Abstract
Till date no community based data on plasma homocysteine is available in North Eastern Region. Hence, the present study was conducted to analyze and correlate the plasma homocysteine level with some life style factors like diet, alcohol intake, smoking habit and body weight, in a cross-section of population. 12 h fasting samples of 970 apparently healthy, Assamese population of both genders in the age group of 35–86 years, mostly from the urban area of Assam were tested for plasma total homocysteine level over a period of 3 years. Out of 970 volunteers, hyperhomocysteinemia was detected in 533 (55%) individuals with a mean value of 18.41 μmol/l. Of that hyperhomocysteinemia, 89.1% were in the range of moderately high and rest 10.9% were intermediate high. Another finding was that males had a tendency towards greater value (mean = 20.36 μmol/l) than females (mean = 16.37 μmol/l). It was observed that the relationship of homocysteine levels to gender and some of the life style factors were also significant.
Keywords: Homocysteinemia, Age, Gender, Dietary habit, Alcohol, Smoking, Obesity, Life style
Introduction
Homocysteine is a sulfhydryl (thiol) containing amino acid produced by the intracellular demethylation of dietary methionine. Numerous case–control and prospective studies have shown a relation between moderate hyperhomocysteinemia and atherothrombotic vascular disease [1, 2]. Individuals with a nutritional deficiency are at risk of developing hyperhomocysteinemia [3]. It was suggested that about two-thirds of hyperhomocysteinemia in an elderly population in the United States resulted from inadequate blood concentrations of the vitamins [4, 5]. Various studies have illustrated that besides the traditional risk factors of stroke like hypertension, hyperlipidemia, smoking and obesity, hyperhomocysteinemia is also considered as a major risk factor for coronary heart disease and stroke [6, 7]. And various life style factors like alcohol, diet, obesity, smoking etc. have been associated with increased level of homocysteine [8, 9]. Despite the obstacles to the modification of life style factors, identification of such factors can improve our ability to prevent stroke.
Materials and Method
Target Population
This study was conducted for a period of 3 years from 2002 to 2005 in the Biochemistry Lab., GNRC Hospitals, Dispur, Guwahati-6, Assam.
A total of 970 healthy volunteers of both genders in the age group of 35–86 years, of different socio-economic status were enrolled in the study, from the population in and around GNRC Hospitals, after excluding the subjects as per the Exclusion criteria like Cardiovascular disease, Diabetes Mellitus, Dyslipidemias, Endocrine Disorders, Hypertension, Liver obstruction, Medication, Pregnancy, Renal Disease and Use of Oral Contraceptives. At recruitment, a face-to-face interview was conducted in the subject’s home by trained interviewer who used a standard questionnaire. The questionnaire included a detailed dietary, medical and family history along with the information on lifetime use of tobacco, alcoholic beverages and current physical activity.
Sampling
12 h overnight fasting 2 ml EDTA venous blood samples were collected irrespective of seasonal variations throughout the year. Immediately after blood collection, the specimens were separated and then stored at −20°C until analyzed. The selected population was advised to avoid high protein diet late in the day prior to sampling.
Method of Estimation
Plasma total homocysteine was determined by HPLC (High Performance Liquid Chromatography) based on fluorimetric detection. Total plasma homocysteine represents the sum of Homocysteine, Homocystine (Homocysteine–Homocysteine disulfide), and Cysteine–Homocysteine disulfide both in free and protein bound forms.
The present method allows rapid, simple and specific determination of total homocysteine [10, 11].
Results
In the present study, a cross section of the community was selected and the target population is classified into different age groups and the results of homocysteine are tabulated accordingly. To ensure the accuracy and precision of the test results, quality control (CHROMSYSTEMS Homocysteine Plasma Control, lyophilized) check was done everyday and Standard Deviation (SD) and Coefficient of Variation (CV) were calculated accordingly (as shown in Table 1). It gives an overview of the quality control material used for the evaluation of the assay along with the day to day coefficient of variation.
Table 1.
