizes them to differentiation and establishes a signalling role for ROS, in the regulation of haematopoietic cell. Our results lead to a model that could be extended to reveal a probable signaling role for ROS, in the differentiation of common myeloid progenitors in mammalian haematopoetic development and oxidative stress response.”
Fig. 2.
Physiological role of ROS and RNS
Fig. 3.
Redox modulated pathways of signal transduction (Adams & Adams)
Fig. 4.
Mechanism of oxidant-antioxidant regulated apoptosis
All these finding taken collectively impress for a radical thinking and rationale approach to antioxidant-intake, therapy and supplement. With regard to FR the statement of de Maghalaes and Church seems to be quite convincing “In the same way fire is dangerous and nonetheless humans learned how to use it, it now appears that cells evolved mechanisms to control and use ROS.” Nevertheless, till date we cannot refute that FR could be co-players in many situations especially age related conditions or diseases such as aging, cardiovascular diseases, diabetes mellitus, carcinogenesis, neurological disorders and some other conditions. Their adverse effects even in obesity cannot be ignored. We have also to keep in mind the compartmentalized role of AO and that the deregulation of antioxidant defence system such as seen with decline of age usually, if not always, leads to slow and progressive rise in OS resulting in oxidative damage. In conclusion, gradually the concept of dangerously threatening role of OS in human system is getting tenuous, where as to, benevolent, and beneficial behavior is gaining momentum. In conclusion we are inclined to believe that the raised OS in many instances could possibly be due to increased metabolic activity in the disease process representing the phenomenon of “Smoke After Fire” rather than “Inflicting Effects of Fire”.
Antioxidant Juggernaut
The pandemonium created by the discovery of FR in human body for their evil temper got invigorating solace with the rediscovery of super oxide dismutase (SOD) by Fridovich [147, 148] for its antioxidant activity-an enzyme otherwise reported back in 1939. The comforting relief was that SOD eliminated the “primary free radical” superoxide anion O2−. (other free radicals are called “Secondary Free Radicals” as they arise from O2−.) to form hydrogen peroxide; and that catalase and glutathione peroxidase (GPx) whose presence was already well known, effectively detoxified hydrogen peroxide to molecular oxygen and water. In the intervening period several other molecules present in body and those supplied in diet were under screening for their radical scavenging effect. All these endogenous and exogenous compounds possessing AO property were collectively termed as “Antioxidants”. Among dietary AO vitamins C, E and β-carotene were highlighted for their sacrosanct antioxidant action. Vitamin A, though to some lesser extent, was also included in the team. In subsequent years, many dietary AO such as carotenoids, flavonoids and polyphenols were reported to be important members to strengthen the antioxidant cabinet. Thus AO quickly rose to a luminous pedestal. Some called them “brooks of life” others called them “superpower of the cell” and a few zealots called them “elixirs of life”. Suddenly the AO became “Molecules of Miracle”. Everybody felt that they should be abundantly consumed in the diet and that they should be liberally taken as supplements, if one could afford them, to envigorate the health. Ten years ago Pryor stated that in USA 50% physicians were taking AO pills as supplement, Hathock et al. [149] only 5 year later said that daily intake of vitamin E and C in as high dose as 1000 mg and 2000 mg, respectively was safe. While enzyme AO are busy in detoxifying superoxide anion or hydrogen peroxide the non-enzyme AO, acting as reductants, sacrifice themselves to save cellular species from prooxidizing elements and to terminate chain reaction. This underscored their role as “Paragon” but in due course of time, their role as “Elixirs of Life” was summarily rejected. In midst of massive literature piling up with superlative praises of AO, it is indeed commendable that Halliwell and his group kept on issuing warning against exaggerated praises, which now seems to be quite justified.
Presently, AO are defined as any substance that when present in low concentration compared to any oxidizable substrate (the term oxidizable substrate in biological system includes almost every thing except water) significantly delays or prevents oxidation of substrates. Broadly they are categorized in two groups (i) enzyme AO and (ii) non-enzymic AO. The latter are classified into two: endogenous and exogenous (dietary) AO. By definition enzyme AO are proteins that limit OS by catalyzing a redox reaction with a reactive oxidant. They are unambiguous in their activity. The non-enzymic AO are redox active compounds that limit OS by reacting non-enzymatically with a reactive oxidant. There is a lot of ambiguity in action of the non-enzymic AO. All the three antioxidant enzymes SOD, catalase and GPx, are inducible enzymes and therefore their activity in vivo in humans and animals variates as per needs. Their manipulation has been tried for AO benefits, but therapeutic manipulation has neither been successful nor advisable. The therapeutic use of SOD in the management of myocardial ischaemia and cancer came as a bang, but ended up in whimper because SOD is a large protein molecule which can not cross cell membrane. Hence, non-enzymatic AO have been focus of attention for therapeutic and supplemental purposes [150, 151]. Although large number of dietary AO have been examined and recommended from time to time but central focus has been on vitamin E [51] which intercalates in membrane and vitamin C which acts in cytosol. For quite some time beta-carotene too was center of attraction but went sideways soon. α-Lipoic acid and reduced glutathione (GSH) are excellent endogenous AO and their supplements have shown many benefits. Both these species have wide range of non-antioxidant functions also. At many places in cellular milieu, their antioxidant and non-antioxidant actions are well distinguishable. The flourishing literature on the benefits of these AO is available in plenty in the last quarter of twentieth century. However, the benefit of their therapeutic or supplemental value is still a debatable issue.
It would be prudent to define the ideal merits of an antioxidant. These are; (i) should have general property to quench all types of FR or specific quenching property. The latter will be more useful from clinical point of view. (ii) nullify or retard the action of redox active non-radical species. (iii) chelate redox metals especially iron and copper. (iv) easily absorbed and distributed in tissues. (v) should be tissue specific because in many diseases specific tissue is involved such as liver, heart, brain or skeletal muscle etc. (vi) effectively enters in the proper slot of the antioxidant network because electron flows from higher energy level to low energy level and not vice versa. (vii) preferably soluble in both water and lipid medium which will make it equally effective in cytosol as well as membrane domains. (ix) possesses wide pecking order and lastly. (x) as per requirement, it exerts positive or negative effect on gene expression. In addition to these, preferably, it should have capability to repair damaged molecule e.g. glutathione repairs DNA damage and that it should destroy the acutely damaged molecules and replacing them with new ones as done by several enzymes. Unfortunately, due to evolutionary and environmental constraints, none of endogenous or dietary AO known so far are amalgamated with all these qualities. Inherently, it is inconceivable that any single antioxidant can serve as an ideal AO in all cellular settings. It is also doubtful if a combination of few AO would be imbued with all these qualities. It is, therefore, increasingly felt that the best solution is to consume antioxidant rich diet for this purpose with liberal inclusion of fruits and vegetables (400–600 g/d) [152–157], Expectedly this should optimize AO defence network to take care of redox homeostasis. There is gathering evidence that excess supplement in form of antioxidant tablets may cause adverse effects due to lopsided disturbances in antioxidant network or deregulating other metabolic activities. Thus while in vitro, cell culture and animal studies have shown great potential of many AO in health and many diseases, the same promise has not been seen in many clinical trials. On the contrary, as pointed out earlier there have been several drubbing outcomes including increased morbidity and mortality. Ironically, the initial juggernaut image, as impeccable devotee of health and longevity, without considering their merit, started crumbling. Suddenly antioxidant supplements are reported wearing black hat.
Where Have We Faultered
Why reputation of AO as “House Keepers” of human body has dwindled? The disappointing outcomes in clinical trials have also tainted their image as “Security Force” against disease. It is, therefore, high time to deliberate to find out where we have committed mistakes understandably to preclude confusion in future studies. In this regard three points about AO need elaboration. (i) selection (ii) reducing potential and (iii) selection of dose, duration of administration and safety.
(i) Selection of Antioxidant
While selecting a single AO or antioxidant cocktail following points should be kept into consideration. Selection of AO should be judicious because none of the antioxidant is omnipotent and omniscient. Each antioxidant has specific qualities [5, 13, 18]. Numerous oxidative processes continue incessantly in human tissues, such as oxidation of vast variety of molecules in metabolism and electron leak in transport chain. They are well regulated and nurtured. ROS are also one of the regulatory processes of electron transport chain (to certain degree) and other process as stated earlier. Excess accumulation of AO in tissues either from exogenous source by supplement or from endogenous source by manipulation may lead to deleterious effects due to their undesirable intervention. An example is given in Table 2 [158, 159].
Table 2.
Planning of antioxidant therapies
| 1. Blocking free radical generation | Alleviating antioxidant strength |
| 2. Decompartmentalization of metal complexes | Restructuring of antioxidant defenses |
| 3. Chelation of excess of iron and copper | Using chelating agents |
| 4. Inhibition of | |
| Arachidonic acid and metabolism | (a) Use of enhancers of endogenous antioxidants |
| Phagocyte activation | (b) Therapeutic use of synthetic antioxidants |
| Xanthine oxidase | (c) Selective strengthening of membrane and cytosolic antioxidants |
| Nitric oxide synthase | (d) Supplementation of nutrient and other natural antioxidants |
| 5. Dietary modifications | (a) Caloric restriction |
| (b) Regulated PUFA intake |
Complete and comprehensive information about these aspects is still not available but the choice ofAO largely depend on: (i) which site ROS and RNS are getting produced in excess (ii) in which environment raised prooxidant activity is occurring (iii) which target species damage is occurring and lastly but most importantly (iv) to what degree the provoked degree of OS is to be controlled. Wrong choice of AO in such situation will lead to adverse consequences. As regard to target species damage, it is interesting to point out some examples such as when human plasma is examined for its ability to inhibit iron-ion-dependent lipid peroxidation, transferrin and ceruloplasmin exhibit most potent protective effect, when plasma is exposed to nitric oxide, uric acid is most effective protective agent. Interestingly uric acid has almost no effect against hypochlorous acid. Further, if source of ROS is kept same but different targets of oxidative damage are examined, the results may be different, for example, when human plasma is exposed to gas phase smoke ascorbic acid inhibits the lipid peroxidation but not the protein [160, 161]. Finally, the striking observation is that black currant and apple juice inhibited lipid peroxidation in human volunteers but promoted protein oxidation [162]. The literature is replete with such contrasting types of action. Moreover, an AO may exhibit elective behavior.
