Abstract
Objectives
To investigate the anti-kinetoplastid activity of choline-derived analogues with previously reported antimalarial efficacy.
Methods
From an existing choline analogue library, seven antimalarial compounds, representative of the first-, second- and third-generation analogues previously developed, were assessed for activity against Trypanosoma and Leishmania spp. Using a variety of techniques, the effects of choline analogue exposure on the parasites were documented and a preliminary investigation of their mode of action was performed.
Results
The activities of choline-derived compounds against Trypanosoma brucei and Leishmania mexicana were determined. The compounds displayed promising anti-kinetoplastid activity, particularly against T. brucei, to which 4/7 displayed submicromolar EC50 values for the wild-type strain. Low micromolar concentrations of most compounds cleared trypanosome cultures within 24–48 h. The compounds inhibit a choline transporter in Leishmania, but their entry may not depend only on this carrier; T. b. brucei lacks a choline carrier and the mode of uptake remains unclear. The compounds had no effect on the overall lipid composition of the cells, cell cycle progression or cyclic adenosine monophosphate production or short-term effects on intracellular calcium levels. However, several of the compounds, displayed pronounced effects on the mitochondrial membrane potential; this action was not associated with production of reactive oxygen species but rather with a slow rise of intracellular calcium levels and DNA fragmentation.
Conclusions
The choline analogues displayed strong activity against kinetoplastid parasites, particularly against T. b. brucei. In contrast to their antimalarial activity, they did not act on trypanosomes by disrupting choline salvage or phospholipid metabolism, instead disrupting mitochondrial function, leading to chromosomal fragmentation.
Keywords: Trypanosoma brucei, leishmaniasis, protozoan parasite, lipid metabolism, choline
Introduction
Kinetoplastid parasites of the genera Trypanosoma and Leishmania cause some of the most severe and widespread parasitic diseases of man and of livestock,1,2 but the drugs used currently to treat these diseases are mostly old, exhibit many side effects and are in many cases becoming obsolete because of resistance.3,4 The plasma membrane of the kinetoplastid parasites contains high levels of choline phospholipids and sphingomyelin (SM), and sphingolipid metabolism is essential in Trypanosoma brucei brucei,5,6 making choline salvage and/or metabolism potential drug targets.
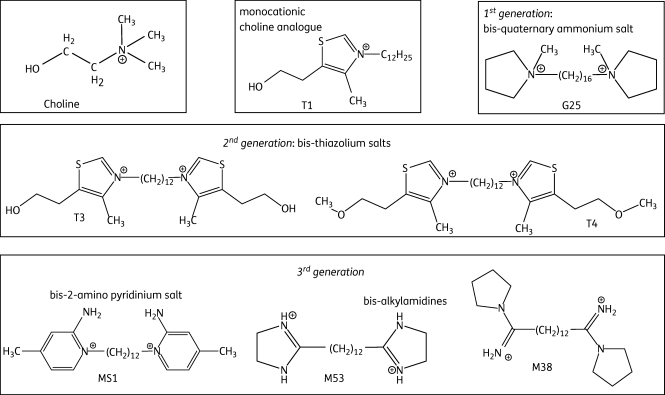
A library of more than 600 compounds derived from the choline scaffold has been systematically developed in recent years.7–10 Three generations of compounds were rationally designed to mimic the choline structure. In monoquaternary ammonium salts such as T1, cationic charges and the presence of a long lipophilic chain on the nitrogen atom appeared crucial for antimalarial activity (see Figure 1 for structures).9,11 However, bis-quaternary ammonium salts exhibited higher activities than mono-ammonium salts by two log scales.9 The first-generation bis-quaternary ammonium lead compound G25 showed powerful in vitro and in vivo antimalarial activity, but also exhibited some toxicity.12,13 In second-generation compounds, we replaced the alkyl-ammonium head with a thiazolium motif that is already present in vitamin B1, with the aim of reducing toxicity. The bis-thiazolium compounds T3 and T4 displayed the most promising activities against Plasmodium falciparum14,15 and T3 is now under clinical development against severe malaria. The third generation includes 2-amino pyridinium salts and bis-alkylamidines. Three derivatives—MS1, M38 and M53—have emerged as lead compounds.16 We determined the major structural motif that mediates the antiparasitic activity and delineated their specific features, notably concerning the CNS.17 Figure 1 summarizes the development of this compound series, which was developed primarily for antimalarial activity.7,9,10,12,18 However, the compounds have also been reported to possess activity against the related Babesia species.19 In the current report we investigate the anti-kinetoplastid activities of some of the most promising compounds from each of the first-, second- and third-generation choline-derived compounds in order to establish whether any of these classes displays sufficient activity to warrant a systematic structure–activity study.
Figure 1.
Chemical structures of choline and various choline-derived analogues.
The mode of action of these and similar choline analogues has been investigated previously in P. falciparum.19–22 Ancelin and Vial20 first demonstrated that some choline analogues inhibit biosynthesis of phosphatidylcholine (PC) in P. falciparum and showed that these compounds acted on the choline transporter of the infected erythrocytes.21,23 This led to impaired de novo PC biosynthesis and parasite death. However, a different compound, T16, enters infected erythrocytes through the so-called new permeation pathway,24 is taken up by the parasites through a choline transporter in the P. falciparum plasma membrane25 and subsequently accumulates in the P. falciparum digestive vacuole, where it binds ferriprotoporphyrin IX—a process critical for its antimalarial activity.24 Different classes of choline analogues thus appear to have distinct antimalarial modes of action. The quaternary ammonium compound G25 was shown to inhibit de novo PC biosynthesis and at higher concentrations also the decarboxylation of phosphatidylserine to phosphatidylethanolamine.22 A report on choline uptake in Leishmania major promastigotes noted that this process is inhibited by various commercially available choline analogues and that some of these display antileishmanial activity in vitro.26
Seven analogues, representing the most active representatives of the successive generations of choline-derived antimalarials (Figure 1), were investigated in this study for effects on kinetoplastid parasites. We found that the test compounds were generally much more active against T. b. brucei than against Leishmania mexicana and mostly concentrated our mode-of-action studies on the former parasite. Our data show that third-generation choline-derived compounds in particular show very considerable promise against kinetoplastid parasites, including multidrug-resistant T. b. brucei, and could be considered as broad-spectrum antiparasitic agents with low toxicity.
Materials and methods
Materials
Resazurin, Dulbecco's modified Eagle's medium, 0.25% trypsin–EDTA solution, diminazene aceturate, digitonin, heparin and choline chloride were obtained from Sigma. Penicillin/streptomycin, gentamicin, HO-minimal essential medium (HOMEM) and Schneider's Drosophila Medium were from Gibco. Heat-inactivated fetal calf serum (FCS) was purchased from Biosera. Propidium iodide (PI) and dichlorofluorescein were from Fluka. Troglitazone was from Biomol. [ring-3H]pentamidine (88 Ci/mmol) and [methyl-3H]choline chloride (83 Ci/mmol) were purchased from Amersham.
Cell lines and cultures
Three clonal lines of trypanosomes were used: T. b. brucei 427 wild-type (WT); TbAT1-knockout (KO) derived from WT;27 and the pentamidine-adapted clonal line TbAT1-KO B48.28 All were cultured in HMI-9 medium supplemented with 10% FCS and 2 mM β-mercaptoethanol (pH 7.4) at 37°C and 5% CO2. For transport assays, bloodstream trypanosomes were isolated from infected female Wistar rats (Harlan UK Ltd, Bicester, UK) as described previously.29 Promastigote forms of L. mexicana (MNYC/BZ/62/M379 strain) were cultured at 25°C in HOMEM (pH 7.4) with 10% FCS. Axenic cultures of L. mexicana amastigotes were maintained at 32°C and 5% CO2 in Schneider's Drosophila Medium with 20% heat-inactivated FCS, 0.3% gentamicin and 10 µL of 1.0 M hydrochloric acid per mL (pH 5.5). Human embryonic kidney (HEK) cells were cultured at 37°C and 5%–10% CO2 in Dulbecco's modified Eagle's medium supplemented with newborn calf serum (Gibco) and 1% of both l-Glutamax (200 mM, Gibco) and penicillin (10 000 units/mL)/streptomycin (10 000 µg/mL) solution (Gibco).
Test compounds
Test compounds (Figure 1) were dissolved in DMSO at 20 mM, except M53, which was dissolved in DMSO at 0.5 mM due to limited solubility.
Drug susceptibility assays
Drug susceptibility was assessed using Alamar blue, adapting the procedure developed by Räz et al.,30 as described previously.31 Assays were conducted in 96-well plates with wells containing 200 µL of the appropriate medium, with 1 × 105 cells/mL for T. brucei for 48 h or with 1 × 106 cells/mL for L. mexicana for 72 h, respectively, in the presence of the test compounds (doubling dilutions starting at 100 µM). An aliquot of 20 µL of Alamar blue solution (12.5 mg of resazurin/100 mL of PBS) was added to each well and plates were incubated for another 24 (T. brucei) or 48 (L. mexicana) h before analysis using a FLUOstar OPTIMA plate reader at 544 nm excitation and 590 nm emission.