The overview of the quality control material
| Analytes | Reference mean | Mean obtained in laboratory | SD | % of CV |
|---|---|---|---|---|
| Homocysteine (μmol/l) | 13.4 | 13.39 | 0.51 | 3.8 |
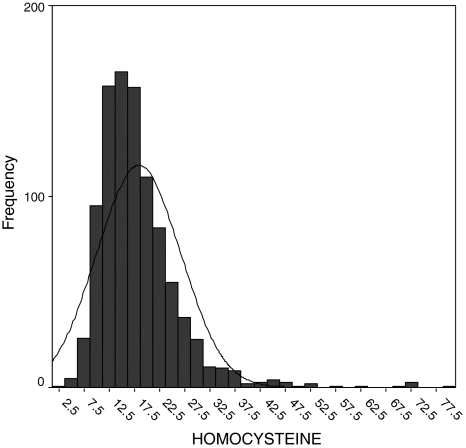
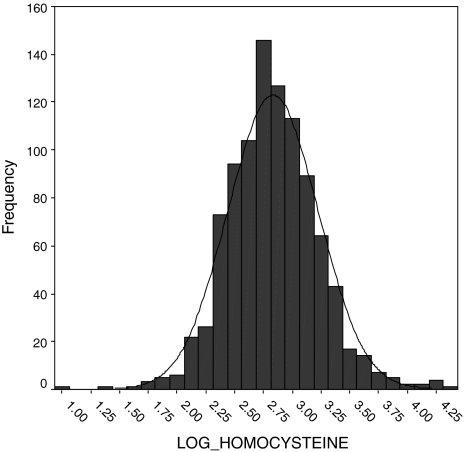
All statistical calculations were performed on the SPSS version 7.5 software (SPSS Inc, Chicago, IL, USA). The distributions of plasma homocysteine concentrations are positively skewed; therefore, they are transformed logarithmically to approximate normal distribution (Figs. 1 and 2).
Fig. 1.
Skewed distribution of homocysteine
Fig. 2.
Normal distribution of HM after transformed logarithmically
The distribution of selected subject characteristics and plasma total homocysteine concentrations are shown for all subjects by age and gender in Table 2.
Table 2.
Results of homocysteine obtained in the study
| Age in years | Gender | Frequency with % | Homocysteine (μmol/l) | Observation within normal | Observation above normal | ||
|---|---|---|---|---|---|---|---|
| Mean | Total (M + F) | Range | |||||
| 31–40 | M | 63 (6.49%) | 20.75 ± 10 | (17.74) | 5.8–72 | 24 (5.5%) | 39 (7.3%) |
| F | 78 (8.04%) | 15.31 ± 6 | 6.7–44.3 | 51 (11.7%) | 27 (5.1%) | ||
| 41–50 | M | 88 (9.07%) | 19.54 ± 6 | (17.63) | 2.7–37.8 | 26 (5.9%) | 62 (11.6%) |
| F | 176 (18.14%) | 16.67 ± 8.7 | 3.9–79.1 | 105 (24%) | 71 (13.3%) | ||
| 51–60 | M | 146 (15.05%) | 21.4 ± 10 | (18.78) | 8.2–71.9 | 44 (10.1%) | 102 (19.1%) |
| F | 145 (14.95%) | 16.15 ± 5.6 | 6.3–42.7 | 77 (17.6%) | 68 (12.8%) | ||
| 61–70 | M | 149 (15.36%) | 20.08 ± 8.5 | (19.10) | 8.1–69.8 | 51 (11.7%) | 98 (18.4%) |
| F | 64 (6.6%) | 16.81 ± 6.4 | 7.9–33.6 | 35 (8%) | 29 (5.4%) | ||
| 71–80 | M | 47 (4.85%) | 19.19 ± 5.9 | (19.29) | 6.4–33.5 | 17 (3.9%) | 30 (5.6%) |
| F | 9 (0.93%) | 19.85 ± 12 | 5.7–46.1 | 5 (1.1%) | 4 (0.8%) | ||
| >81 | M | 5 (0.52%) | 18.7 ± 6.5 | (18.74) | 12.1–28.5 | 2 (0.5%) | 3 (0.6%) |
| F | – | – | – | – | |||
| Total | M | 498 (51.3%) | 20.36 ± 8.7 | 2.7–72 | 164 (37.5%) | 334 (62.7%) | |
| F | 472 (48.7%) | 16.37 ± 7.3 | 3.9–79.1 | 273 (62.5%) | 199 (37.3%) | ||
| Overall | M + F | 970 (100%) | 18.41 ± 8.3 | 2.7–79.1 | 437 (45%) | 533 (55%) | |
| (M:F 498:472 = 1.05:1) | |||||||
In our study, we find a very wide range of plasma total homocysteine level ranging from 2.7 to 79.1 μmol/l with an overall mean of 18.41 ± 8.3. It is observed that the mean homocysteine is gradually increases up to the age group 71–80 and then decreases. There is no significant relation of homocysteine with the age (F = 1.186, P = 0.314).