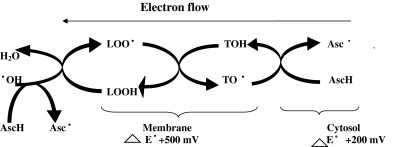
Still more, an AO may be soluble in lipid medium or in aqueous medium or in both. For example, vitamin-E intercalates in membrane due to lipid soluble activity, vitamin C in aqueous phase and α-lipoic acid in both. Though there is no tight compartmentalization in their activity, clinician has to be cautious to choose the AO as per clinical requirement to get optimal results.
The alpha-tocopherol may exhibit three patterns of its behaviour, first, it may exert beneficial effect if its deficiency exists or its requirement is more due to increased FR generation, second it may have no effect, if it stays as a bystander, third, it may exert harmful effect if its prooxidant form α-tocopheryl radical accumulates in excess due to excessive formation or slower removal especially by ascorbic acid [8, 13, 22]. Ascorbic acid may also similarly exert these three type of effects.
(ii) Reducing Potential
The role of enzyme and non-enzyme AO is largely different. The former directly eliminate primary free radicals whereas non-enzymic AO such as vitamin C, E glutathione, lycopene, polyphenols, carotenoids and many others can directly exchange electron (or hydrogen atoms) with FR. The non-enzymic antioxidant vary widely in their thermodynamic properties ranging from strong oxidizing to strong reducing character [163]. These thermodynamic characteristics can be used to predict the “Pecking Order” or “Hierarchy” for radical reactions. For examples, tocopheryl radical can be reduced by ascorbic acid but not vice versa. Similarly, GSH can reduce ascorbyl radical but not vice versa. Thus, these non-enzymic molecules primarily function as chain breaking agents and determine the course of movement of electrons from cell membrane to cytosol and in cytosol from one molecule to another with slow and graded dissipation of energy till the last molecule attains more or less ground level energy and becomes physiologically harmless molecules. In the biological system free hydroxyl radical (OH) is most oxidizing radical. Being at the top of hierarchy or pecking order (E°/mv at PH-7-2310) it can oxidize almost any cellular molecule [20, 163]. Recent evidences indicate that it may be produced in DNA matrix and may mutate it if not taken care off very quickly and efficiently. Buettner [163] has listed the hierarchy of various FR and AO molecules. Innately stronger the reducing power,greater will be their AO activity, Redox homeostasis is a subtle cellular phenomenon requiring a balance between oxidizing and reducing process and oscillates on either side as per requirement of the cell. Administration of an antioxidant with stronger reductant property than required one would cause a shift in electron sink thereby lowering ROS and RNS levels. Obviously this will induce undesirable effects.
(iii) Dose Duration and Safety
Most of the countries formulate their own recommendations for RDA (Recommended Daily Allowance) of nutrients for their populations. For Indian population, ICMR formulates these recommendations and updates them periodically. For nutrient antioxidant vitamins A, E, C and beta-carotene, the RDA is 600 μg/d, 0.8/g essential fatly acid, 40 mg/d and 2400 μg/d, respectively [19]. The RDA for E for most of the populations is between 20 and 30 mg/d. Higher intake of vitamin E (100 mg/d) has been recommended by Pryor [19], as this intake helps to maintain ideal blood level for both antioxidant and non-antioxidant activity. We also agree with them. In clinical studies its supplementation has varied between 50 mg and 2500 mg/d [22, 149]. Most commonly used dose in clinical trials has varied between 400 and 800 mg/d. Some of the long term supplemental studies have reported harmful effects. Pauling, the Nobel Laurette forcefully advocated heavy intake of vitamins C varying from 250 to 4000 mg/d [29]. However recent researches do not support his arguments, because daily intake of 100 mg vitamin C saturates body’s capacity to metabolize it. Any intake higher than this will be excreted [28, 164]. Clinically it has been given in the doses up to 10 gm/d. Creagon et al. [165] in a randomized controlled clinical trial gave 10 gm vitamins C per day to advance cancer patients for 50–210 days and did not notice any side effects. Mortal et al [166] gave the same dose to advance colorectal patients efforts. Several workers [167, 168] noted increased oxalate-excretion on varying dose of vitamin C in humans. Experimental studies also support this concentration [169]. Singh et al. [168.] noted that even massive dose of vitamin C given for 3 months to rats did not induce any side effects nor any change in the urinary tract though oxalate excretion was high [170].In conclusion, it appears that high intake of ascorbic acid is safe but not beneficial. The daily intake of beta-carotene between 2.5 and 3 mg seems to be justified. Higher intake is harmless except in smokers, where its adducts in higher concentration interfere with some signaling systems. Vitamins A play only a minor role as on antioxidant. The earlier concept that both beta-carotene and vitamins A are potent quenchers of singlet oxygen in humans is not now acceptable. Many carotenoids and phenolics especially polyphenols have strong antioxidant activity, but no solid proof is available for their explicit benefits, dose and safety. Both lipoic acid [171–173] and reduced glutathione (GSH) [174–181] are synthesized in human body and are absorbed from the gut also. Both of them are reported to be of supplemental value in some conditions but their dietary intake does not seem to have convincing value.
Among all the dietary AO, vitamins E and C have most comprehensively been examined. Both are potent and effective AO and that their potency is mutually supplemented, so the AO strength of cellular milieu enhances when both of them are present in adequate quantity (Fig. 5). The most exhaustive review in this regard is of Hathock et al. [150] for American population. The Food and Nutrition Board of Institute of Medicine in USA has established a system of dietary reference intake (DRI) values for US population. It has defined safety “presenting no unreasonable risk of illness or injury or a reasonable certainly of no harm”. The DRI system defines three criteria: first daily average requirement, second the RDA and third, the tolerable upper intake level. They recommended that vitamins E supplements are safe for most of the adults in the dose of about 1600 IU/d.(1073 mg RRR-α-tocopherol or molar equivalent of its esters)and ascorbic acid up to 2000 mg/d for adults. Others do not agree with them [85]. Looking to all the evidence, we feel that 100 mg each of vitamin C and E is an ideal dose for humans to attain maximum AO activity. Excess supplement should be given only in deficient conditions. Intake of beta-carotene up to 5 mg/d is absolutely safe and is usually available in this quantity from the diet, hence is additional intake is not warranted. Zinc, copper, manganese and selenium are essential components of different antioxidant enzymes. Selenium, besides being cofactor in other biomolecules is an essential cofactor of GPx. ICMR has recommended the daily intake of zinc—12 mg/d, copper—2.2 mg/d and manganese—5.5 mg/d., selenium is essential for many activities [182]. No recommendation has so far been made for selenium. Further it has been started that food provides sufficient selenium unless soil is deficient in it. Intake above 300 μg/d is usually toxic.
Fig. 5.
Ascorbic acid is extremely efficient as one electron reducing agent
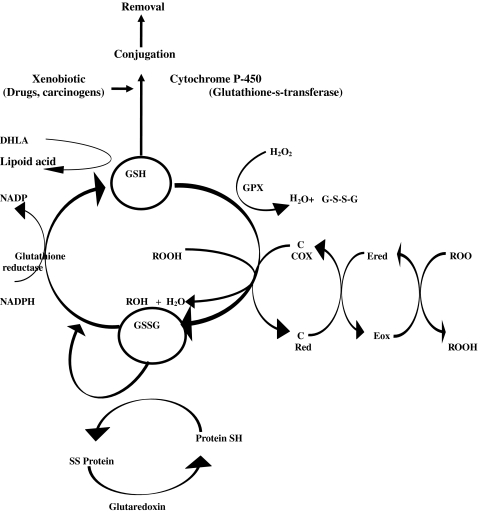
Glutathione (GSH) and lipoid acid are versatile endogenous AO (Fig. 6) [171–181]. In recent years, several reports have advocated their supplements in the management of some diseases. Dietary GSH is absorbed, both by sodium dependent and sodium independent mechanisms. Administration of GSH in the doses of 1-3 gm is reported to raise blood GSH level for a transient period. Dietary GSH has been suggested to provide protective coverage against some cancers but evidence is still inconclusive. In fact the supplemental value of GSH in raising AO strength in human tissues is still an undecided issue. α-lipoic acid is synthesized in human cells. It is a multi-purpose molecule with both AO and non-antioxidant activities involved in regulation of several reactions of lipid and carbohydrate metabolism. As an AO it is a radical scavenger and metal chelator and most importantly regenerates ascorbyl and alpha-tocopheryl radicals. It is easily absorbed and crosses blood brain barrier easily without any serious side effect and that performs many beneficial activities and so has been considered as a promising drug for improving nerve conduction velocity and in many other diseases. Since it is synthesized in human body in adequate quantity there is no dietary requirement. In clinical practice it has been used as a drug only.
Fig. 6.