Cell survival assays
Two different types of cell survival assays were performed in this study, using either a spectrophotometer-based assay or a PI/fluorescence assay. The former was performed using a UV/visible spectrophotometer (HP-8453, Hewlett Packard) exactly as described previously.28 For the reversibility assessment, at a predetermined incubation time the samples were spun at 2500 g for 10 min and resuspended in fresh medium, and incubated further in the presence or absence of the same concentration of test compound. The PI assay was performed using 96-well plates as described previously.32 Each well of a 96-well plate contained 1 × 106 cells, 9 µM PI and a variable concentration of test compound. The plates were incubated in a FLUOstar OPTIMA fluorimeter at 37°C with 5% CO2 atmosphere for bloodstream forms and at 25°C for promastigotes; fluorescence was monitored at 544 nm excitation and 620 nm emission.
Transport assays
Transport assays for [3H]pentamidine and [3H]choline by T. b. brucei bloodstream forms and L. mexicana promastigotes were performed exactly as described previously.33–35 Briefly, trypanosomes isolated from blood taken from infected rats or promastigotes from a culture were washed twice in assay buffer (33 mM HEPES, 98 mM NaCl, 4.6 mM KCl, 0.3 mM CaCl2, 0.07 mM MgSO4, 5.8 mM NaH2PO4 and 14 mM glucose, pH 7.3) and then resuspended at 108 cells/mL. Cells (100 µL) were then incubated, in 1.5 mL microfuge tubes containing 300 µL of an oil mixture, with 100 µL of radiolabel in the presence or absence of potential effectors. Transport was terminated by adding 1 mL of ice-cold stop solution followed by rapid centrifugation through the oil layer. Radioactivity in the cell pellet was determined using a scintillation counter. All experiments were performed in triplicate.
Flow cytometry
T. b. brucei bloodstream forms were incubated in HMI-9 medium (10 mL, 37°C, 5% CO2) with various concentrations of test compounds for up to 72 h. At different timepoints, 1 mL was taken from each culture for assessment of DNA content, cellular permeability and mitochondrial membrane potential (Ψm).
DNA content was assessed as described previously.36,37 Briefly, 1 mL of treated and non-treated T. brucei bloodstream forms (2 × 106 cells) was centrifuged at 1000 g for 10 min at 4°C. The pellet was resuspended and fixed in 1 mL of 70% methanol and 30% PBS (pH 7.4) and left at 4°C overnight. The cells were washed twice with 1 mL of PBS by spinning at 2500 g for 10 min at 4°C, and resuspended in 1 mL of PBS containing PI and RNAse (Sigma), both at 10 µg/mL. The samples were incubated in the dark at 37°C for 45 min and analysed with a Becton Dickinson fluorescence-activated cell sorter (FACSCalibur) using the FL2-Area detector.
Cellular permeability was measured as described previously38,39 using PI. One millilitre of treated and untreated cells (2 × 106 cells) was taken and spun at 2500 g for 10 min at 4°C and resuspended in the same volume of HMI-9/FCS medium containing PI. After 10 min in the dark the samples were analysed with the FACSCalibur using 6 µM digitonin as a positive control.
The Ψm was evaluated using tetramethylrhodamine ethyl ester (TMRE).40,41 Following incubation of bloodstream trypanosomes with and without test compounds for a predetermined time, 1 mL of cells (106 cells) was centrifuged for 10 min at 2500 g and washed once in PBS. The pellet was resuspended in 1 mL of PBS containing 25 nM TMRE and incubated at 37°C for 30 min. Valinomycin (100 nM) and troglitazone (10 µM) were used as positive controls. The samples were analysed by flow cytometry using the FL2-Height detector.
Measurement of reactive oxygen species (ROS)
This assay was performed using black-bottomed 96-well plates; 200 µL of test compounds, at twice the final concentration, were added to the wells in the first column in the plate. Then 100 µL of PBS, pH 7.4, was added to all wells and doubling dilutions of the test compound were performed, after which 2 × 105 trypanosomes in 100 µL of PBS were added to each well, to which also 2 µL of 1 mM dichlorofluorescein was immediately added in the dark. The plates were incubated in a FLUOstar OPTIMA fluorimeter at 37°C, and the fluorescence was monitored at 485 nm excitation and 520 nm emission. Four wells were included in each plate as controls: (i) 2 × 105 cells in PBS without test compound; (ii) 2 × 105 cells in PBS incubated with 100 µM H2O2; (iii) PBS without cells or test compound; and (iv) 100 µM H2O2 in PBS without cells.
Effect on lipid content in T. brucei
Lipids were extracted according to the method of Bligh and Dyer42 and subsequently processed according to Richmond et al.43 Samples were loaded into thin-wall nanoflow capillary tips (Waters) and analysed by electrospray mass spectrometry (ES-MS) in both positive and negative ion modes using a capillary voltage of 0.9 kV and cone voltages of 50 V. The choline-containing phospholipid species PC and SM were observed in positive ion mode, using parent-ion scanning of m/z 184; 45 V. Peaks were annotated based on their [M + HNMe3(+)]+, [M-140] and [GPA-H] daughter ion derivatives, respectively, and compared with that of their theoretical values and previous analyses.43,44 In negative ion mode, all other glycerophospholipids were detected by parent-ion or neutral loss scanning as described previously.45 Annotation of all phospholipids was also based upon comparison with their theoretical values and other ES-MS and electrospray tandem mass spectrometry (ES-MS-MS) analyses conducted on whole cell extracts.43 Each spectrum encompassed at least 50 repetitive scans.
Analysis of intracellular calcium response
Intracellular calcium concentrations were assayed using the Screen Quest™ Fluo-8 Calcium Kit (ABD Bioquest) following the manufacturer's instructions. T. b. brucei bloodstream forms were grown for 48 h in HMI-9/10% FCS, at 37°C and 5% CO2. The cells were spun at 2500 g for 10 min at 4°C, resuspended to a final density of 4 × 106 cells/mL in Fluo-8 loading solution and incubated at 37°C for 30 min. The cells were then washed twice with assay buffer to remove any extracellular dye, and 90 µL, containing 4 × 105 cells, was added to each well of a black-bottomed 96-well plate. The plate was incubated in a FLUOstar OPTIMA fluorimeter at 37°C and fluorescence was recorded for 2 min at 485 nm excitation and 520 nm emission, at which point test compounds were added (10 µL at 10× concentration in assay buffer containing ≤10% DMSO). The fluorescence was then recorded for a further 250 cycles of 4 s each. Calcium ionophore A23187 (Sigma) at 10 µM served as a positive control; 10 µL of assay buffer was used as a negative control. The experiment was repeated with additional aliquots from the same cell culture, loaded with Fluo-8 for 30 min directly before recording fluorescence, but after a total time of exposure to the choline analogues of 4 or 8 h. Cell counts were performed and densities were adjusted for each culture before dye loading to ensure exactly the same cell densities for all groups.
Analysis of cyclic adenosine monophosphate (cAMP) levels in trypanosomes
The effect of compound MS1 on the cAMP level in T. b. brucei 427 was investigated using the Direct Cyclic AMP Enzyme Immunoassay Kit (Assay Designs, USA). Briefly, trypanosomes at a final concentration of 5 × 106 cells/mL were incubated with 0.15 µM MS1 for 15 min. The mixture was centrifuged to pellet the cells and the cAMP level in the cell pellet was determined following the manufacturer's instructions. Cells with no exposure to drug but treated similarly were used in parallel and used as a negative control. Each experiment was performed in duplicate and the results presented are from two independent experiments.
Data analysis
Assay results were analysed using the GraphPad Prism 4 software package using non-linear regression. All transport experiments were performed in triplicate, and kinetic parameters and IC50 values are presented as the means and SEM of at least three independent experiments. Data obtained by flow cytometry were analysed using CELLQuest; 10 000 cells were analysed per run and the percentage of cells in each cell cycle phase was determined after excluding cell autofluorescence and cell debris.
Results
In vitro efficacy of choline-derived analogues
EC50 values were determined for the trypanocidal and leishmanicidal effects of the choline-derived analogues using the Alamar blue assay.31,46 All experiments were performed at least four times for each test compound; mean values and SEM are shown in Table 1.
Table 1.