The male and female ratio of the target population is almost equal (M = 51.3%, F = 48.7%; M:F = 1.05:1). Though the range of homocysteine is wider in case of females than in case of males, the overall mean value of homocysteine is higher in males (20.36 ± 8.7) than in females (16.37 ± 7.3) which is evident in all the age groups except in the age group of 71–80 years. (M = 19.19 ± 5.9, F = 19.85 ± 12) where the difference is very minimal and can be ignored.
In the studied group, 55% of the population shows hyperhomocysteinemia, of which 62.7% are male and 37.3% female. On the other hand, of the 45% population having normal homocysteine level, 62.5% are female and rest 37.5% are male. So it is observed in our study that in all the age groups males are more affected than the females except in the age group of 41–50 years. (M = 11.6%, F = 13.3%). The Student’s t test result indicates that there is a statistically significant difference between mean homocysteine for males and females (t = 7.708, P = 0.001). On the other hand females in the age group below 61 years tend to have normal homocysteine level than the females of the higher age group i.e. above 61 years. Overall, hyperhomocysteinemia is more common in the age group of 41–60 years. Almost 56.8% of homocysteinemia is detected in the age group of 41–60 years.
In our study we have found that, of the detected hyperhomocysteinemia, 89.1% are in the range of moderately high and rest 10.9% are intermediate high as shown in Table 3.
Table 3.
Pattern of distribution of homocysteine level in different age groups
| Age groups in years | Gender | Moderately high (M.H.) | Intermediately high (I.M.H.) | Total |
|---|---|---|---|---|
| 31–40 | M | 35 (6.6%) | 4 (0.8%) | 39 (7.3%) |
| F | 25 (4.7%) | 2 (0.4%) | 27 (5.1%) | |
| 41–50 | M | 59 (11.1%) | 3 (0.6%) | 62 (11.6%) |
| F | 61 (11.4%) | 10 (1.9%) | 71 (13.3%) | |
| 51–60 | M | 85 (15.9%) | 17 (3.2%) | 102 (19.1%) |
| F | 64 (12%) | 4 (0.8%) | 68 (12.8%) | |
| 61–70 | M | 89 (16.7%) | 9 (1.7%) | 98 (18.4%) |
| F | 24 (4.5%) | 5 (0.9%) | 29 (5.4%) | |
| 71–80 | M | 28 (5.3%) | 2 (0.4%) | 30 (5.6%) |
| F | 2 (0.4%) | 2 (0.4%) | 4 (0.8%) | |
| >81 | M | 3 (0.6%) | 0 | 3 (0.6%) |
| F | 0 | 0 | 0 | |
| Total | M | 299 (56.1%) | 35 (6.6%) | 334 (62.7%) |
| F | 176 (33%) | 23 (4.3%) | 199 (37.3%) | |
| Overall | M + F | 475 (89.1%) | 58 (10.9%) | 533 (100%) |
In both the groups males (M.H. = 56.1% and I.M.H. = 6.6%, respectively) are more affected than the females (M.H. = 33% and I.M.H. = 4.3%, respectively) and that is observed in all the age groups except in the age group of 41–50 years. where females are more affected (In M.H; M = 11.1%, F = 11.4% and in I.M.H.; M = 0.6%, F = 1.9%). Not a single person of the community shows severely high homocysteine level.