Interaction among reduced glutathione, antioxidants, E, C and Proteins
Initially, the thrust was to assess dietary intake of AO, but gradually it has shifted to assessment of blood levels as they provide better idea for both diagnostic and prognostic purposes. The normal blood level of nutrient AO vitamins A, E and C and beta-carotene for different age groups are given in Tietz [183]. For adults the normal serum levels are: vitamin A—1.05–2.80 μg/l (30–80 μg/dl), vitamin E—12–42 μmol/l (0.5–1.8 mg/dl), vitamin C—23–85 μmol/l (0.4–1.5 mg/dl) and beta-carotene—0.19–1.58 μmol/l (10–85 μg/dl). These are different from those suggested earlier by WHO in 1966 [184]. Gay et al. [185, 186] all have recommended following values for obtaining the optimal AO activity-vitamin A—2.2–2.8 μmol/l, vitamin E—27.5–30 μmol/l, vitamin C—40–50 μmol/l and beta-carotene—0.4–0.5 μmol/l. Although these levels seem to be satisfactory but require further authentication, more so in relation to cancer and CVD [187].
Mechanism of Adverse Actions
Majority of adverse affects of AO are due to biphasic relationship between dose and activity. Some of the mechanisms known for unwanted actions are given below.
Low levels of ROS and RNS enhance the antioxidant strength of the cells. They do so by stimulating oxidative response genes. In this was they are able to adapt themselves to counter the OS by augmenting the endogenous AO. For example, a cell line initially treated by low doses of hydrogen peroxide increased 40 fold their resistance to cytotoxic dose of hydrogen peroxide due to increase in catalase and glutathione peroxidase activity. High doses of AO many suppress the FR thereby abolishing this effect [187].
Ever since beta-carotene started getting a comprehensive drubbing for its no effects or harmful effects, if consumed liberally, there have been a spate of reports to avoid its supplements both in health and in disease. Lipoxygenase is a FR generating enzyme. Beta-carotene in physiological doses inhibits the activity of this enzyme thereby regulating FR generation, whereas in higher concentration it stimulates the activity of this enzyme, thus enhancing the pro-oxidant environment [188].
Apoptosis is largely a genetically determined process, regulating programmed cell death and their disappearance [28]. It is rightly said that apoptosis leads to altruistic suicide of the cells and protects the human tissues against malignancy and immune dysfunction. Free radical nitric oxide and active non-radial hydrogen peroxide are strong regulators of apoptosis. Beta-carotene in high doses is suggested to prevent this process [28].
Many dietary AO are metabolized in body. The metabolized adducts may exert harmful effects. For example, the break down products of beta-carotene have been noted to interfere with retinoid signaling [189]. Further, the risk of toxicity was found to be greater among smokers who were also drinkers. It has been speculated that ethanol and beta-carotene may cooperate in induction of enzymes that activate smoke derived carcinogens. It has also been suggested that beta-carotene decreases bioavailability of other carotenoids. Interestingly beta-carotene at high pressure increases lipid peroxidation and protein carbonyl generation.
The toxicity of high dose of some vitamins is well known for a long time. Although human body has good tolerance of vitamin-C and E but prolonged intake of high doses of these vitamins has now been demonstrated to have many ill effects including increased mortality [52, 53, 56]. This is attributed to their accumulation of their toxic prooxidant forms in tissues. Lee et al. [190] have clearly demonstrated that ascorbic acid when in excess, induces lipid hydroperoxide decomposition to DNA reactive bifunctional electrophiles 4-oxo-2-nonenal, 4-oxo-2-noxenal and 4,5,epoxy-2 (E)-decenal. The latter is highly mutagenic lesion in human DNA. In all probability ascorbic acid induced formation of genotoxins from lipid hydroperoxides is the cause of failure of this vitamin to function cancer preventive agent.
The experimental studies have shown that overdose of vit-E stimulates the production of cytochrome P450-3-A4 and multidrug resistance protein-I. They can metabolize a large number of drugs. Hence, they will decrease the sensitivity of all those drugs which are metabolized by them [191–193].
Genes are indeed fortune tellers of molecular and physiologic activities. The genetic probes are acceptably gold standard to derive direct evidence regarding the involvement of gene in action, However, the genetic modification of animal species may induce “Clandestine Cinematic Artifacts” which may affect the experimental modification. Further, without any doubt the human genome response may differ from other animals at several points and switches. As such, due to differences in interspecies heterogeneity, the data obtained in animals may not apply in totality in humans and may be quite different. This is one of the reasons as to why the benefits to AO when tested in clinical trials have failed to achieve same response as seen in animal and cell culture studies.
The process of programmed cell death ‘apoptosis’ is redox regulated. OS and antioxidants duly participate in the mechanism. A specific level of OS is required to participate in multiple mechanism of initiation and completion. Feinendegen [194] has claimed that low ROS concentration during antioxidant supplementation may prevent apoptosis in favour of cell survival and proliferation also in neoplastic cells and thus rather promote cancer than protect against it.
Some workers have suggested that antioxidant block ROS-mediated protection of nervous system evoked by preconditioning [195].
However, precise mechanism for many other adverse actions are still to be elucidated. Needless to say to antioxidant jargon is too complex.
Inconsistencies: Antioxidant Supplements are Boon or Bane
The resolved issues for AO are: (a) essential for human life (b) organized in a network, which follows the rule of hierarchy, and (c) the network functions best when the strength of individual AO is optimized. The unresolved critical issue requiring focused attention is whether additional supplements of non-enzymic AO are useful in health and disease when AO status is normal and that the diet is sufficiently providing them. The opinions are still divided and inconclusive. In view of exponential growth of literature on AO and FR, it is not possible to review all of them but some salient examples are worth citing. Abounding literature exists on “Helpful Effects” of AO, especially based on in vitro, cell culture, animal studies and short term clinical studies with large confounding variables [1, 3, 15, 16, 18, 22]. Adding further confusion to these reports, are some punning statements or advertisements. For example, in July 1998 issue of “VERIS”, Blumberg stated in his lecture titled “Antioxidant Diffuse the Demographic Time Bomb” “One way of explaining this is that with vitamin E we can turn off “Off Switch” that turn on with age”. Praising the vitamin further, he said that if you did not know, you were looking at 70 years old; you would probably think it was 40 year old. Almost simultaneously the cover page of world famous Time magazine read “The real power of vitamins; new research shows they may help fight cancer, heart and ravages of aging” [13, 15, 16] A pamphlet on Lyco-Red claiming lycopene as wonder antioxidant captioned “From prostate cancer prevention to sun-protection, lycopene is the world’s most powerful antioxidant”. The advertising literature on SELACE antioxidant pill (beta-carotene + vitamin-E + vitamin-C + magnesium + zinc + copper + selenium) said that it helped in management and protection of atherosclerotic artery disease, diabetes mellitus, neoplastic diseases cancer, leukoplakea, smoking induced lung diseases, sub mucous fibrosis caused by tobacco, cataract, retinopathy, parkinsonism, senile dementia, Alzheimer’s disease, multiple scelerosis, age related changes such as wrinkling, immune deficiency rheumatoid arthritis, contact dermatitis and renal graft rejection. These claims were attractive enough to tempt clinicians to include AO in their prescription frequently.
We do not deny that all the claims stated above are completely hollow but are not epithet as well. Many statements in lectures and adds are usually based on in vitro, cell culture, desperate animal experiments and imperfect clinical studies which do not simulate human situations [85]. Therefore they failed in high quality clinical trials [22, 23, 54, 55]. The initial verve for AO therapy and supplement got a disheartening jostle with the claim that beta-carotene supplement did not provide any benefits, rather may prove harmful. Some of the sophisticated long term clinical studies showed that beta-carotene increased the risk of lung cancer in smokers [196]. In an animal study using smoke induced lung cancer it was observed that the toxicity of beta-carotene was exerted by its oxidative adducts which interfered with retinoid signaling resulting in precancerous (squamous metaplasia) lesions [197]. It almost startled the scientific community though the randomized, double blind, placebo controlled trial of beta-carotene by Hennekenset.al. [198] gave some relief. This study enrolled 22,071 male physicians in 40–84 years age in USA for beta-carotene supplement in the doses of 50 mg on alternate days for 12 years. It did not show benefit nor harm in terms of CVD, cancer or mortality from all causes. ATBC (The α-Tocopherol, beta-Carotene Cancer Prevention Study) enrolled 29,133 male smokers in Finland, They received beta-carotene (20 mg/d) and vitamin E (50 mg/d) for 6 years [196]. The CARET (The beta-Carotene and Retinol Trial) enrolled 18,000 men and women at high risk for lung cancer because of smoking habits or occupation exposure to asbestos (4 years trial) [199]. Both these studied observed no significant benefits of these supplements in terms of CVD and cancer. Paradoxically both studies noted a little higher rate of lung cancer and CVD among subjects receiving beta-carotene. Among all the dietary AO vitamin E consisting of a family of eight members is most studied molecule wherein alpha-tocopherol has received maximum attention as it is retained in human body and has an excellent antioxidant action in stabilizing membrane structure; and that it has strong potential against oxidative stress [7, 8, 10, 11, 19, 200–202]. Contrary to earlier prevailing concept that supplementation with alpha-tocopherol confers protection against a large spectrum of diseases such as CVD, cancer, diabetes mellitus, Parkinsonism, Alzheimer disease pre-eclampsia and aging, the recent observations from large prospective, randomized placebo controlled trials have largely been negative [22]. The supplementation has been shown to aggravate blood pressure and most disappointingly increased mortality. Although yet to be established in humans, the in vitro studies on human cell cultures and animal models suggests that vitamin E may increase the production of cytochrome p 450 s and MDRI in liver. Induction of cytochrome p450 3 A4 or Multidrug Resistance Protein -1 (MDRI) may lower the efficacy of any drug metabolized by them [191–193]. Very recently, Dotan et al. [54] used a very recent statistical method Markov model analysis to analyse the effects of vitamin E supplementation. Markov-model is superior to Chochrane meta-analysis in several ways. They used a microsimulation to select 200000 subjects (100,000 with supplemented and 100000 non-supplemented).Their study revealed that non-selective supplementation of vitamin E to the general public is harmful in terms of Quality-Adjusted Life Year (QALY). A literature search processing 156 articles that contained the results from 159 reports on 144 trials (of 159 reports one-third were of high quality), the results indicated : (a) a pooled analysis of smaller studies did not show evidence of any effect of vitamin E alone or in combination with other agents on all cause mortality, CVD mortality, fatal or non-fatal myocardial infarction or blood lipid levels, (b) a number of large clinical trials not included in the pooled analysis also supported these conclusions (c) ascorbic acid in combination with other AO showed no favourable effects on prevention of CVD and (d) Co-enzyme Q did not show any beneficial effect in CVD. Few years ago, Lawlor et al. [203] excellently analyzed the reasons for variables conclusions of researchers on vitamin supplement. For example, antioxidant vitamins showed protective effects on cancer, CVD and all cause mortality in observational studies. On the contrary, randomized controlled trials did not show these effects. He analysed the effects of vitamin C in a considerably large randomized controlled trial and observational study. Both the studies are methodologically sound yet their conclusions are contradictory. Several plausible reasons are possible but the most likely reasons are confounding social and behavioral factors. It is not practically feasible to eliminate all these factors completely even in randomized trials, but they should be minimized to click conclusive answers. Till then, we will have to wait for the final verdict.