Effective concentration (EC50) of choline compounds for various kinetoplastid parasites evaluated by Alamar blue assay
| EC50 (µM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | T. brucei 427 WT | T. brucei TbAT1-KO | T. brucei B48 | L. mexicana (promastigote) | L. mexicana (amastigote) | P valuea | HEK EC50 (µM) | SI (427) | SI (amastigotes) |
| T1 | 1.8 ± 0.1 | 2.2 ± 0.5 | 2.3 ± 0.2 | 0.28 ± 0.08 | 1.7 ± 0.4 | <0.02 | 42 ± 4 | 23 | 25 |
| T3 | 7.9 ± 2 | 11 ± 2 | 10 ± 0.9 | 33 ± 3 | 38 ± 10 | NS | >600 | >76 | >16 |
| T4 | 0.61 ± 0.2 | 2.0 ± 0.9 | 2.5 ± 0.2b | 6.9 ± 0.5 | 13 ± 4 | NS | >500 | >820 | >39 |
| M38 | 0.17 ± 0.06 | 0.28 ± 0.1 | 0.23 ± 0.01 | 0.95 ± 0.1 | 2.9 ± 0.9 | <0.05 | 250 ± 50 | 1470 | 86 |
| G25 | 0.20 ± 0.04 | 0.37 ± 0.1 | 0.34 ± 0.03 | 2.1 ± 0.3 | 4.8 ± 1 | NS | 360 ± 100 | 1800 | 75 |
| MS1 | 0.13 ± 0.02 | 0.25 ± 0.04 | 0.18 ± 0.01 | 0.42 ± 0.03 | 3.0 ± 0.6 | <0.01 | 65 ± 9 | 500 | 22 |
| M53 | 2.6 ± 0.7 | 4.0 ± 0.2 | 4.7 ± 0.6 | NE, 5 µM | 3.4 ± 1.7 | ND | >10c | >3.8 | >2.9 |
| Pentamidine | 0.0021 ± 0.0002 | 0.0079 ± 0.0011 | 0.27 ± 0.02 | 2.9 ± 0.5 | 14 ± 3 | NS | ND | ND | ND |
| Diminazene | 0.072 ± 0.024 | 1.3 ± 0.3 | 0.53 ± 0.11 | ND | ND | ND | ND | ND | ND |
NS, not significant; ND, not determined; NE, no effect at indicated concentration; SI, in vitro selectivity index, defined as EC50 (HEK)/EC50 (parasite).
Values for HEK cells are averages ± SEM of three independent experiments; EC50 values for activity against the parasite strains are averages ± SEM of four to five determinations.
aComparison of activities against cultures of promastigotes and amastigotes, using Student's t-test.
bSignificant difference between T. brucei 427 WT and B48 strains (P < 0.01).
cM53 was not sufficiently soluble to obtain data above 10 µM.
The antiparasitic activity of the seven choline analogues was evaluated against three clonal strains of T. b. brucei with increasing drug resistance profile: drug-susceptible 427 WT; the 427-derived TbAT1-KO (strongly resistant to some diamidines and mildly resistant to others and to arsenicals);27 and the pentamidine-adapted B48 clonal line derived from TbAT1-KO (strongly resistant to diamidines and arsenicals).28 Four out of seven compounds displayed submicromolar activity against all three T. brucei strains and no cross-resistance to the existing trypanocides pentamidine and diminazene was observed (Table 1), the sole exception being reduced activity of T4 against B48 (P < 0.01). Compound MS1 performed the best against trypanosomes, with an EC50 value of 0.13 ± 0.02 µM for WT trypanosomes. Both MS1 and M38 performed better than pentamidine against the multidrug-resistant line B48. For WT trypanosomes, promastigotes and amastigotes, statistically identical EC50 values were obtained using a PI-based assay recently described by Gould et al.32 (data not shown).
The choline-derived analogues tested here were generally less active against promastigote and axenic amastigote L. mexicana than against T. b. brucei (Table 1). The compounds were more active against the promastigote than against the axenic amastigote form. Very similar results were obtained with L. major promastigotes (data not shown). With the exception of T3 and T4, all the test compounds performed better against L. mexicana promastigotes and amastigotes than the clinically used treatment pentamidine (Table 1). Representative results of the Alamar blue assays are available in Figure S1 (available as Supplementary data at JAC Online).
The toxic effect of up to 400 µM choline-derived analogues on HEK cells was evaluated after an incubation time of 40 h, using the Alamar blue method. The compounds showed much lower levels of toxicity to human cells than to kinetoplastid parasites; for T3, T4 and M53 no EC50 value could be determined up to the limit of solubility or assay conditions (Table 1). Several of the test compounds thus display a very promising selectivity index (SI) [EC50 (human cells)/EC50 (parasite)]. M38 and G25, in particular, displayed excellent selectivity against trypanosomes and Leishmania promastigotes, but the SI against L. mexicana amastigotes was lower.
Effect of lead compounds on cellular integrity
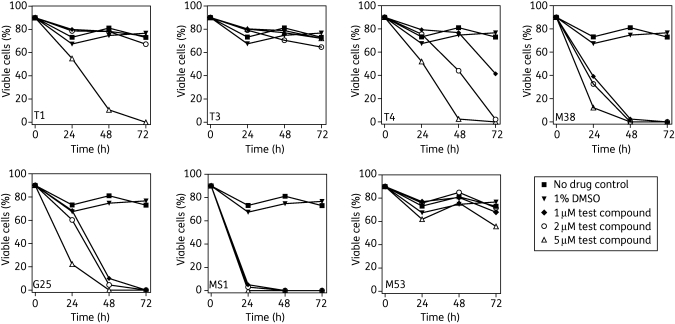
The permeability of the plasma membrane was assessed in cultures containing 2 × 106 cells/mL incubated in the presence or absence of 1, 2 or 5 µM of each compound. PI was added to samples at 4, 24, 48 and 72 h and fluorescence was measured by flow cytometry. Digitonin (6 µM), which rapidly induces 100% plasma membrane permeability (data not shown), was used as a positive control. None of the compounds displayed a noticeable effect on plasma membrane permeability after a 4 h incubation (data not shown). Figure 2 summarizes the results at 24, 48 and 72 h for all seven choline analogues, plotting the percentage of non-permeable (‘intact’) cells against incubation time. The percentage of intact cells was defined as the percentage of cells with one or two nuclei with apparently normal DNA content in the digitonin-treated population minus the percentage of such cells in the non-digitonized population.
Figure 2.
Flow cytometry analysis of cellular integrity of T. brucei treated with choline analogues. Cells incubated with the compounds for 24, 48 or 72 h were stained with 5 µg/mL PI and analysed by flow cytometry for cellular integrity as described in the Materials and methods section. Ten thousand cells were counted and those stained with PI were taken to have lost membrane integrity. Data are presented as percentages of cells with intact cell membranes (non-stained population).
T3 displayed very little effect on T. brucei permeability at concentrations up to 5 µM (Figure 2). T1 also displayed only a marginal effect up to 72 h at 1 or 2 µM, but displayed a much greater effect at 5 µM. Compounds T4, M38 and G25 similarly displayed dose-dependent and time-dependent effects on permeability to PI. At the lowest concentration of 1 µM, MS1 permeabilized almost all trypanosomes within 24 h, reflecting its low EC50 value of 0.13 µM.
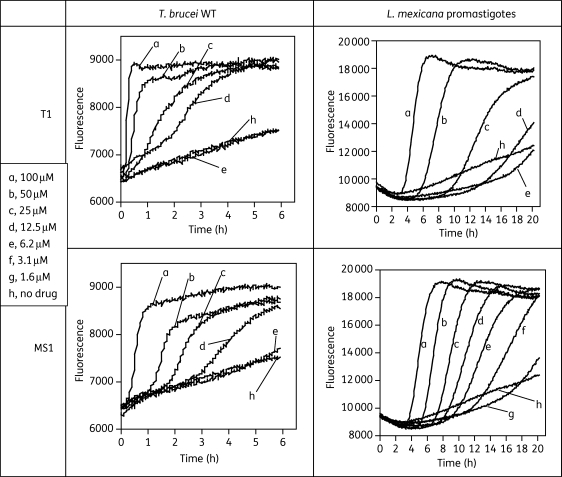
Real-time cell permeability assays
Because the above results show that even a modest concentration of MS1 dramatically increases the permeability of the T. brucei cell membrane within 24 h, presumably reflecting reduced parasite viability, this was further investigated using real-time monitoring of fluorescence. Concentrations of 100, 50, 25 and 12.5 µM T1 killed T. brucei within 15, 30, 90 and 180 min, respectively, and correspondingly within 30, 90, 150 and 270 min, respectively, for MS1 (Figure 3). M38 and G25 showed trypanocidal activity only at 100 and 50 µM, after 3 and 4 h, respectively (data not shown). From Figures 2 and 3 it thus appears that this class of compound is capable of rapidly affecting T. brucei plasma membrane integrity in a concentration- and time-dependent manner. The same protocol was also used to measure dose-dependent plasma membrane permeation in promastigotes of L. mexicana, in which T1 and MS1 showed activity similar to that in T. b. brucei (Figure 3).
Figure 3.
Effects of T1 and MS1 on plasma membrane integrity of T. brucei WT and L. mexicana promastigotes. Cells were cultured in the appropriate medium and treated with the indicated drugs at different concentrations in the continuous presence of PI at 9 µM. Fluorescence was monitored in real time at 544 nm excitation and 620 nm emission.