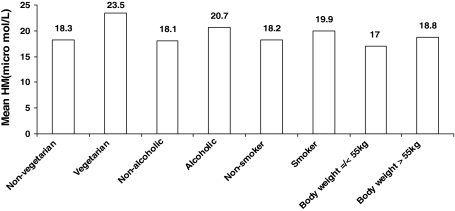
We have also examined the relation of plasma total homocysteine concentrations and some of the life style factors of the target population and the results are tabulated in Table 4 and Fig. 3. After comparison we have found that among alcoholics, smokers and vegetarians, hyperhomocysteinemia is more common than the non-alcoholics, non-smokers and non-vegetarian, 69.5% of the alcoholics show hyperhomocysteinemia in comparison to non-alcoholics, where 52.9% subjects are affected. Similarly among smokers = 62.9%, non-smokers = 53.9%; vegetarians = 70.6% and non-vegetarians = 54.7% are hyperhomocysteinemic. The mean homocysteine level of alcoholics and non-alcoholics are 20.7 ± 10 and 18.1 ± 8, respectively. There is statistically significant difference between the means of the two groups (t = 2.960, P = 0.003). On performing Chi-square test we find that there is a significant relationship between hyperhomocysteinemia and drinking habit. Here Chi-square with 1 df (degree freedom) = 11.478, P = 0.001. The odds that a person who consumes alcohol having high homocysteine is 2.025 times more than the persons who never consumes alcohol.
Table 4.
Distribution of subjects with some life style factors in relation to homocysteine level
| Factors | Homocysteine within normal | Homocysteine above normal | Total | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | ||||
| Alcohol intake | Yes | 36 (30.5%) | 0 (0%) | 36 (30.5%) | 80 (67.8%) | 2 (1.7%) | 82 (69.5%) | 118 (12.2%) | 0.003 (t = 2.96) |
| No | 128 (15%) | 273 (32.1%) | 401 (47.1%) | 254 (29.8%) | 197 (23.1%) | 451 (52.9%) | 852 (87.8%) | ||
| Dietary habit | Veg | 1 (5.9%) | 4 (23.5%) | 5 (29.4%) | 7 (41.2%) | 5 (29.4%) | 12 (70.6%) | 17 (1.8%) | 0.075 (t = −1.784) |
| Non-veg | 163 (17.1%) | 269 (28.2%) | 432 (45.3%) | 327 (34.3%) | 194 (20.4%) | 521 (54.7%) | 953 (98.2%) | ||
| Smoking habit | Yes | 43 (37.1%) | 0 (0%) | 43 (37.1%) | 72 (62.1%) | 1 (0.9%) | 73 (62.9%) | 116 (12%) | 0.043 (t = 2.027) |
| No | 121 (14.2%) | 273 (31.9%) | 394 (46.1%) | 262 (30.7%) | 198 (23.2%) | 460 (53.9%) | 854 (88%) | ||
| Body weight | >55 kg | 134 (18.2%) | 175 (23.7%) | 309 (41.9%) | 286 (38.8%) | 143 (19.4%) | 429 (58.1%) | 738 (76.1%) | 0.004 (t = 2.915) |
| ≤55 kg | 30 (13%) | 98 (42.2%) | 128 (55.2%) | 48 (20.7%) | 56 (24.1%) | 104 (44.8%) | 232 (23.9%) | ||
Fig. 3.
Showing the relation of homocysteine with different life style factors
While comparing the mean homocysteine level between the smokers (19.9 ± 9) and nonsmokers (18.2 ± 8) we have found a statistically significant relationship (t = 2.027 and P = 0.043). The odds that a person who smokes having high homocysteine is 1.454 times more than the nonsmokers. In case of non-vegetarian the mean homocysteine level is 18.3 ± 8 and in case of vegetarian it is 23.5 ± 15. However, there is no statistically significant difference between means of the true groups. Performing Students’s t test we get, t = −1.784 and P = 0.075. The odds of having hyperhomocysteinemia in vegetarians is 1.990 times more than those of the non-vegetarians. So there is a probability that diet may have some role in producing hyperhomocysteinemia. The studied population is also evaluated by body weight and it is seen that 23.9% of the population have body weight ≤55 kg (mean homocysteine = 17.03 ± 7.16) and 76.1% have weight >55 kg (mean homocysteine = 18.84 ± 8.6). The mean homocysteine when compared between the two groups, it is found that there is a statistically significant difference between the means of the two groups (t = 2.915, P = 0.004).