Some papers and opinions published in 2005 require special mention. Johansen et al. [132] summarized the results of clinical trials in diabetes mellitus. Several randomized small trials showed beneficial effects of vitamin C and E supplements but large scale trials such as Heart Outcome Prevention Study Evaluation (HOPE) [204], changes in patients treated with ramipril and vitamin E (SCURE TRIAL) [205], Primary Prevention Projects (PPP TRIAL) [206], no effect of vitamin E was observed. On the other hand in Secondary Prevention with AO of Cardiovascular Disease in End Stage Renal Disease (SPACE TRIAL) [207] beneficial effects of vitamin E were observed in hemodialysis patients with preexisting CVD. In STENO-2 TRIAL [208] the effects of the multi therapy (vitamin E + vitamin C + folic and + chromium piconilate) was examined in DM-2 patients with CVD complications. The results showed 50% decrease in CVD events suggesting that multi-drug treatment is better than single antioxidant only for reducing OS. William and Fisher [133] critically analyzed benefits and harms of various dietary AO in relation to CVD. They concluded “Guidelines for diet should adhere closely to what has been clinically proved and by this standard there is no basis to recommended AO use, beyond what is inherent to the “heart healthy” diet in order to benefit cardiovascular health”. Blomhoff [130] concluded that some compounds in plants, other than vitamin C and E, play two roles as AO. First, they donate electrons to FR to neutralize them, and second they in turn get converted into very little harmful or harmless radicals. This may be good for cell as it may be activating gene expression of AO as well as phase-2-antioxidant enzymes. Further, he said in his concluding remarks “Although experimental studies in cell cultures and animals have indicated that AO such as beta-carotene, ascorbic acid or alpha-tocopherol may reduce oxidative stress, human intervention studies do not support beneficial effects. Governmental and non-governmental organizations such as the US Food and Drug Administration, the Institute of Medicine (Dietary Reference Intake), The American Heart Association or the Report by the World Cancer Research Funds, therefore, do not recommend intake of single or combination of supplemental AO”. In an excellent editorial review, Lichtenstein [131] has aptly said, “The past few years have brought some disappointing outcomes regarding the potential benefits of single nutrients or nutrient cocktails and cardiovascular risk” and concluded with the remark “We need to be cautious in what we say and claim about preliminary findings so as not to put the carriage in front of the horse and for all intents and purposes let the scientific community rather than market, determine direction”. In this context we would like mention that the elaborate articles of Pryor [19] on vitamin E and Tewari [11] on AO in general do not seem to be vexed by any vested interest but tend to derive many deductions on premature presumptions. Pauling was greatest believer in healing and health quality of vitamin C [29]. It is undoubtedly an essential nutrient. In a recent article Verrax and Calderon [23] have said “Thus, for many people, vitamin C is believed to prevent or cure viral respiratory infections and to be beneficial in both cardiovascular diseases and cancer. Although there is no clinical evidence as yet that vitamin C can be beneficial in any one of these indications, it is still perceived by the public as a miracle-pill”. They are frank enough to confess that the popularity of vitamin C has been exaggerated and over embroidered by expensive advertising campaigns. The Bijelakovic group [52] has been engaged in an extensive analysis of the effects of various dietary AO in cancer and other diseases. In a recent Chochrane Meta-analysis including 68 randomized trials with 232, 666 participants they concluded: (a) vitamin supplements increase mortality, (b) retinol, vitamin E and beta-carotene either given alone or in combination increase the chances of premature death (c) no evidence that vitamin C enhances lifespan and lastly (d) findings confirm that trials with inadequate bias control overestimate the intervention effects. Several other studies more or less support these deductions [2, 28, 129]. Thus, antioxidants are boon when consumed in normal quantity but bane when taken in excess as supplement.
Corollary
In the past three decades one of the grotesque fads in health science has been an enamor for AO. On one hand, these have been credited to improve physical appearance (e.g. ads claim that AO improve texture and complexion of skin, keep hairs black etc.), boost health and ruffles of aging and on the other hand, they are claimed to provide protection against numerous diseases. At one time, their usefulness was so strongly felt that health scientists pleaded to develop stronger synthetic AO to combat the OS in diseases. OS was thought to be the root cause of the diseases. However today the debatable question before us is whether this fondness for AO supplements is right or wrong.
In summary the current opinion is that as long as body has adequate levels of AO and that the dietary intake is normal, additional supplement are not needed. On the contrary, such supplements may do more harm than good. Most importantly, all the AO should be present in proper proportion in the defence network-a problem yet to be resolved because AO, being cell specific, may discharge their duties only when present at suitable site and in conducive environment. Inappropriate selection ofAO or indiscriminate dosing may induce side effects. The benefits of AO observed in vitro may, therefore, not necessarily be applicable in humans. Further, AO and ROS are neither exclusive determinant of all cell processes nor solo-monitors in redox homeostasis. Even their major impact on redox balance in humans is yet to be demonstrated. Above all selective interactivity of an AO in defense network is of paramount importance. Failure to appreciate these points is that AO theory of benefits in health and disease in humans when tested in observational, randomized and interventional clinical trials has come out with conflicting and often disappointing answers. Thus, no one can deny the vitality of AO in human physiology, but antioxidant conundrum is in morass. The “Chochrane Metaanalysis” and “Markov Analysis” models are indeed recently developed excellent tools of statistics to analyze data to derive conclusions. But they too are not perfect. In fitness of the things Harvard Health Letter [209] stated “The bottom line is that there is wide spread agreement that fruits and vegetables are indeed good for health and may help in prevention of CVD, some types of cancers and age related diseases. As regard to AO, the key question is whether supplementation has proven to do more good than harm. So far answer is “No”. That is why FDA, USA will not permit any of these substances to be labeled or marketed which claims that they can prevent the diseases”. The involvement of AO in human physiology is undoubtedly implicit but to get explicit answers for their role in prevention or cure of diseases, the only solution is to avoid wanton praise to wean out cock and bull stories based on presumptions and fantasies and to move placidly in the planned direction to get impeccable results. Just to begin with, the one basic clue is provided by an old proverb “Those who fail to read history are destined to suffer from repetition of its mistakes.”
References
- 1.El-Hossary GG, Hassan A, El-Shazly M, Ahmed ES, El-Gohary AA, Metwally FG, et al. Efficacy of alpha-lipoic acid against diabetes induced deterioration of blood antioxidants and diabetic retinopathy in experimental animals Aust. Aust J Basic Appl Sci. 2010;4:127–134. [Google Scholar]
- 2.Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010;131:276–86. [DOI] [PubMed]
- 3.Singh PP, Mahdi F, Roy A, Sharma S. Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type 2. Ind J Clin Biochem. 2009;24:324–342. doi: 10.1007/s12291-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh PP, Sharma P. Antioxidant basket: do not mix apples and oranges. Ind J Clin Biochem. 2009;24:211–214. doi: 10.1007/s12291-009-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh PP, Gupta G, Barjatia MK, Adhikari D. Oxidants antioxidants dovetail hypothesis: let us not sprint before we stand. In: Singh PP et al., editors. Free radicals and antioxidants in health and disease: concordance and discordance. Chowdhary Offset Pvt. Ltd. Udaipur; 2007. p. 1–31.
- 6.Singh PP, Pendse AK, Bomb BS, Barjatiya MK, Ghosh R. Free radicals and antioxidant: sort out facts from fiction (Editorial). 1999. p. XV–XlX.