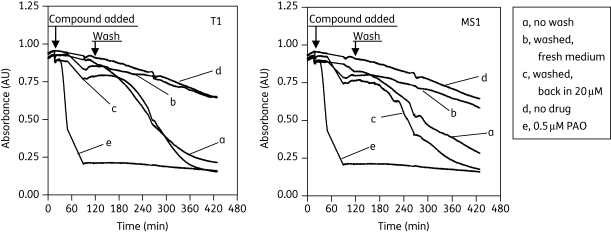
A light scatter assay was also used to explore the minimal duration of contact for compounds T1 and MS1 required for a non-reversible trypanocidal effect. This assay is based on the ability of the flagellated trypanosomes to scatter light; the effect of incubation with an agent that reduces motility or viability is visualized as reduced absorption in a spectrophotometer. Cells were incubated with these compounds and absorbance was monitored for up to 120 min; the cells were then washed twice in fresh HMI-9 medium and incubated for a further 5 h with or without drugs. The results in Figure 4 show that incubation with T1 or MS1 did not affect cell viability when the cells were subsequently resuspended in fresh medium without drug. The tentative conclusion from this result is that the trypanosomes must be continuously exposed to these test compounds until cell death occurs, or at least for a protracted amount of time.
Figure 4.
Reversibility of drug effect on T. brucei bloodstream forms with 20 µM T1 and MS1. Drugs were added to cells suspended at 5 × 107 cells/mL in HMI-9 medium after 15 min of recording and absorbance was monitored for another 2 h at 750 nm. Cells were then washed twice and resuspended in fresh medium and monitored again for another 5 h. Traces: a, no wash; b, cells washed after 2 h and resuspended in fresh medium; c, cells washed after 2 h and resuspended in fresh medium with 20 µM compound; d, no drug control; and e, 0.5 µM phenylarsine oxide (PAO).
Choline uptake in T. brucei and L. mexicana
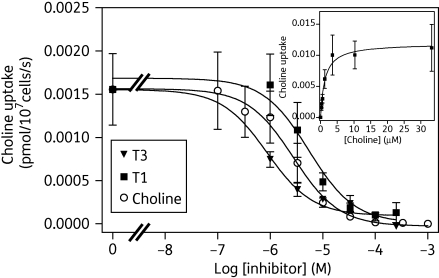
We investigated choline transport by the two kinetoplastid species. In bloodstream-form trypanosomes, we could not detect any mediated choline uptake; transport of 0.25 µM [3H]choline, monitored over 60 s, was not statistically different from zero (linear regression; P = 0.44, F-test) and was not inhibited by 1 mM unlabelled choline (Figure S2A, available as Supplementary data at JAC Online). This suggests that T. b. brucei bloodstream forms do not express a choline transporter, in keeping with previous stable isotope labelling with D9-choline.43 In contrast, uptake of the same concentration of [3H]choline in L. mexicana promastigotes was rapid and highly significant, with a rate of 0.0069 ± 0.0001 pmol (107 cells)−1 s−1 over the first 20 s (linear regression; r2 = 0.999; P < 0.0001, F-test), and fully saturable in the presence of 1 mM unlabelled choline; uptake under those conditions was not statistically different from zero (linear regression; P = 0.79, F-test) (Figure S2B, available as Supplementary data at JAC Online). Using an incubation period of 10 s, consistently within the linear phase of uptake (n = 4), we determined a Km value of 0.49 ± 0.2 µM and Vmax of 0.11 ± 0.04 pmol (107 cells)−1 s−1 for choline uptake in these cells (n = 4) (Figure 5, inset). Transport of [3H]choline in L. mexicana promastigotes, assessed after 10 s of radiolabel incubation, was dose-dependently inhibited by T1 and T3 (Figure 5). These two compounds were effective inhibitors of [3H]choline transport, with average Ki values of 4.0 ± 0.3 and 0.41 ± 0.1 µM (n = 3), respectively.
Figure 5.
Effects of T1 and T3 on [3H]choline uptake by L. mexicana promastigotes. Uptake of 0.25 µM [3H]choline was determined in the presence or absence of various concentrations of unlabelled choline, T1 and T3. Inset shows conversion of the inhibition curve with unlabelled choline into a Michaelis–Menten plot, revealing a Km value of 1.3 µM and a Vmax of 0.012 pmol (107 cells)−1 s−1 for the experiment shown. The data shown are representative of three similar experiments, each performed in triplicate; bars indicate SEM of the internal replicates.
T. b. brucei pentamidine transporters do not mediate uptake of choline-derived analogues
As T. b. brucei lacks choline transporters, we investigated alternative uptake modes for this class of compound. The best characterized drug transporters in T. brucei are the high-affinity pentamidine transporter (HAPT1) and the low-affinity pentamidine transporter (LAPT1), which are implicated in the uptake of diamidine drugs such as pentamidine.28,33,47 With the exception of the monocation T1, the compounds are structurally similar to pentamidine in that they are dications in which the positively charged end groups are joined through a flexible aliphatic linker chain.17 In order to determine whether these compounds can interfere with the diamidine transporters in T. brucei, pentamidine uptake assays were performed using [3H]pentamidine at 40 nM for HAPT1 and 1 µM for LAPT1, as their respective Km values are 36 nM and 56 µM,33 allowing the two transporters to be studied in virtual isolation.27,28,33 G25, T4 and M38 did not inhibit [3H]pentamidine uptake at the concentrations at which they are pharmacologically active, and are poor substrates of T. brucei diamidine transporters. Ki values are given in Table S1 (available as Supplementary data at JAC Online).
Effect of choline-derived analogues on the cell cycle
To investigate whether the analogues have an effect on the cell cycle, the DNA content was measured, with emphasis on the ratio between cells with one diploid set of chromosomes (2C; G1 phase of cell cycle) and those with a double set (4C; G2/M phase of cell cycle), as well as on the appearance of any cells with higher chromosome copy numbers (>4C; polyploids).
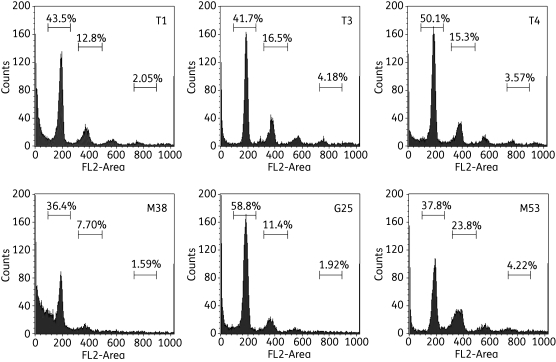
The results show no clear effect of any of the tested compounds on chromosome copy number after 8 and 24 h of incubation (Table S2, available as Supplementary data at JAC Online). The proportion of cells in G1 and G2/M phases did not change over a 24 h incubation time, with gated cells 60%–70% in G1 and 20%–30% in G2/M phases, even at the highest concentration of compound tested (10 µM). None of the 24 h incubations with choline analogues led to a significant proportion of cells with more than a 4C amount of DNA. Cells that contained <2C DNA content were excluded from the calculation of the 2C/4C ratios. DNA damage was particularly pronounced after 24 h of incubation with 5 or 7.5 µM M38 (Figure 6), although 10 µM T1 also produced very substantial DNA degradation at 24 h (data not shown).
Figure 6.
Analysis of DNA content in T. b. brucei bloodstream forms incubated with the indicated compounds for 24 h. Flow cytometry analysis of T. b. brucei 427 WT after incubation with 7.5 µM of the indicated choline test compounds. Cells were permeabilized with digitonin and stained with the fluorescent nucleic acid stain PI after treatment with RNAse. The main peak at ∼200 units represents cells with a normal diploid DNA content (2C), whereas the peak at 400 units represents cells with double DNA content, in the G2/M phase of the cell cycle (4C). Peaks with higher fluorescence represent abnormal cells with higher ploidy and cells with lower fluorescence appear to show DNA degradation and/or fragmentation;40,41 this is especially clear for cells treated with M38. The data are representative of multiple similar experiments with various incubation times and compound concentrations.