On performing Chi-square test we find that there is a significant relationship between hyperhomocysteinemia and weight. Here Chi-square with 1 df Chi-square = 12.618 and P = 0.001. The odds that a person with more weight having high homocysteine is 1.709 times more than the persons whose weight is less.
To see the relation of individual risk factors with the homocysteine levels we perform the multiple linear regression analysis adjusted for age and gender and results are shown in Table 5. Weight shows the strongest significance. Drinking habit is also found to be one of the significant determinants of homocysteine.
Table 5.
Showing the relation of individual life style factors with homocysteine
| Variables | Corelation coefficient (β) | 95% confidence interval (CI) | Significance (P) | |
|---|---|---|---|---|
| Lower | Upper | |||
| Smoking habit | −0.035 | −0.121 | 0.037 | 0.300 |
| Drinking habit | −0.071 | −0.164 | −0.005 | 0.036 |
| Dietary habit | 0.059 | −0.011 | 0.364 | 0.064 |
| Weight | 0.090 | 0.001 | 0.006 | 0.006 |
We have also analyzed the cumulative effect of various life style factors on homocysteine level, but we do not found any statistically significant result (Table 6).
Table 6.
Showing the cumulative effect of the risk factors on homocysteine
| Variables | Mean | F | P |
|---|---|---|---|
| Alcoholic + >55 kg | 20.7 ± 10 | 0.273 | 0.602 |
| Alcoholic + Vegetarian | a | a | a |
| Alcoholic + Smoker | 20.4 ± 11.5 | 1.255 | 0.263 |
| Vegetarian + >55 kg | 23.7 ± 16.9 | 0.03 | 0.863 |
| Vegetarian + Smoker | 22.4 ± 3.8 | 0.232 | 0.630 |
| Smoker + >55 kg | 20.3 ± 9.8 | 0.271 | 0.603 |
| Alcoholic + >55 kg + Vegetarian | a | a | a |
| Alcoholic + >55 kg + Smoker | 20.9 ± 12 | 1.163 | 0.281 |
| Alcoholic + Vegetarian + Smoker | a | a | a |
| Vegetarian + Smoker + >55 kg | 19.7 | 0.496 | 0.481 |
| Alcoholic + Vegetarian + Smoker + >55 kg | a | a | a |
aThere is no such combinations
Discussion
The demographic scenario of Assam today presents a mosaic pattern which is the settling ground for many civilizations. From time immemorial different tribes and ethnic groups have lived in Assam. Before going into the intricate details of the study, it needs to be mentioned here that the conglomeration of the Assamese population which has been mentioned earlier is maintained even in this study. Coming back to the technicalities, the present study shows a very wide range of plasma homocysteine level in the community ranging from 2.7 to 79.1 μmol/l. Almost 55% of the population shows hyperhomocysteinemia and males (mean = 20.36 ± 8.7) are more affected than the females (mean = 16.37 ± 7.3). There is a significant relation of homocysteine and gender (P = 0.001). Similar findings were also observed in the Hordaland Homocyseine Studies conducted between 1995 and 2006 [12–18]. But we have not observed any direct relation of homocysteine with age. Hyperhomocysteinemia is more common in the age group of 41–60 years. Almost 56.8% of hyperhomocysteinemia is detected in the age group of 41–60 years. Other recent study also established that blood homocysteine level was higher in the older group than the younger group [19]. Another finding is that, in case of females the more affected age group is 41–50 years. That can be due to some hormonal affect as, sex steroid hormones (mainly oestrogen) which can modulate plasma homocysteine levels and that is why the post menopausal state has been found to be associated with a higher value [20].