- 7.Singh PP, Gupta S, et al. Antioxidants and cardiovascular system. In: Singh PP, et al., editors. Free radicals and antioxidants in health and disease: concordance and discordance. Udaipur: Chowdhary Offset Printer Pvt. Ltd.; 2007. pp. 119–128. [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Joshua T. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Sies H, Stahl W. Vitamins E and C, β-carotene and other carotenoids antioxidants. Am J Clin Nutr. 1995;62(suppl):1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 10.Seifried HE, Anderson DE, Trisher EI, Milner JA. A review of interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Tewari AK. Antioxidants: new generation therapeutic base for treatment of polygenic disorders. Curr Sci. 2004;86:192–212. [Google Scholar]
- 12.Cadenas E, Packer L. Hand book of antioxidants. New York: Marcel Dekker Inc.; 2002. [Google Scholar]
- 13.Halliwell B. Food derived antioxidant: how to evaluate their importance in food and in vivo. In: Cadenas E, Packer L, editors. Handbook of antioxidants. New York: Marcel, Inc.; 2002. pp. 1–45. [Google Scholar]
- 14.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3. New York: Oxford University Press; 1999. [Google Scholar]
- 15.Halliwell B. Antioxidants and human disease. Nutr Rev. 1997;55:S44–S52. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B.Antioxidants and human disease Am J Clin Nutr 2005821141–1142.16280453 [Google Scholar]
- 17.Halliwell B. Drug antioxidant effects. Drugs. 1991;42:569–581. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutteridge JMC, Halliwell B. Antioxidants in nutrition, health and disease. New York: Oxford University Press; 1996. [Google Scholar]
- 19.Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trails. Free Radic Biol Med. 2000;28:141–164. doi: 10.1016/S0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 20.Narsinga Rao BS. Nutrient requirement and safe dietary intake for Indians. NFI Bull (Bulletin of the Nutrition Foundation of India). 2010;31:1–5. [Google Scholar]
- 21.Loscalzo J. Membrane redox state and apoptosis death by peroxide. Cell Metab. 2008;8:182–183. doi: 10.1016/j.cmet.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Clarke MW, Burnett JR, Croft KD. Vitamin E in human health and disease. Crit Clin Lab Sci. 2008;45:417–450. doi: 10.1080/10408360802118625. [DOI] [PubMed] [Google Scholar]
- 23.Buettner GR. Commentary on: faster plasma disappearance in smokers is normalization by vitamin C supplementation. Free Radic Biol Med. 2006;40:1–4. doi: 10.1016/j.freeradbiomed.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Droge W. Free radicals in physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 25.Dalle–Donne L, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 26.Baxi M, Baxi J, Adhikari D, Singh PP. Clinicopathological profile of fibrocystic disease of breast with and without malignancy and assessment of efficacy of vitamin E supplementation in mastalgia due to fibrocystic disease: results from a tertiary care centre in western Nepal. 42nd World Congress of the Int. Soc. Surgery Montreal Canada. 2007; p. 148.
- 27.Brigelius-Flohe R, Traber GM. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 28.Murray RK, Bender DA, Bothan KM, Kennelly PJ, Rodwell VW, Weid PA. Harper’s illustrated biochemistry 28 in Ed. McGraw Hill. Lange. N.Y.P. p. 468–469.
- 29.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophilla hemaetopoitic progenitors for differentiation. Nature. 2009;461:537–542. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azzi A, Aratri E, Boscoboinik D, Clement S, Ozer NK, Ricciarelli R, Spychers S. Molecular basis of alpha-tocopherol control of smooth muscle cell proliferation. Biofactors. 1998;7:3–14. doi: 10.1002/biof.5520070102. [DOI] [PubMed] [Google Scholar]
- 31.Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol: role of protein kinase C. J Biol Chem. 1991;266:6181–6194. [PubMed] [Google Scholar]
- 32.Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q. J Bioenerg Biomeds. 1994;26:349–357. doi: 10.1007/BF00762775. [DOI] [PubMed] [Google Scholar]
- 33.Vasudevan DM, Sreekumari S, Vaidyanathan K. Text book of biochemistry for medical students. 6. New Delhi: Jaypee Brothers Med Pub; 2011. pp. 407–410. [Google Scholar]
- 34.Freedman JE, Farhat JH, Loscalzo J, Keanney JF., Jr Alpha-tocopherol inhibits aggregation of human platelets by a protein-Kinase-C-dependent mechanism. Circulation. 1996;94:2434–2440. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 35.Keaney JF, Jr, Guo Y, Cunningham D, Shwaery GT, Xu A, Vita JA. Vascular micarporation of alpha-tocopherol prevents endothelial days function due to oxidized LDL by inhibiting protein kinase C stimulation. J Clin Invest. 1996;88:386–394. doi: 10.1172/JCI118804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasinato A, Boscoboink D, Bartoli G, Maroni P, Azzi A. Alpha-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentration, correlates with protein Kinase C inhibition and is independent of its antioxidant properties. Proc Natl Acad Sci USA. 1995;92:12190–12194. doi: 10.1073/pnas.92.26.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theopold U. A bad boy comes good. Nature. 2009;461:486–487. doi: 10.1038/461486a. [DOI] [PubMed] [Google Scholar]
- 38.Loh K, Derg H, Fukushina A, Cai X, Boivin B, Glalic G, et al. Reactive oxygen enhance insulin sensitivity. Cell Met. 2009;10:27–260. doi: 10.1016/j.cmet.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathewa J, Galarneu L, Loranger A, Gilbert S, Marceu N. Kertatin-protein Kinase interaction in reactive oxygen species-induced hepatic cell death through mitochondrial signaling. Free Radic Biol Med. 2008;45:413–424. doi: 10.1016/j.freeradbiomed.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Veal EA, Day AM, Morgan BA. Hydrogen sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 41.D’Autreaux B, Toledano M. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 42.Seilar A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/16-lipoxygenase dependent and AIF mediated cell death. Cell Met. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Juranek L, Bezek S. Controversy of free radical hypothesis reactive oxygen species hypothesis cause of consequence of tissue injury? Gen Physiol Biophys. 2005;24:263–278. [PubMed] [Google Scholar]
- 44.Poli G, Leonarduzzi G, Biasi F, Chairpotto E. Oxidative stress and cell signaling. Curr Med Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 45.Thannickal VJ, Fanburg Bl. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L 1005–L 1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 46.Brooks PS, Yoon Y, Robothan JL, Andres MW, Sheu SS. Calcium, ATP and ROS: a mitochondrial love hate triangle. Am J Phys. 2004;287:c817–c823. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 47.Mehta G, Kotharie S, Singh PP. Breast cancer in developing population a nutrition caveat. Ind J Clin Biochem. 2001;16:65–71. doi: 10.1007/BF02867570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective or an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Risal S, Adhikari D, Alurkar VM, Singh PP. Oxidative stress and antioxidant status in Cardiovascular diseases in population of western Nepal. Kathmandu Univ Med J. 2006;4:270–274. [PubMed] [Google Scholar]
- 50.Maharajan BR, Jha JC, Vishwanath P, Alurkar M, Singh PP. Oxidant and antioxidant status and lipid profile in hypertension patients. J Nepal Health Res Comc. 2008;6:63–68. [Google Scholar]
- 51.Singh PP, Mahdi F. Free radical and antioxidant covenant in etiopathogenesis of diabetes mellitus. International Conference on Advances in Free Radical Research; Natural Products Antioxidants Protectors & 8th Ann. Meeting of Society Free Radical Research in India. Lucknow Abstract, 2009; p. 8.