Effect on plasma membrane permeability and chromosomal integrity
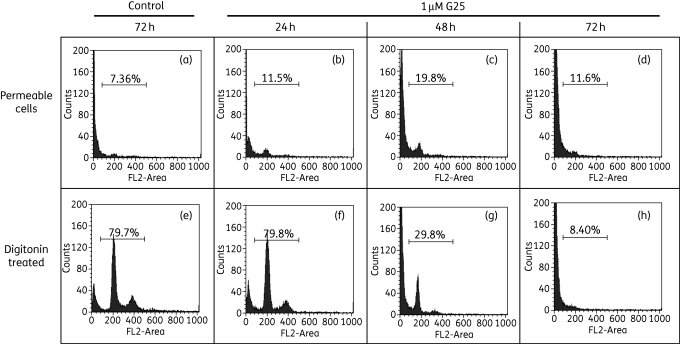
Being derivatives of choline with long alkyl chains, reminiscent of lipid molecules, the compounds were tested for whether they displayed any detrimental effect on the integrity of the plasma membrane using flow cytometry with the nucleic acid dye PI, to which intact membranes are impenetrable. If increased permeability of the plasma membrane was an early feature of treatment with the compounds, flow cytometry would have displayed a classical 2C/4C ratio, as seen in Figure 6. Instead, Figure 7 illustrates that treatment with 1 µM G25 progressively visualizes DNA degradation over 72 h, but not permeable cells containing intact DNA. Digitonin (6 µM; 10 min) was added to parallel samples to visualize the DNA content of the cells. At 24 h, 79.8% of cells treated with G25 displayed normal DNA content (Figure 7f), but almost none of these was permeable (Figure 7b). At 48 h only 29.8% of cells were still displaying a 2C or 4C fluorescence (Figure 7g), which again did not show up without digitonin treatment (Figure 7c); at 72 h of treatment only cells with highly degraded DNA were apparent, all of which were permeable to PI. These results clearly establish that G25 causes widespread DNA degradation prior to cell death and plasma membrane permeation. Very similar results were obtained with the other compounds (Figure S3, available as Supplementary data at JAC Online).
Figure 7.
Analysis of cellular permeability of T. brucei 427 WT after treatment with G25. Parasites treated with 1% DMSO (v/v) or 1 µM G25 were stained for 10 min with 5 µg/mL PI and analysed by flow cytometry. Samples from the same culture were either analysed directly (top row) or additionally treated with 6 µM digitonin for 10 min prior to analysis. The data shown are representative of three independent experiments with three different concentrations of all seven choline compounds (see Figure S3).
Effects of choline-derived analogues on T. brucei bloodstream-form lipid content
The major T. brucei phospholipid classes and their molecular species from total lipid extracts were characterized using nano-ES-MS-MS after treatment with the choline analogues at high concentrations (see legend to Figure 8) for approximately one generation (8 h). The concentration chosen was the highest concentration of test compound that does not affect cellular viability at 8 h (as assessed in earlier sections, above). Cymelarsan, a water-soluble analogue of the arsenic-based trypanocide melarsoprol, was used as a control trypanocidal compound that does not act on lipid metabolism.
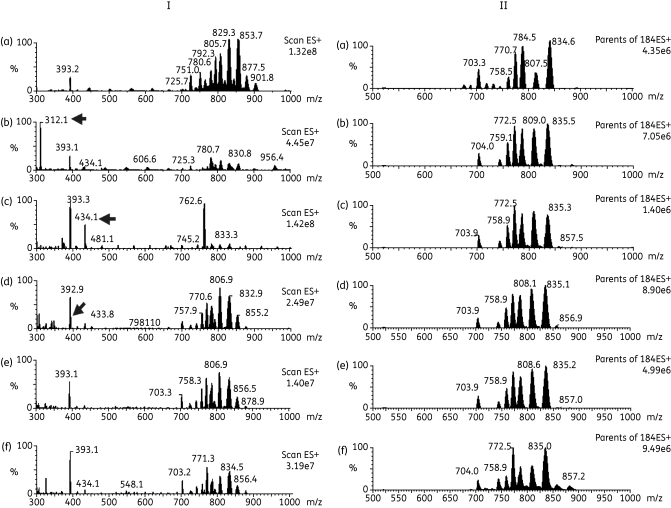
Figure 8.
Positive survey ES-MS (I) and parents of 184 m/z by ES-MS-MS (II) scans of lipid fraction of untreated and treated T. b. brucei. Lipids extracted from untreated T. b. brucei (a) or treated with 7.5 µM T1 (b), 10 µM M38 (c), 10 µM G25 (d), 5 µM MS1 (e) or 0.05 µM cymelarsan (f). (I) Samples were analysed by positive ion mode ES-MS (300–1000 m/z) and arrows indicate the presence of the potential inhibitor that has been co-extracted with the lipids. (II) Samples were analysed by parent ion scanning of 184 m/z ES-MS-MS for PC-containing phospholipids (600–1000 m/z).
The positive ion survey spectrum (300–1000 m/z) of untreated WT T. brucei [Figure 8a (I)] shows multiple molecular phospholipid species between 650 and 950 m/z. The corresponding positive ion survey spectra of cells treated with the test compounds T1, M38, G25 and MS1 [Figure 8b–e (I), respectively] initially look different from untreated cells [Figure 8a (I)]. However, most of these differences can be attributed to the presence of additional molecular species in the lipid extract samples, which ionize better than the phospholipids, resulting in the relative suppression of phospholipid ionization and thus their relative signal; this is an artefact known as ion suppression. For instance, this occurs in Figure 8(b) (I), where the positively charged molecular ion of T1 can clearly be observed at 312 m/z, in Figure 8(c) (I), where the positively charged molecular ion of M38 can clearly be observed at 434 m/z and in Figure 8(d) (I), where the positively charged molecular ion of G25 can be observed at 395 m/z, very close to the background non-related ion at 393 m/z (observed in all samples). No positively charged ions are observed for MS1 or cymelarsan [Figure 8e and f (I)].
As the analogues were suspected to interfere with the metabolism of PC and SM, these were further analysed by positive ion precursor scanning for m/z 184 (phosphocholine) to detect all PC and SM [M+H]+ ions. Numerous PC and SM molecular species were observed in a total lipid extract of untreated bloodstream-form 427 WT [Figure 8a (II)]. Annotation of these species is provided in Richmond et al.43 The relative composition of the choline-containing lipid species in untreated cells was remarkably similar to the lipid composition in cells treated with potential inhibitors [compare Figure 8a (II) with Figure 8b–f (II)].
Negative ion survey spectra (600–1000 m/z) and ES-MS-MS analysis of all of the other major phospholipid classes—phosphatidylinositol, phosphatidylserine, phosphatidylethanolamine and phosphatidylglycerol—were conducted in both untreated and treated parasites. These analyses show that there were no significant changes in lipid metabolism of the parasite after 8 h of incubation with maximum tolerated concentrations of the choline-derived analogues.
Effect of choline-derived compounds on Ψm
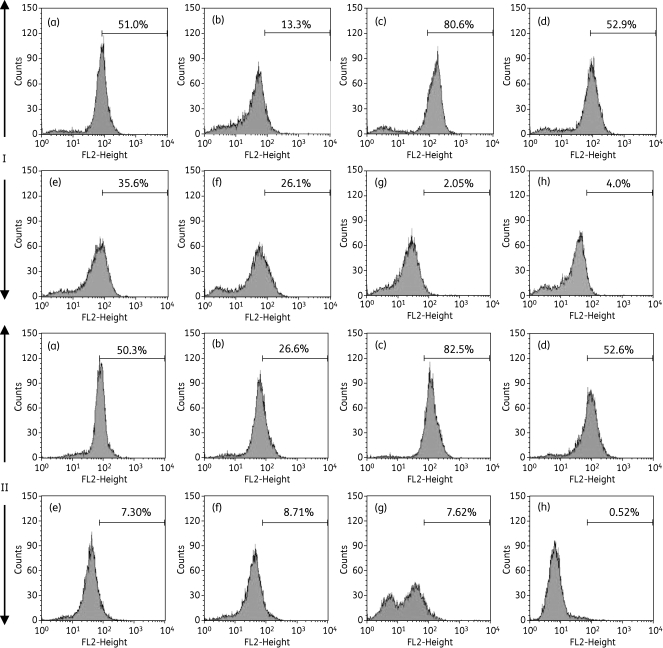
Bloodstream forms of T. brucei were cultured for up to 24 h in the presence and absence of 2 or 5 µM of the compounds, stained with TMRE and analysed by flow cytometry for analysis of Ψm. Valinomycin was used as the control for depolarization and troglitazone as the control for hyperpolarization.41 Figure 9 depicts histograms of TMRE fluorescence, with Figure 9(a) (I) and Figure 9(a) (II) displaying the control cells, incubated without test compound for 8 and 16 h, respectively. Values are given as the percentage of cells with fluorescence above 100 arbitrary units (AU), which was approximately 50% for the controls. A shift to higher fluorescence signifies increased Ψm; lower fluorescence signifies depolarization of the mitochondrial membrane.41
Figure 9.
Effect of choline-derived analogues on Ψm of T. brucei bloodstream forms. Effects on Ψm were measured by flow cytometric analysis of parasites loaded with 25 nM TMRE. Control (a) and treated cells [(d) 5 µM T1; (e) 5 µM T4; (f) 2 µM M38; (g) 5 µM G25; (h) 2 µM MS1] were harvested at (I) 8 and (II) 16 h and prepared for Ψm analysis as described in the Materials and methods section. Positive controls were performed with 100 nM valinomycin (b) and 10 µM troglitazone (c) to decrease and increase the Ψm, respectively.