The values of plasma homocysteine are analyzed by distributing them into four categories like Normal (5–15 μmol/l), Moderately high (16–30 μmol/l), Intermediate high (31–100 μmol/l) and Severely high (>100 μmol/l) as reported in some previous studies [21, 22]. And we have noticed that 89.1% of the hyperhomocysteinemia falls in the category of moderately high and rest 10.1% in the intermediate high. When we compare some of the life style factors like diet, alcohol intake, smoking habit and body weight with plasma homocysteine level, we have found that alcoholics, smokers and persons having more body weight are having significantly higher mean homocysteine than the nonalcoholics, nonsmokers and the persons having less body weight. Data on the determinants of homocysteine concentrations in Asian population are scarce and till date there is no community based study on homocysteine in this North Eastern Region [23–25]. When we compare our data with some recent studies of other region, we find that our findings do not differ much with that of those studies. Recently a study conducted on Korean people, established that upper-body fat distribution, hyperhomocysteinemia, and depletion of antioxidants were identified as important cardiovascular disease risk factors in addition to excessive alcohol consumption and cigarette smoking. They found that plasma homocysteine levels were higher in heavy drinker–heavy smoker than in nondrinker–nonsmoker [26, 27].
In our study when we analyze and compare the dietary habit with plasma homocysteine level of the community, we find that 70.6% of the vegetarian community shows hyperhomocysteinemia with a mean homocysteine of 23.5 μmol/l, in comparison to non-vegetarians (54.7% and mean of 18.3 μmol/l).Though it is not statistically significant, diet may have some role in producing hyperhomocysteinemia. Vegetarians are more likely to be deficient in vitamin B6, vitamin B12, and folate which are the essential cofactors for homocysteine metabolism. Recent evidence strongly suggests that too little vitamin B12 can lead to high level of homocysteine [28–33]. In our study, we have seen that weight and alcohol intake are statistically significant predictors of homocysteine (P = 0.006 and P = 0.036, respectively). It has been already established that the lifestyle factors most strongly associated with plasma total homocysteine were number of cigarette smoked and alcohol [34]. There is no significant effect of multiple risk factors on plasma homocysteine level, though individually most of them are significant. So the present study identified gender, tobacco and body weight as the important predictors of plasma total homocysteine level of the people of Assam.
Conclusion
The findings of this population based, 3 years study, suggest that the prevalence of hyperhomocysteinemia is high in this population sample showing a mean of 18.41 μmol/l. Elderly group (41–60 years) and males are more affected. The findings also suggest that some of the life style factors have role in developing hyperhomocysteinemia and needs to be further looked into for its association or propensity to developing stroke and coronary disease, as a number of investigations also support the theory that the elevated plasma homocysteine is associated with occlusive vascular disease. From a public health viewpoint, it is important to identify the modifiable factors that influence plasma total homocysteine levels and at the same time to increase the public’s awareness that feasible changes in life style may favorably alter plasma total homocysteine levels, which in turn might reduce the risk of cardiovascular and cerebrovascular diseases.