- 52.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gulshan K, Rovinsky SA, Coleman ST, Moye-Rowley WS. Oxidant specific folding of Yap 1-p regulates both transcriptional activation and nuclear localization. J Biol Chem. 2005;280:40524–40533. doi: 10.1074/jbc.M504716200. [DOI] [PubMed] [Google Scholar]
- 54.Dotan Y, Pinchuk I, Litchenberg D, Leshno M. Decision analysis supports the paradigm that indiscriminate supplementation of vitamin E does more harm than good. Arterioscle Thromb Vasc Biol. 2009;29:1304–1309. doi: 10.1161/ATVBAHA.108.178699. [DOI] [PubMed] [Google Scholar]
- 55.Bielakovic G, Nikolova D, Simonetti RG, Gludd C. Antioxidant supplements for preventing cancers. USA: The Cochrane Collaboration John Wiley & Sons Ltd.; 2008. pp. 1–79. [Google Scholar]
- 56.Bjalakovic G, Nikolova D, Gludd LL, Smonetti RG, Gludd C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 57.Bejelakovic G, Nikolova D, Simonetti RG, Gludd C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 58.Marchivdi R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, et al. Vitamin E increases the risk of increasing heart failure after myocardial infraction: results from the GISSI Preventive Trial. J Cardiovasc Med (Hagesgerstown) 2006;6:347–350. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- 59.Daga MK, Mohan A. Antioxidants and disease–current status. Assoc Phys Ind. 1996;44:703–714. [PubMed] [Google Scholar]
- 60.Buettner GR. Commentry on: faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40:1–3. doi: 10.1016/j.freeradbiomed.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta G, Singh PP, et al. Breast cancer scenario in north- west India with special reference to oxidant antioxidant juxtaposition. In: Singh PP, et al., editors. Free radical and antioxidants in health and disease: concordance and discordance. Udaipur: Chowdhary Offset Printers; 2007. pp. 94–112. [Google Scholar]
- 62.Valko M, Rhodes CJ, Marcol Izakonic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Slorz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1886. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 64.Adhikari D, Baxi J, Risal S, Singh PP. Oxidative stress and antioxidant status in cancer patients and healthy subject: a case controlled study. Nepal Med J. 2005;7:112–115. [PubMed] [Google Scholar]
- 65.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/B:MCBI.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 66.Serrano M, Blasco MA. Cancer and aging: convergent and divergent mechanisms. Nature. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 67.Krishnaswamy K, Polasa K. Diet, nutrition and cancer-the Indian scenario. J Med Res. 1995;102:200–209. [PubMed] [Google Scholar]
- 68.Maharjan BR, Jha JC, Adhikari D, Akila Risal S, Singh PP, et al. Oxidant stress antioxidant status and lipid profile in ischemic heart disease patients from western Nepal. Nepal Med J. 2008;10:20–24. [PubMed] [Google Scholar]
- 69.Serdar Z, Aslan K, Dirican M, Sarandol E, Yesilbursa D, Serdar A. Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clin Biochem. 2006;48:1–11. doi: 10.1016/j.clinbiochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Bachorun T, Soobrattee MA, Luximom- Ramma V, Aruoma OI. Free radicals and antioxidants in cardiovascular health and disease. IJMU. 2006;11:1–21. [Google Scholar]
- 71.Heistad DD. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2006;26:689–695. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- 72.Anh N. Hyperlipidemia and cardiovascular disease: oxidative damage and atherosclerosis. Curr Opin Lipidol. 2006;17:92–94. [PubMed] [Google Scholar]
- 73.Burrel CJ, Blake DR. Reactive oxygen metabolites and the human myocardium. Br Heart J. 1989;61:4–8. doi: 10.1136/hrt.61.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curtis LK. Reversing atherosclerosis? New Eng J Med. 2009;360:1144–1146. doi: 10.1056/NEJMcibr0810383. [DOI] [PubMed] [Google Scholar]
- 75.Schleicher E, Friess U. Oxidative stress, AGE, and atherosclerosis. Kid Int. 2007;72:S.17–S.26. doi: 10.1038/sj.ki.5002382. [DOI] [PubMed] [Google Scholar]
- 76.Devaraj S, Tang R, Adams-Huet B, Harris A, Sreenivasan T, de-Lemos JA. Effects of high dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary atery disease. Am J Clin Nutr. 2007;86:1392–1398. doi: 10.1093/ajcn/86.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stocker R, Kenny JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2003;35:117–132. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 78.Li D, Mehta JL. Oxidized LDL, a critical factor in atherogenesis. Cardiovas Res 2005;68:353–4. [DOI] [PubMed]
- 79.Mueller CFH, Laude K, McNally JS, Harrison DG. Redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 80.Jialal I, Devraj S. Antioxidant and atherosclerosis. Don’t throw out the baby with bath water. Circulation. 2003;107:926–928. doi: 10.1161/01.CIR.0000048966.26216.4C. [DOI] [PubMed] [Google Scholar]
- 81.Ross R. Atherosclerosis: an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 82.Resende R, Moreira PI, Proenca T, Deshpande A, Bnsciglio S, Pereira C, et al. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Tanagno E, Bardini P, Guglielmotto M, Danni O, Tabaton M. The various aggregation states of beta-amyloid 1-42 mediate different effects on oxidative stress, neurodegeneration and BACE-1-expression. Free Radic Biol Med. 2006;41:202–212. doi: 10.1016/j.freeradbiomed.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 84.Tretter L, Sips L, Adam-vizzi I. Initiation of neuronal damage by complex I deficiency and oxidative stress in Parkinson’s disease. Neurochem Res. 2004;29:569–577. doi: 10.1023/B:NERE.0000014827.94562.4b. [DOI] [PubMed] [Google Scholar]
- 85.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53:S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 86.Butterfield DA, Castegra A, Lauderback CM, Drake J. Evidence that amyloid beta- peptide induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurotriol Aging. 2002;23:655–664. doi: 10.1016/S0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 87.Montesa MJP, Rico MAG, Salguero MAS, Maicas IT, Munoz MCT, Tormo GS, et al. Study of oxidative stress in advanced kidney disease. Nefrologica. 2009;29:464–473. doi: 10.3265/Nefrologia.2009.29.5.5493.en.full. [DOI] [PubMed] [Google Scholar]
- 88.Sathyapriya K, Vijayachandrika V, Paxameswari CS. Antioxidant status in polycystic end stage renal renal diseased patients and antihemolytic effect of Boerhaavia diffusa. Ind J Biochem Biophys. 2009;46:272. [Google Scholar]
- 89.Barjatiya MK, Nagamma T, Ahmed S, Mathur M, Singh PP, et al. Status and intradialytic changes in reactive oxygen species activity and total antioxidants strength in chronic renal failure patients from India and Nepal. In: Singh PP, et al., editors. Free radicals and antioxidants in health and diseases: concordance and discordance. Udaipur: Chowdhary Offset Print; 2007. pp. 238–245. [Google Scholar]
- 90.Diez BG. Progression of chronic renal failure and oxidative stress. Elect J Biomed. 2003;1:5–11. [Google Scholar]
- 91.Singh R, Singh S, Tripathi AK, Singh RK. Circadian periodicity of human circulating lipid peroxide and antioxidant enzymes as putative markers in cirrhosis of liver. In: Singh PP, editor. Free radic antioxidants: sort out facts from fiction. Udaipur: Chowdhary Offset Print; 1999. pp. 223–237. [Google Scholar]
- 92.Fecher J, Lengyel G, Blazovics A, Hegedus E, et al. Antioxidants therapy in liver disease. In: Singh PP, et al., editors. Free radic antioxidants. Sort out facts from fiction. Udaipur: Chowdhary Offset Print; 1999. pp. 168–175. [Google Scholar]
- 93.Feher J, Cosmos G, Vereckel A. Role of free radicals in liver disease. In: Cosmos G, Feher J, editors. Free radical and the liver. Berlin: Springer Verlag; 1992. pp. 1–12. [Google Scholar]
- 94.Paravicini TM, Touyz RN. NADPH oxidases, reactive oxygen species and hypertension: clinical implicators and therapeutic possibilities. Diab Care. 2008;31:S70–S180. doi: 10.2337/dc08-s247. [DOI] [PubMed] [Google Scholar]
- 95.Sowers JR. Hypertension, angiotensin II and oxidative stress. New Eng J Med. 2002;346:1999–2001. doi: 10.1056/NEJMe020054. [DOI] [PubMed] [Google Scholar]
- 96.Maurice MM, Nakamura H, Vander Voort EAM, Van Vliet A, Staal FJ, T, Tak PP et al. An altered redox state in hyporesponsiveness of synovial T cells in rheumatoid arthritis. J Immunol. 1997;158;1458–65. [PubMed]
- 97.Cunnane G, Fitz Gerald O, Beeton C, Cawston TE, Bresnihan B. Early joint erosions and serum levels of matrix metalloproteinase 1, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 in rheumatoid arthritis. Arth Rheumat. 2001;44:2263–2274. doi: 10.1002/1529-0131(200110)44:10<2263::AID-ART389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 98.Bauerova K, Bezek S. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys. 1999;18:15–20. [PubMed] [Google Scholar]
- 99.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Geren DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthiritis synovium. Proc Natl Acad Sci USA. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alvardo C, Alvarez P, Puerto M, Gauwsseres N, Jinenez L, Fuente MDe. Dietary supplementation with antioxidants improve functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition. 2006;22:767–777. doi: 10.1016/j.nut.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Finkel T, Holbrook J. Oxidants, oxidative stress and the biology of aging. Nature. 2000;9:239–246. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 102.Ghosh R, Mehta A, Ramavatram DVVS, Barjatia MK, Singh PP, et al. Pro oxidant antioxidant balance in aging. In: Singh PP, et al., editors. Free radical and antioxidants. Sort out facts from fiction. Udaipur: Chowdhary Offset Print; 1999. pp. 127–137. [Google Scholar]
- 103.Beckman KB, Ames BN. Free radical theory of aging natures. Phys Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 104.Freanzini L, Ardigo D, Zavaroni I. Dietary antioxidant and glucose metabolism. Curr Opin Clin Nutr Met Care. 2008;11:471–476. doi: 10.1097/MCO.0b013e328303be79. [DOI] [PubMed] [Google Scholar]
- 105.Mootha UK, Lindgren CM, Errikson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately down regulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 106.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 107.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endo Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 108.Goh SY, Cooper ME. The role of advanced glycation end products in progression and complication of diabetes. J Clin Erdocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 109.Aruoma OI, Neergheen VS, Bahourn T, Jen L. Free radicals, antioxidants and diabetes mellitus: embryopathy, retinopathy, neuropathy, nephropathy and cardiovascular complications. Neuroembryol Aging. 2006/2007; 4:117–37.