There was no effect on Ψm with 5 µM of compounds T1 [Figure 9(d) (I) and Figure 9(d) (II)], T3 and M53 (not shown). In contrast, 5 µM T4, 2 µM M38, 5 µM G25 and 2 µM MS1 each caused a clear decrease in Ψm within 8 h of incubation [Figure 9e–h (I)]. These compounds clearly reduced the proportion of cells with fluorescence >100 AU, from ∼50% at t = 0 h to 35.6%, 26.1%, 2.1% and 4.0%, respectively, at 8 h. The mitochondrial membrane was completely depolarized after 16 h by T4 (7.30%) and M38 (8.71%). Cells incubated with G25 for 16 h displayed two fluorescence peaks, that at approximately 50 AU representing cells with a highly depolarized mitochondrial membrane, and the peak below 10 AU possibly representing dead cells.
Effects of choline-derived compounds on intracellular calcium levels and cAMP
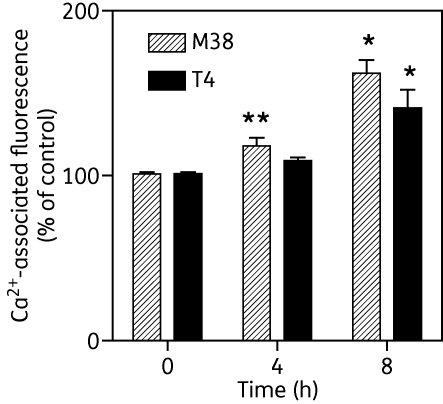
It was speculated that the depolarization of the mitochondrial membrane may lead to the release of Ca2+ from mitochondria as these organelles have a large capacity for Ca2+ sequestration, a process that is driven by Ψm.48,49 We therefore tested the effect of 20 µM of the choline test compounds on intracellular levels of Ca2+ after loading T. b. brucei 427 WT bloodstream forms with Fluo-8. MS1, G25, M38, T1, T3 and T4 did not elicit any calcium response over a period of 15 min, but the positive control, 10 µM of the standard calcium ionophore A23187, induced a massive increase in calcium-associated fluorescence (Figure S4, available as Supplementary data at JAC Online). Nor did we find any evidence that any of these compounds induced alterations in cellular cAMP levels as an alternative signalling event, as 0.15 µM MS1 did not significantly change basal cAMP concentrations in WT T. b. brucei [5.8 ± 1.3 pmol cAMP/108 cells (control) versus 6.0 ± 0.5 in treated cells; P = 0.90, paired Student's t-test]. Yet intracellular calcium did appear to be elevated after several hours of incubation with 2 μM M38 or 5 μM T4. The effect was faster in onset and more robust with M38, leading to significant increases in Ca2+-associated fluorescence after 4 h (Figure 10).
Figure 10.
Treatment with T4 and M38 increases intracellular calcium levels in bloodstream-form T. brucei. Parasites were loaded with Fluo-8 fluorescent indicator after 0, 4 or 8 h of incubation with 2 µM M38 or 5 µM T4, as described in the Materials and methods section, and fluorescence intensity was determined. Data are presented as percentages of non-treated control, for which calcium-associated fluorescence was measured in parallel cultures at the same timepoints; they are average values and SEM of four independent experiments. *P < 0.05; **P < 0.01 (paired Student's t-test).
Choline-derived compounds reduce the levels of ROS in T. b. brucei
We assessed the production of ROS in T. brucei bloodstream forms in response to incubation with the compounds, using the ROS-sensitive fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DH).40 The results show that trypanosomes in culture generate a substantial amount of ROS, which is greatly increased in the presence of H2O2. The rate at which ROS were generated over 6 h was dose-dependently reduced by incubation with T1 and MS1 (Figure S5A and B, available as Supplementary data at JAC Online). The same trends continued for up to 12 h (not shown).
Discussion
A large series of choline-derived analogues has been developed and optimized for antimalarial activity,7–11 culminating in the clinical development of T3 by Sanofi-Aventis, but hardly any assessment has been made of the utility of these compounds for other protozoan infections. In this study representative members of the monocationic choline analogues (T1) and of the first-generation (G25), second-generation (T3 and T4) and third-generation (MS1, M53 and M38) symmetrical, dicationic choline analogues were tested for anti-kinetoplastid activity. The compounds displayed strong in vitro activities against kinetoplastid species. In particular, the activities of M38, G25 and MS1 against bloodstream forms of both drug-susceptible and multidrug-resistant strains of T. b. brucei were very promising and close to the level of the positive control, diminazene aceturate. The compounds displayed only moderate to low effects on a human cell line. These activities are highly encouraging, considering the severe problems with drug resistance in the field, particularly to diamidines and melaminophenyl arsenicals.3
Uptake of radiolabelled choline by L. mexicana promastigotes (Km = 0.49 µM) was inhibited by unlabelled choline and by the choline test compounds T1 and T3, with Ki values of 4.0 and 0.41 µM, respectively—consistent with similar reports for P. falciparum and Babesia divergens.8,19,21,22 The compounds are accumulated to a high intracellular level by these intra-erythrocytic parasites, explaining both their potency and their specificity;13,14,19 monoquaternary ammonium compounds are not accumulated (H. J. Vial, unpublished observation). The observation that T1 and T3 inhibit [3H]choline uptake in L. mexicana does not in itself prove that the compounds are themselves accumulated through the choline carrier, as this would require studies with radiolabelled compounds. The identification of several of the compounds in mass spectra (Figure 8) does confirm, however, that the compounds enter the cells, as expected from their anti-kinetoplastid activities. But entry into Leishmania promastigotes may not be primarily dependent on the choline carrier, as there was no correlation between affinity for the transporter (Ki for T1 was 4.0 ± 0.3 µM; Ki for T3 was 0.41 ± 0.1 µM) and antileishmanial activity (EC50 for T1 was 0.28 ± 0.08 µM; EC50 for T3 was 33 ± 3 µM). However, this could also reflect a more favourable high-affinity interaction of T1 with the intracellular target. More conclusively, EC50 values for the choline-derived compounds were unchanged when tested in the presence or absence of 100 µM choline (data not shown), which indicates that the uptake of the choline-derived analogues is not significantly inhibited by saturation of the carrier.
For T. brucei, we show that bloodstream forms do not express specific choline transporters acting in the micromolar concentration range, consistent with previous results.50 Three different transporters have been implicated in the uptake of dicationic drugs in T. b. brucei; the P2 adenosine/adenine transporter,51–53 HAPT1 and LAPT1.27,33,47 Since uptake of pentamidine into the parasites was not inhibited by the compounds tested here at pharmacologically relevant concentrations, it is unlikely that their uptake is mediated by HAPT or LAPT. Uptake through P2 was not specifically addressed, but is highly unlikely, given the structures of the compounds, which do not contain the well-established P2 recognition motif,54,55 and the absence of resistance to choline analogues in the TbAT1-KO line (Table 1). How the choline-derived analogues traverse the plasma membrane of kinetoplastid parasites is therefore a question that remains to be answered and necessarily awaits studies with radiolabelled analogues. It is unlikely that these dications diffuse across the plasma membrane, despite their long aliphatic linker chain, as the closely related thiazolium compound T16 was completely unable to penetrate human erythrocytes until these were infected with Plasmodium parasites,24 which generate a channel-like permeation pathway in the red cell plasma membrane.56
It has been shown that quaternary ammonium salts,8,13,21 bisthiazoliums14,19 and bisalkylamidines (H. J. Vial, unpublished observation) deprive haematozoan cells of choline by blocking its entry and thereby disrupt malarial and babesial de novo PC synthesis, while the serine-decarboxylase-phosphoethanolamine-methyltransferase pathway, a source of compensatory choline, is also blocked.15 Bloodstream T. b. brucei are believed to salvage choline in the form of lyso-PC,57 abundant in the human bloodstream.58 Analysis of the treated bloodstream trypanosomes revealed no dramatic change in the composition of choline-containing lipids after treatment with T1, M38, G25 or MS1, showing that the test compounds do not act principally through disruption of lipid metabolism; however, we cannot rule out the possibility of subtle or localized changes to lipid content.
One alternative mode of action for these molecules would be disruption of the plasma membrane as, given the physical–chemical characteristics of the compounds with polar head groups and long hydrophobic chains, association or partial immersion in membranes would seem possible, especially given their detection in T. brucei lipid extracts by mass spectrometry (see above). However, we found no evidence that the choline-derived analogues used in this study caused plasma membrane defects leading to cell death. Even when a large proportion of trypanosomes had died after exposure to 1 µM G25, the remaining cells were non-permeable to PI. Nor did we detect any direct effects on cell cycle progression. Instead, the choline-derived analogues appear to induce extensive DNA fragmentation within 24 h, probably before the integrity of the cell is compromised.