References
- 1.Gheye S, Lakshmi AV, Krishna TP, Krishnaswamy K. Fibrinogen and homocysteine levels in coronary disease. Indian Heart J. 1999;51(5):499–502. [PubMed] [Google Scholar]
- 2.Bhargava S, Parakh R, Manocha A, Ali A, Srivastava LM. Prevalence of hyperhomocysteinemia in vascular disease: comparative study of thrombotic venous disease vis-a-vis occlusive arterial disease. Vascular. 2007;15(3):149–153. doi: 10.2310/6670.2007.00031. [DOI] [PubMed] [Google Scholar]
- 3.Biswas A, Ranjan R, Meena A, Akhter MS, Yadav BK, Munisamy M, et al. Homocysteine levels, polymorphisms and the risk of ischemic stroke in young Asian Indians. J Stroke Cerebrovasc Dis. 2009;18(2):103–110. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Seang-Mei S, Jian-Min Y Y, Choon-Nam O, Kazuko A, Hin-Peng L, Gerhard CA, et al. Genetic, dietary, and other lifestyle determinants of plasma homocysteine concentrations in middle-aged and older Chinese men and women in Singapore. Am J Clin Nutr. 2001;73(2):232–239. doi: 10.1093/ajcn/73.2.232. [DOI] [PubMed] [Google Scholar]
- 5.Egil A, Helga R, Kaare BH, Mahne UP, Olar FH, Jan NE. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995;24(4):704–709. doi: 10.1093/ije/24.4.704. [DOI] [PubMed] [Google Scholar]
- 6.Yoo JH, Chung CS, Kang SS. Relation of plasma homocysteine to cerebral infarction and cerebral atherosclerosis. Stroke. 1998;29(12):2478–2483. doi: 10.1161/01.str.29.12.2478. [DOI] [PubMed] [Google Scholar]
- 7.Graham IM, Daly LE, Refsum HM, Robinson K, Brattstrom LE, Ueland PM, et al. Plasma homocysteine as a risk factor for vascular disease. JAMA. 1997;277(22):1775–1781. doi: 10.1001/jama.277.22.1775. [DOI] [PubMed] [Google Scholar]
- 8.de Bree A, Monique Verschuren WM, Blom HJ, Kromhout D. Lifestyle factors and plasma homocysteine concentrations in a general population sample. Am J Epidemiol. 2001;154(2):150–154. doi: 10.1093/aje/154.2.150. [DOI] [PubMed] [Google Scholar]
- 9.Krishnaswamy K, Lakshmi AV. Role of nutritional supplementation in reducing the levels of homocysteine. J Assoc Physicians India. 2002;50(Suppl):5–8. [PubMed] [Google Scholar]
- 10.Schreiner R, Gobel-Schreiner B, Walch S. Homocysteine. Clin Lab. 1995;41:1007–1011. [Google Scholar]
- 11.Ueland PM, Refsum H. Plasma homocysteine, a risk factor for vascular disease: plasma levels in health, disease and drug therapy. J Lab Clin Med. 1989;114:473–501. [PubMed] [Google Scholar]
- 12.Refsum H, Nurk E, Smith AD, Peters J, Foresti R, Heaton N, et al. The Hordaland Homocysteine Study: a community based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136:1731S–1740S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- 13.Ueland PM, Nygard O, Vollset SE, Refsum H. The Hordaland Homocysteine Studies. Lipids. 2001;36:S33–S39. doi: 10.1007/s11745-001-0679-7. [DOI] [PubMed] [Google Scholar]
- 14.Nygard O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1526–1533. doi: 10.1001/jama.274.19.1526. [DOI] [PubMed] [Google Scholar]
- 15.Nurk E, Refsum H, Tell GS, Engedal K, Vollset SE, Ueland PM, et al. Plasma total homocysteine and cognition in the elderly. The Hordaland Homocysteine Study. Ann Neurol. 2005;58:847–857. doi: 10.1002/ana.20645. [DOI] [PubMed] [Google Scholar]
- 16.Guttormsen AB, Ueland PM, Nesthus I, Nygård O, Schneede J, et al. Determinants and vitamin responsiveness of intermediate hyperhomocysteinemia (40 μmol/l). The Hordaland Homocysteine Study. J Clin Invest. 1996;98:2174–2183. doi: 10.1172/JCI119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nygard O, Refsum H, Ueland PM, Vollset SE. Major lifestyle determinants of plasma total homocysteine distribution: the Hordaland Homocysteine Study. Am J Clin Nutr. 1998;67:263–270. doi: 10.1093/ajcn/67.2.263. [DOI] [PubMed] [Google Scholar]