- 110.Gratt GM, Schoutern EG, Kok FJ. Effect of daily vitamin E and multivitamin supplementation on acute respiratory tract infection in elderly persons: a randomized controlled trial. JAMA. 2002;288:715–721. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 111.Hemila H, Virtamo J, Albances D, Kaprio J. Vitamin E and beta carotene supplementation and hospital treated pneumonia incidence in male smokers. Chest. 2004;125:537–565. doi: 10.1378/chest.125.2.557. [DOI] [PubMed] [Google Scholar]
- 112.Singh PP, Kothari S, Bhasin S, Bhargav U. Diminished endogenous antioxidant defence in the etiology of senile cataract formation. SFRR-India Bull. 2004;3:11–14. [Google Scholar]
- 113.Babizhayev MA. Failure to withstand oxidative stress induced by phospholipids hydroperoxides as a possible cause of the lens opacities in systemic diseases and aging. Biochem Biophys Acta. 1996;1315(2):87. doi: 10.1016/0925-4439(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 114.Tinahone FJ, Murri-Pierri M, Garrido-Sanchez L, Garca-Almeida JM, Garcia-Serrano S, Garcia-Ames J, Garcia-Fuentes E. Oxidative stress in severely obese person is greater in those with insulin resistance. Obesity. 2009;17:240–246. doi: 10.1038/oby.2008.536. [DOI] [PubMed] [Google Scholar]
- 115.Katakan PV, Domoki F, Snipes JA, Busija AR, Jarajapu YP, Bushija DW. Impaired mitochondria dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R289–R298. doi: 10.1152/ajpregu.90656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nathan C. Epidemic inflammations: Pondoring obesity. Molecular Med. 2009;14:485–492. doi: 10.2119/2008-00038.Nathan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wells GD, Noseworthing MD, Hamilton J, Tarnopolska M, Teir I. Skeletal muscle metabolic dysfunction in obesity and metabolic syndrome. Can J News Sci. 2008;35:31–40. doi: 10.1017/s0317167100007538. [DOI] [PubMed] [Google Scholar]
- 118.Farooqui IS, O’Rahilly S. Genetics of obesity in humans. End Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 119.Foster-Schubert KE, Cummings DE. Emerging therapeutic strategies for obesity. Endo Rev. 2006;27:779–793. doi: 10.1210/er.2006-0041. [DOI] [PubMed] [Google Scholar]
- 120.Buonocore G, Groenendaal F. Antioxidant strategies. Sem Fetal Neonat Med. 2008;12:287–295. doi: 10.1016/j.siny.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 121.Smith AR, Shenvi SV, Lipoice Widlansky M, Suh JH, Hgen TM. Lipoic acid as a potential therapy for chronic disease associated with oxidative stress. Curr Med Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 122.Lynd DD, Gunnet CA, Chu Y, Brooks RM, Faraci FM, Heistad DD. Gene transfer of extra cellular superoxide dismutase improves relaxation of aorta after treatment with endotoxin. Am J Physiol Heart Circ Physiol. 2004;56:H805–H811. doi: 10.1152/ajpheart.00907.2003. [DOI] [PubMed] [Google Scholar]
- 123.Cleveland JL, Kastan MB. A radical approach to treatment. Nature. 2000;407:309–311. doi: 10.1038/35030277. [DOI] [PubMed] [Google Scholar]
- 124.Maxwell SRJ. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 125.Blunberg JB. Considerations of scientific substantiation for antioxidant vitamins and beta-carotene in disease prevention. Am J Clin Nutr. 1995;62(suppl):1521S–1526S. doi: 10.1093/ajcn/62.6.1521S. [DOI] [PubMed] [Google Scholar]
- 126.Rice-Evans CA, Diplock AT. Current status of antioxidant therapy. Free Radic Biol Med. 1993;15:77–96. doi: 10.1016/0891-5849(93)90127-G. [DOI] [PubMed] [Google Scholar]
- 127.Downey J, Onar B, Ooiwa H, Mc Cord J. Superoxide dismutase therapy for myocardial ischemia. Free Radic Res Commun. 1991;703:12–13. doi: 10.3109/10715769109145850. [DOI] [PubMed] [Google Scholar]
- 128.Pocobelli G, Peters U, Peters U, Kristal AR, White E. Use of supplements of multivitamins, vitamin C and vitamin E in relation to mortality. Am J Epidemiol. 2009;170:472–483. doi: 10.1093/aje/kwp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller ER, Pastor –Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high dosage vitamin E supplementation may increase all cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 130.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr Opin Lipidol. 2005;16:47–54. doi: 10.1097/00041433-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 131.Lichtenstein AH. Nutrient and cardiovascular disease no easy answers. Curr Opin Lipidol. 2005;16:1–3. doi: 10.1097/00041433-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 132.Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:1–11. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Williams KJ, Fisher EA. Oxidation, lipoproteins and atherosclerosis: which is wrong, the antioxidants or theory? Curr Opin Clin Nutr Care. 2005;8:139–146. doi: 10.1097/00075197-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 134.Singh S, Farzana M, Singh PP. Insinuating role of free radicals and placating behaviour of antioxidant in diabetes mellitus. J Physiol. 2009;3:5–8. [Google Scholar]
- 135.Sokol RJ. Vitamin E toxicity. Pediatrics. 1984;74:564–565. [PubMed] [Google Scholar]
- 136.Bensuasson RV, Land EJ, Truscott TG. Excited states and free radicals in biology and medicine: contribution from flash photolysis and PulseRadiolysis. New York: Oxford University Press; 1993. pp. 101–141. [Google Scholar]
- 137.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Dwyer P, Fenn WO. Oxygen poisoning and X-irradiation, A mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 138.Harman D. Aging; a theory based on free radical and radiation chemistry. J Gerontol. 1956;2:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 139.Harman D. Free radical theory of aging. In: Johnson JE Jr, et al., editors. Free radicals, aging and degenerative disease. NY. 1982. p. 3–49.
- 140.Magalhes JP ole, Church GM. Cell discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2005;41:1–10. doi: 10.1016/j.exger.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 141.Bujisse B, Feskens EJM, Schlettwein-Gsell D, Ferry M, Kok FJ, Kromhout D, et al. Plasma carotene and alpha-tocopherol in relation to 10 yr all-cause and cause-specific mortality in European elderly: the survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA) Am J Clin Nutr. 2005;82:879–886. doi: 10.1093/ajcn/82.4.879. [DOI] [PubMed] [Google Scholar]
- 142.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Denberg MV. Vitamin C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;10:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- 144.Halliwell B, Whitemam M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Brit J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Araki E, Nishikawa N. Oxidative stress: a cause and therapeutic target of diabetic complications. J Diab Invest. 2010;1:90–96. doi: 10.1111/j.2040-1124.2010.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 147.Fridovich I. Superoxide radical and superoxide dismutase. Ann Red Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 148.Mc Cord JM, Fridovich I. Superoxide dismutase. An enzyme function for erythrocuprin. J Biol Chem. 1969;224:6049–6055. [PubMed] [Google Scholar]
- 149.Hathock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 150.Sies H, Krinsky NI. The present status of antioxidant vitamins and beta-carotene. Am J Clin Nutr. 1995;62(suppl):1299-S–1300-S. doi: 10.1093/ajcn/62.6.1299S. [DOI] [PubMed] [Google Scholar]
- 151.Diplock AT. Antioxidant nutrients and disease prevention: an overview. Am J Clin Nutr. 1991;53:S189–S193. doi: 10.1093/ajcn/53.1.189Sb. [DOI] [PubMed] [Google Scholar]
- 152.Potter JD. Vegetable, fruits and cancer. Lancet. 2005;366:527–530. doi: 10.1016/S0140-6736(05)67077-8. [DOI] [PubMed] [Google Scholar]
- 153.Cao G, Both SL, Sadowski JA, Prior RL. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1998;68:1081–1087. doi: 10.1093/ajcn/68.5.1081. [DOI] [PubMed] [Google Scholar]
- 154.Serafini M, Bellocco R, Wolk A, Ekstram AM. Total antioxidant potential of fruits and vegetables and risk of gastric cancer. Gastroenterology. 2002;123:985–991. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 155.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsourer KJ. Fruit vegetable and antioxidant intake and all cause cancers and cardiovascular disease mortality in a community dwelling population in Washington country Maryland. Am J Epidemiol. 2004;160:1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 156.Kelemen LE, Cohan JR, Lim U, Davis S, Cozen W, Schank M, et al. Vegetables, fruits and antioxidant related nutrients and risk of non-Hodghkin lymphoma: a National cancer Institute-Surveillance epidemiology and results population-based case control study. Am J Clin Nutr. 2006;83:1401–1410. doi: 10.1093/ajcn/83.6.1401. [DOI] [PubMed] [Google Scholar]
- 157.Lock K, Pomerleau J, Causer L, Altman DR, Mckee M. The global burden of disease attributable to low consumption of fruits and vegetables: implications for global strategy an diet. Bull. W.H.O. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- 158.Szeeto Y-T, Benzie IFF. Effects of dietary antioxidants and human DNA ex vivo. Free Radic Biol Res. 2002;36:113–118. doi: 10.1080/10715760210161. [DOI] [PubMed] [Google Scholar]
- 159.Perrig WJ, Perrig P, Stahelin HB. The influence of antioxidants on cognitive decline in the elderly. In: Basu TK, et al., editors. Antioxidants in human health. Willingford, Oxon, Uk: CAB International; 1999. p. 335–49.
- 160.Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Biochem J. 1991;247:133–138. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reznick AZ, Cross CE, Hu M, Suzuki YJ, Khwaja S, Safadi A, et al. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl. Biochem J. 1992;286:607–611. doi: 10.1042/bj2860607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Young JF, Nielsen SE, Haraldsdottir J. Effect of fruit juice on urinary querchetin excretion and biomarkers of oxidative stress. Am J Clin Nutr. 1999;69:87–94. doi: 10.1093/ajcn/69.1.87. [DOI] [PubMed] [Google Scholar]
- 163.Buttner GR. The pecking orders of free radicals and antioxidants: lipid peroxidation, alpha tocopherol and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 164.Padyatty SJ, Daruwala R, Wang Y, Eck PK, Song J, Koh WS, et al. et al. Vitamin C: from molecular actions to optimum intake. In: Cadenas E, Packer L, et al.et al., editors. Handbook of antioxidants. New York, USA: Marcel Dekker Inc.; 2002. pp. 117–145. [Google Scholar]
- 165.Creagan ET, Moertel CG, O’ Fallon JR. Failure of high dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. New Eng J Med. 1979;301:687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 166.Moertei CG, Flemming TR, Creagon ET, et al. High dose vitamin C verses placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. New Eng J Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 167.Pendse AK, Purohit AK, Ghosh R, Goyal A, Singh PP, et al. The effect of ingestion of mega doses of ascorbic acid on urinary oxalate excretion in normal subjects and store formers. In: Schwille PO, et al., editors. Urolithiaisis and related clinical research. NewYork, USA: Plenum Press; 1984. pp. 225–228. [Google Scholar]
- 168.Singh PP, Mehta A, Rao GS, Pendse AK, Barjatiya MK, Ramavataram DVSS. Antioxidant status peroxidative stress and urinary excretion of calcium and oxalate in kidney diseases with special reference to urolithiasis. In. Pak CYC, et al., editors. Urolithiasis-96. Dallas, USA: The Printer Inc., p. 26–28.