DNA degradation has been linked with programmed cell death (PCD) in many species, but it is unclear whether the observations reported here should be categorized as evidence for apoptosis. Another marker for apoptosis is depolarization of the Ψm. Several of the choline-derived analogues, including M38, G25, T4 and MS1, but not T1, did have very pronounced effects on Ψm as early as 8 h and at concentrations that do not affect cell viability at that point in time (Figure 2). While this is consistent with apoptosis, it may also indicate that the mitochondria are specifically targeted by the dicationic compounds. Dicationic diamidines are also known to predominantly accumulate in the mitochondria,59,60 the Ψm providing the driving force. While the mechanism of action of diamidines on trypanosomes has never been satisfactorily defined and may be multifactorial, it is believed that the mitochondria are the main target for this class of drugs, both in Saccharomyces cerevisiae and in T. b. brucei.60,61 Pentamidine resistance in Leishmania donovani has been linked to exclusion of pentamidine from the mitochondrion.62,63 Fluorescent diamidines can be detected within the T. b. brucei mitochondria within 1 min of administration, but this is much delayed in diamidine-resistant trypanosomes.64 Worthen et al.65 recently identified several trypanocides with a rapid effect on T. b. brucei Ψm as well as several others without such an effect, and divided trypanocides into two categories: (i) drugs that cause loss of Ψm and significant DNA degradation but do not alter the G1/G2 ratio or cellular morphology; and (ii) drugs that affect cellular morphology and increase the number of G2-phase cells. Pentamidine was in the former class and our data appear to also add the choline-derived analogues to that category. The case for mitochondrial targeting is further enhanced by the observation that calcium levels in the trypanosome slowly increase after incubation with M38 and T4, which seems to correlate with mitochondrial damage, both in timing and magnitude.
The fact that all the dicationic choline-derived analogues (but not the monocationic T1) strongly reduced the Ψm, and that these compounds affected DNA integrity before cell death, appears consistent with PCD in trypanosomes, as reported for some prostaglandins.38,40 However, the PCD induced by the prostaglandins was found to be associated with the production of ROS,40 as was PCD in Trypanosoma cruzi.66 Our finding that incubation with the choline-derived analogues actually reduced the production of ROS by trypanosomes seems inconsistent with PCD. Worthen et al.65 similarly did not find any evidence that trypanocides, including prostaglandin D2 and pentamidine, induce PCD in bloodstream-form trypanosomes, as assessed by annexin V binding and activation of caspase-like proteases.
In conclusion, it appears that the dicationic choline-derived analogues, like the structurally related dicationic diamidines pentamidine and DB75,60–62 collapse the Ψm and are thus highly likely to act on an intramitochondrial target, although this in itself does not yet prove that the mitochondrion is the pharmacologically relevant target for this class of drugs. The dications evaluated in this study differ from the bis-benzamidine compounds currently used against African trypanosomes,3 and exert potent and promising activity against T. brucei that warrants further studies of in vivo activity. Of great interest, their antiparasitic activity circumvents current cross-resistance to not just the diamidine trypanocides but also to the melaminophenyl arsenicals.
Funding
H. M. S. I. was supported by a personal studentship from the Libyan government, M. I. A.-S. was supported by a Value-in-People award from the Wellcome Trust and A. A. M. A. was supported by a personal studentship from the Saudi government. This work was supported in part by T. K. S.’s Wellcome Trust Senior Research Fellowship 067441 and Wellcome Trust project grant 086658.
Transparency declarations
None to declare.
Supplementary data
Supplementary Material
Acknowledgements
We are grateful to Sanofi-Aventis for the use of several of the choline-derived analogues, including T3 and T4, and to Mr Alexander D. de Koning for technical assistance.
References
- 1.Stuart K, Brun R, Croft S, et al. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–10. doi: 10.1172/JCI33945. doi:10.1093/jac/dkn371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett MP, Burchmore RJS, Stich A, et al. The trypanosomiases. Lancet. 2003;362:1469–80. doi: 10.1016/S0140-6736(03)14694-6. doi:10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 3.Delespaux V, Koning HP. Drugs and drug resistance in African trypanosomiasis. Drug Resist Updat. 2007;10:30–50. doi: 10.1016/j.drup.2007.02.004. doi:10.1093/jac/dkm405. [DOI] [PubMed] [Google Scholar]
- 4.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–26. doi: 10.1128/CMR.19.1.111-126.2006. doi:10.1159/000097078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutterwala SS, Creswell CH, Sanyal S, et al. De novo sphingolipid synthesis is essential for viability, but not for transport of glycosylphosphatiylinositol-anchored proteins, in African trypanosomes. Eukaryot Cell. 2007;6:454–64. doi: 10.1128/EC.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridberg A, Olson CL, Nakayasu ES, et al. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J Cell Sci. 2008;121:522–35. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 7.Calas M, Cordina G, Bompart J, et al. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. J Med Chem. 1997;40:3557–66. doi: 10.1021/jm9701886. [DOI] [PubMed] [Google Scholar]
- 8.Ancelin ML, Calas M, Vidal-Sailhan V, et al. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob Agents Chemother. 2003;47:2590–7. doi: 10.1128/AAC.47.8.2590-2597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calas M, Ancelin ML, Cordina G, et al. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J Med Chem. 2000;43:505–16. doi: 10.1021/jm9911027. [DOI] [PubMed] [Google Scholar]
- 10.Hamzé A, Rubi E, Arnal P, et al. Mono- and bis-thiazolium salts have potent antimalarial activity. J Med Chem. 2005;48:3639–43. doi: 10.1021/jm0492608. [DOI] [PubMed] [Google Scholar]
- 11.Ancelin ML, Calas M, Bompart J, et al. Antimalarial activity of 77 phospholipid polar head analogues: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood. 1998;91:1426–37. [PubMed] [Google Scholar]
- 12.Ancelin ML, Calas M, Bonhoure A, et al. In vivo antimalarial activities of mono- and bis quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob Agents Chemother. 2003;47:2598–605. doi: 10.1128/AAC.47.8.2598-2605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wengelnik K, Vidal V, Ancelin ML, et al. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science. 2002;295:1311–4. doi: 10.1126/science.1067236. [DOI] [PubMed] [Google Scholar]
- 14.Vial H, Wein S, Farenc C, et al. Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc Natl Acad Sci USA. 2004;101:15458–63. doi: 10.1073/pnas.0404037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calas M, Ouattara M, Piquet G, et al. Potent antimalarial activity of 2-aminopyridinium salts, amidines and guanidines. J Med Chem. 2007;50:6307–15. doi: 10.1021/jm0704752. [DOI] [PubMed] [Google Scholar]
- 16.Salom-Roig XJ, Hamzé A, Calas M, et al. Dual molecules as new antimalarials. Comb Chem High Throughput Screen. 2005;8:47–60. doi: 10.2174/1386207053328219. [DOI] [PubMed] [Google Scholar]
- 17.Nicolas O, Margout D, Taudon N, et al. Pharmacological properties of a new antimalarial bisthiazolium salt, T3, and a corresponding prodrug, TE3. Antimicrob Agents Chemother. 2005;49:3631–9. doi: 10.1128/AAC.49.9.3631-3639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richier E, Biagini GA, Wein S, et al. Potent antihematozoan activity of novel bisthiazolium drug T16: evidence for inhibition of phosphatidylcholine metabolism in erythrocytes infected with Babesia and Plasmodium spp. Antimicrob Agents Chemother. 2006;50:3381–8. doi: 10.1128/AAC.00443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancelin ML, Vial HJ, Philippot JR. Inhibitors of choline transport into Plasmodium-infected erythrocytes are effective antiplasmodial compounds in vitro. Biochem Pharmacol. 1985;34:4068–71. doi: 10.1016/0006-2952(85)90390-9. [DOI] [PubMed] [Google Scholar]
- 20.Ancelin ML, Vial HJ. Quaternary ammonium compounds efficiently inhibit Plasmodium falciparum growth in vitro by impairment of choline transport. Antimicrob Agents Chemother. 1986;29:814–20. doi: 10.1128/aac.29.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roggero R, Zufferey R, Minca M, et al. Unravelling the mode of action of the antimalarial choline analog G25 in Plasmodium falciparum and Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2004;48:2816–24. doi: 10.1128/AAC.48.8.2816-2824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ancelin ML, Parant M, Thuet MJ, et al. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem J. 1991;273:701–9. doi: 10.1042/bj2730701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biagini GA, Richier E, Bray PG, et al. Heme binding contributes to antimalarial activity of bis-quaternary ammoniums. Antimicrob Agents Chemother. 2003;47:2584–9. doi: 10.1128/AAC.47.8.2584-2589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biagini GA, Pasini EM, Hughes R, et al. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood. 2004;104:3372–7. doi: 10.1182/blood-2004-03-1084. [DOI] [PubMed] [Google Scholar]