- 18.Nurk E, Tell GS, Vollset SE, Nygard O, Refsum H, Nilsen RM, et al. Changes in lifestyle and plasma total homocysteine: the Hordaland Homocysteine Study. Am J Clin Nutr. 2004;79:812–819. doi: 10.1093/ajcn/79.5.812. [DOI] [PubMed] [Google Scholar]
- 19.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 20.Hak AE, Polderman KH, Westendrop ICD, Jakobs C, Hofman A, Witteman JCM, et al. Increased plasma homocysteine after menopause. Athersclerosis. 2000;149(1):163–168. doi: 10.1016/S0021-9150(99)00321-4. [DOI] [PubMed] [Google Scholar]
- 21.Kang SS. Hyperhomocysteinemia as a risk factor for occlusive vascular disease. Annu Rev Nutr. 1992;12:279–298. doi: 10.1146/annurev.nu.12.070192.001431. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB. Folate and vit B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–364. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 23.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2:160–166. doi: 10.1007/s11883-000-0111-3. [DOI] [PubMed] [Google Scholar]
- 24.Chacko KA. Plasma homocysteine levels in patients with coronary heart disease. Indian Heart J. 1998;50(3):295–299. [PubMed] [Google Scholar]
- 25.Sastry BK, Indira N, Anand B, Kedarnath, Prabha BS, Raju BS. A case control study of plasma homocysteine levels in South Indians with and without coronary artery disease. Indian Heart J. 2001;53(6):749–753. [PubMed] [Google Scholar]
- 26.Elsevier BV. Influence of alcohol consumption and smoking habits on cardiovascular risk factors and antioxidant status in healthy Korean men. Nutr Res. 2009;20(9):1213–1227. [Google Scholar]
- 27.Chrysohoou C. The associations between smoking, physical activity, dietary habits and plasma homocysteine levels in cardiovascular disease-free people: the “ATTICA” study. Vasc Med. 2004;9(2):117–123. doi: 10.1191/1358863x04vm542oa. [DOI] [PubMed] [Google Scholar]
- 28.Bissoli L, Francesco V, Ballarin A, Mandragona R, Trespidi R, Brocco G, et al. Effect of vegetarian diet on homocysteine levels. Ann Nutr Metab. 2002;46:73–79. doi: 10.1159/000057644. [DOI] [PubMed] [Google Scholar]
- 29.Kumar J, Das SK, Sharma P, Karthikeyan G, Ramakrishnan L, Sengupta S. Homocysteine levels are associated with MTHFR A1298C polymorphism in Indian population. J Hum Genet. 2005;50(12):655–663. doi: 10.1007/s10038-005-0313-1. [DOI] [PubMed] [Google Scholar]
- 30.Nair KG, Nair SR, Ashavaid TF, Dalal JJ, Eghlim FF. Methylenetetrahydrofolate reductase gene mutation and hyperhomocysteinemia as a risk factor for coronary heart disease in the Indian population. J Assoc Physicians India. 2002;50:9–15. [PubMed] [Google Scholar]
- 31.Chandalia M, Abate N, Cabo-Chan AV, Jr, Devaraj S, Jialal I, Grundy SM. Hyperhomocysteinemia in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88(3):1089–1095. doi: 10.1210/jc.2002-021133. [DOI] [PubMed] [Google Scholar]
- 32.Kumar J, Garg G, Sundaramoorthy E, Prasad PV, Karthikeyan G, Ramakrishnan L, et al. Vitamin B12 deficiency is associated with coronary artery disease in an Indian population. Clin Chem Lab Med. 2009;47(3):334–338. doi: 10.1515/CCLM.2009.074. [DOI] [PubMed] [Google Scholar]
- 33.Misra A, Vikram NK, Pandey RM, Dwivedi M, Ahmad FU, Luthra K, et al. Hyperhomocysteinemia, and low intakes of folic acid and vitamin B12 in urban North India. Eur J Nutr. 2002;41(2):68–77. doi: 10.1007/s003940200010. [DOI] [PubMed] [Google Scholar]
- 34.Panagiotakos D, Pitsavos C, Zeimbekis A, Chrysohoou C, Stefanadis C. The association between lifestyle related factors and plasma homocysteine levels in healthy individuals from the “ATTICA” study. Int J Cardiol. 2005;98(3):471–477. doi: 10.1016/j.ijcard.2003.12.036. [DOI] [PubMed] [Google Scholar]