- 169.Singh PP, Rajbala, Ramavataram DVSS, Pendse AK, Barjatia MK. Extended experimental studies to evaluate the role of ascorbic acid in lithogenesis under different set of conditions. In: Pak CYC, et al., editors. Urolithiasis. Dallas, USA: Millet the Printer Inc.; 1996. p. 349–50.
- 170.Singh PP, Sharma DC, Rathore V, Surana SS. An investigation into the role of ascorbic acid in renal calculosis in albino rats. J Urol. 1988;139:156–157. doi: 10.1016/s0022-5347(17)42343-3. [DOI] [PubMed] [Google Scholar]
- 171.Aw TY, Wierzbicka G, Jones DP. Oral glutathione increases tissue glutathione in vivo chem. Biol Interact. 1991;80:89–97. doi: 10.1016/0009-2797(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 172.Tirosh O, Roy S, Packer L. Lipoic acid, cellular metabolism, antioxidant activity and clinical relevance. In: Cadenas E, Packer L, editors. Hand book of antioxidants. 2. New York: Marcel Dekker Inc.; 2002. pp. 473–487. [Google Scholar]
- 173.Moreau R, Znang W, Hgen TM. Cellular effects of lipoic acid and its role in aging. In: Cadenas E, Packer L, editors. Handbook of antioxidants. 2. New York: Marcel Dekker Inc.; 2002. pp. 489–510. [Google Scholar]
- 174.Jones DP. Bioavailability of glutathione. In: Cadenas E, Packer L, editors. Handbook of antioxidants. New York: Marcel Dekker Inc.; 2002. pp. 549–564. [Google Scholar]
- 175.Jones DP, Coates RJ, Flagg EW, Eley JW, Block G, Greenberg RS, et al. Glutathione in food listed in National Cancer Institute Health Habits and History Food Frequency Questionnaire. Nutr Cancer. 1992;17:57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- 176.Flagg EW, Coates RJ, Jone DP, Byers TE, Green berg RS, Gridley G, et al. Dietary glutathione intake and risk of oral and pharangeal cancer. Am J Epidemiol. 1994;139:453–465. doi: 10.1093/oxfordjournals.aje.a117028. [DOI] [PubMed] [Google Scholar]
- 177.Panpilla A, Visviki A, Paolicchi A, DeTata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/S0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 178.Livingstone C, Davis J. Targeting therapeutics against glutathione depletion in diabetes and its complications. Brit J Diab Vas Dis. 2007;7:258–265. doi: 10.1177/14746514070070060201. [DOI] [Google Scholar]
- 179.Johnston CS, Meyer CG, Srilakshmi JC. Vitamin C elevates red blood cell glutathione in healthy adults. Am J Clin Nutr. 1993;140:3–5. doi: 10.1093/ajcn/58.1.103. [DOI] [PubMed] [Google Scholar]
- 180.Deneka SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol Lung Cell Mol Physiol. 1989;257:E790–E800. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 181.Meister A. Glutathione metabolisms and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 182.Cay M, Naziroglu M, Koylu H. Selenium and vitamin E modulate cigarette smoke exposure-induced oxidative stress in blood of rats. Biol Trace Elem Res. 2009;131:62–70. doi: 10.1007/s12011-009-8347-4. [DOI] [PubMed] [Google Scholar]
- 183.Burtis CA, Ashwood ER, Bruns DE. Tietz text book of clinical chemistry and molecular diagnosis. 4th ed. St Louis, USA: Saunders; 2006.
- 184.Jellifee DB. The Assessment of the Nutritional Status of the Community. WHO Monograph Series No. 53.
- 185.Gey KF. Vitamin E plus C and interacting coutrients required for optional health. A critical and constructive review of epidemiology and supplementation data regarding cardiovascular diseases and cancer. Biofactors. 1998;7:113–174. doi: 10.1002/biof.5520070115. [DOI] [PubMed] [Google Scholar]
- 186.Gey KF, Moser UK, Jordon P, Stahelin HB, Eichholzor M, Ludin E. Increased risk of cardiovascular disease at suboptimal plasma concentrations of essential antioxidants: an epidemiological update with special attention with carotene and vitamins. Am J Clin Nutr. 1993;57(Supp):787 S–799 S. doi: 10.1093/ajcn/57.5.787S. [DOI] [PubMed] [Google Scholar]
- 187.Lonn E, Bosch J, Yusuf S, et al. Effects of long term supplementation on cardiovascular events and cancer a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 188.Adam BD, Adams WW. Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2149–2153. doi: 10.1126/science.1078002. [DOI] [PubMed] [Google Scholar]
- 189.Wang XD, Liu C, Bronson RT, et al. Retinoid signaling and activator protein 1 expression in ferrets given beta carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 190.Lee SH, Oe T, Blair IA. Vitamin C induced decomposition of lipid hydroperoxide to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 191.Mustacih DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG. Alpha-tocopherol regulation of hepatic cytochrome. P-450 and ABC transporters in rats. Free Radic Biol Med. 2006;41:1069–1078. doi: 10.1016/j.freeradbiomed.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 192.Brigelius-Flohe R. Induction of drug metabolism enzymes by vitamin E. J Plant Physiol. 2005;162:797–802. doi: 10.1016/j.jplph.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 193.Landes N, Pfuluger P, Kluth D, Birringer M, Rahur R, Bol GF, et al. Vitamin E activates gene expression via the pregnane X receptor. Biochem Pharmacol. 2003;65:269–273. doi: 10.1016/S0006-2952(02)01520-4. [DOI] [PubMed] [Google Scholar]
- 194.Finendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol. 2002;21:85–90. doi: 10.1191/0960327102ht216oa. [DOI] [PubMed] [Google Scholar]
- 195.Ravati A, Ahlemayer B, Becker A, Krrieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species. Brain Res. 2002;866:23–32. doi: 10.1016/S0006-8993(00)02210-1. [DOI] [PubMed] [Google Scholar]
- 196.ATBC Study (Alpha-Tocopherol, Beta-carotene Cancer Prevention Study) Incidence of cancer and mortality following ά-tocopherol and β-carotene supplementation. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 197.Northrop-Clewes CA, Thurnhan N. Monitoring micronutrients in cigarette smokers. Clin Chim Acta. 2007;377:14–38. doi: 10.1016/j.cca.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 198.Hennekens CH, Buring JE, Mason JE, Stamper M, Rosner B, Cook NR, et al. Lack of long term supplementation with beta-carotene on the incidence of malignant neoplasma and cardiovascular disease. New Eng J Med. 1996;334:1145–1155. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 199.Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL, Jr, Omenn GS, Valanis B, Williams JH., Jr The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96(23):1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 200.Rodrigo R, Prat H, Prassalacqua W, Aryas J, Bachler JP. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction of blood pressure in patients with essential hypertension. Clin Sci. 2008;114:625–634. doi: 10.1042/CS20070343. [DOI] [PubMed] [Google Scholar]
- 201.Kappus H, Diplock AT. Tolerance and safety of E up to 3200 I.V/day vitamin E: a toxicological position report. Free Rad Biol Res. 1992;13:55–74. doi: 10.1016/0891-5849(92)90166-E. [DOI] [PubMed] [Google Scholar]
- 202.Traber MG. How much vitamin E? Just enough. Am J Clin Nutr. 2006;84:959–960. doi: 10.1093/ajcn/84.5.959. [DOI] [PubMed] [Google Scholar]
- 203.Lawlor DA. Those confounded vitamins: what can we learn from differences between observational versus randomized trial evidence. Lancet. 2004;363:1724–1727. doi: 10.1016/S0140-6736(04)16260-0. [DOI] [PubMed] [Google Scholar]
- 204.Heart Outcomes Prevention Evaluation study Investigators Vitamin E supplementation and cardiovascular events in high risk patients. New Eng J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 205.Loan E, Yusuf S, Dzavik V. Effcets of rampril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with rampril and vitamin E (SECURE Trial) Circulation. 2001;103:919–925. doi: 10.1161/01.cir.103.7.919. [DOI] [PubMed] [Google Scholar]
- 206.Gaetano G. Collaborative Group of Primary Prevention Project Low dose aspirin and vitamin E in people with cardiovascular risk: a randomized trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/S0140-6736(00)03539-X. [DOI] [PubMed] [Google Scholar]
- 207.Boaz M, Smetana S, Wemistein T. Secondary prevention with antioxidants of cardiovascular disease in end stage renal disease (SPACE Trial): randomized placebo controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/S0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 208.Gaede P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pederson O. Multifunctional intervention and cardiovascular disease in patients with type 2 diabetes. New Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 209.Harvard Health Letter. Taking stock of antioxidants. 2003;27(4):283–4. [PubMed]