- 25.Zufferey R, Ben Mamoun C. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol Biochem Parasitol. 2002;125:127–34. doi: 10.1016/s0166-6851(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 26.Matovu E, Stewart M, Geiser F, et al. The mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell. 2003;2:1003–8. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges D, Gould MK, Nerima B, et al. Loss of the high affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol. 2007;71:1098–108. doi: 10.1124/mol.106.031351. [DOI] [PubMed] [Google Scholar]
- 28.De Koning HP, Jarvis SM. Purine nucleobase transport in bloodstream forms of Trypanosoma brucei brucei is mediated by two novel transporters. Mol Biochem Parasitol. 1997;89:245–58. doi: 10.1016/s0166-6851(97)00129-1. [DOI] [PubMed] [Google Scholar]
- 29.Le Roch KG, Johnson JR, Ahiboh H, et al. A systematic approach to understand the mechanism of action of the bisthiazolium compound T4 on the human malaria parasite, Plasmodium falciparum. BMC Genomics. 2008;9:513. doi: 10.1186/1471-2164-9-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Räz B, Iten M, Grether-Bühler Y, et al. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997;68:139–47. doi: 10.1016/s0001-706x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 31.Rodenko B, Van der Burg AM, Wanner MJ, et al. 2,N6-Disubstituted adenosine analogs with antitrypanosomal and antimalarial activity. Synthesis, uptake studies and in vivo evaluation. Antimicrob Agents Chemother. 2007;51:3796–802. doi: 10.1128/AAC.00425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gould MK, Vu XL, Seebeck T, et al. An improved assay to monitor antiprotozoal drug action in vitro. Anal Biochem. 2008;382:87–93. doi: 10.1016/j.ab.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 33.De Koning HP. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters. Implications for crossresistance with arsenicals. Mol Pharmacol. 2001;59:586–92. doi: 10.1124/mol.59.3.586. [DOI] [PubMed] [Google Scholar]
- 34.Wallace LJM, Candlish D, De Koning HP. Different substrate recognition motifs of human and trypanosome nucleobase transporters: selective uptake of purine antimetabolites. J Biol Chem. 2002;277:26149–56. doi: 10.1074/jbc.M202835200. [DOI] [PubMed] [Google Scholar]
- 35.Al-Salabi MI, Wallace LJM, De Koning HP. A Leishmania major nucleobase transporter responsible for allopurinol uptake is a functional homologue of the Trypanosoma brucei H2 transporter. Mol Pharmacol. 2003;63:814–20. doi: 10.1124/mol.63.4.814. [DOI] [PubMed] [Google Scholar]
- 36.Mutomba MC, To WY, Hyun WC, et al. Inhibition of proteasome activity blocks cell cycle progression at specific phase boundaries in African trypanosomes. Mol Biochem Parasitol. 1997;90:491–504. doi: 10.1016/s0166-6851(97)00197-7. [DOI] [PubMed] [Google Scholar]
- 37.Hammarton TC, Mottram JC, Doerig C. The cell cycle of parasitic protozoa: potential for chemotherapeutic exploitation. Prog Cell Cycle Res. 2003;5:91–101. [PubMed] [Google Scholar]
- 38.Figarella K, Rawer M, Uzcategui NL, et al. Prostaglandin-induced programmed cell death in Trypanosoma brucei bloodstream form. Cell Death Differ. 2005;12:335–46. doi: 10.1038/sj.cdd.4401564. [DOI] [PubMed] [Google Scholar]
- 39.Uzcátegui NL, Carmona-Gutierrez D, Denninger V, et al. Antiproliferative effect of dihydroxyacetone on Trypanosoma brucei bloodstream forms: cell cycle progression, subcellular alterations, and cell death. Antimicrob Agents Chemother. 2007;51:3960–8. doi: 10.1128/AAC.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figarella K, Uzcategui NL, Beck A, et al. Prostaglandin-induced programmed cell death in Trypanosoma brucei involves oxidative stress. Cell Death Differ. 2006;13:1802–14. doi: 10.1038/sj.cdd.4401862. [DOI] [PubMed] [Google Scholar]
- 41.Denninger V, Figarella K, Schoenfeld C, et al. Troglitazone induces differentiation in Trypanosoma brucei. Exp Cell Res. 2007;313:1805–19. doi: 10.1016/j.yexcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 43.Richmond GS, Gibellini F, Young SA, et al. Lipidomic analysis of bloodstream and procyclic form Trypanosoma brucei. Parasitology. 2010;137:1357–92. doi: 10.1017/S0031182010000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibellini F, Hunter WN, Smith TK. The ethanolamine branch of the Kennedy pathway is essential in the bloodstream form of Trypanosoma brucei. Mol Microbiol. 2009;73:826–43. doi: 10.1111/j.1365-2958.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Salabi MI, De Koning HP. Purine nucleobase transport in amastigotes of Leishmania mexicana: involvement in allopurinol uptake. Antimicrob Agents Chemother. 2005;49:3682–9. doi: 10.1128/AAC.49.9.3682-3689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Koning HP. Ever-increasing complexities of diamidine and arsenical crossresistance in African trypanosomes. Trends Parasitol. 2008;24:345–9. doi: 10.1016/j.pt.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Moreno SNJ, Docampo R. Calcium regulation in protozoan parasites. Curr Opin Microbiol. 2003;6:359–64. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 48.Xiong Z-H, Ruben L. Trypanosoma brucei: the dynamics of calcium movement between the cytosol, nucleus and mitochondrion of intact cells. Exp Parasitol. 1998;88:231–9. doi: 10.1006/expr.1998.4249. [DOI] [PubMed] [Google Scholar]
- 49.De Koning HP, Stewart M, Anderson L, et al. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights in diamidine resistance in African trypanosomes. Antimicrob Agents Chemother. 2004;48:1515–19. doi: 10.1128/AAC.48.5.1515-1519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rifkin MR, Strobos CA, Fairlamb AH. Specificity of ethanolamine transport and its further metabolism in Trypanosoma brucei. J Biol Chem. 1995;270:16160–66. doi: 10.1074/jbc.270.27.16160. [DOI] [PubMed] [Google Scholar]
- 51.Carter NS, Fairlamb AH. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature. 1993;361:173–76. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- 52.De Koning HP, Jarvis SM. Adenosine transporters in bloodstream forms of T. b. brucei: substrate recognition motifs and affinity for trypanocidal drugs. Mol Pharmacol. 1999;56:1162–70. doi: 10.1124/mol.56.6.1162. [DOI] [PubMed] [Google Scholar]
- 53.Collar CJ, Al-Salabi MI, Stewart ML, et al. Predictive computational models of substrate binding by a nucleoside transporter. J Biol Chem. 2009;284:34028–35. doi: 10.1074/jbc.M109.049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Koning HP, Bridges DJ, Burchmore R. Purine transporters of protozoa: from biology to therapy. FEMS Microbiol Rev. 2005;29:987–1020. doi: 10.1016/j.femsre.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Ginsburg H, Krugliak M, Eidelman O, et al. New permeability pathways induced in membranes of Plasmodium falciparum-infected erythrocytes. Antimicrob Agents Chemother. 1983;8:177–90. doi: 10.1016/0166-6851(83)90008-7. [DOI] [PubMed] [Google Scholar]
- 56.Bowes AE, Samad AH, Jiang P, et al. The acquisition of lyso-phosphatidylcholine by African trypanosomes. J Biol Chem. 1993;268:13885–92. [PubMed] [Google Scholar]
- 57.Zeisel SH. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr. 1981;1:95–121. doi: 10.1146/annurev.nu.01.070181.000523. [DOI] [PubMed] [Google Scholar]
- 58.Lansiaux A, Tanious F, Mishal Z, et al. Distribution of furamidine analogues in tumor cells: targeting of the nucleus or mitochondria depending on the amidine substitution. Cancer Res. 2002;62:7219–29. [PubMed] [Google Scholar]
- 59.Mathis AM, Holman JL, Sturk LM, et al. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob Agents Chemother. 2006;50:2185–91. doi: 10.1128/AAC.00192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanteri CA, Tidwell RR, Meshnick SR. The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob Agents Chemother. 2008;52:875–82. doi: 10.1128/AAC.00642-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanteri CA, Trumpower BL, Tidwell RR. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2004;48:3968–74. doi: 10.1128/AAC.48.10.3968-3974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basselin M, Denise H, Coombs GH, et al. Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob Agents Chemother. 2002;46:3731–8. doi: 10.1128/AAC.46.12.3731-3738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee A, Padmanabhan PK, Sahani MH, et al. Roles for mitochondria in pentamidine susceptibility and resistance in Leishmania donovani. Mol Biochem Parasitol. 2006;145:1–10. doi: 10.1016/j.molbiopara.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Stewart ML, Krishna S, Burchmore RJ, et al. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet. 2005;366:486–7. doi: 10.1016/S0140-6736(05)66793-1. [DOI] [PubMed] [Google Scholar]
- 65.Worthen C, Jensen BC, Parsons M. Diverse effects on mitochondrial and nuclear functions elicited by drugs and genetic knockdowns in bloodstream stage Trypanosoma brucei. PLoS Negl Trop Dis. 2010;4:e678. doi: 10.1371/journal.pntd.0000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piacenza L, Irigoin F, Alvarez MN, et al. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J. 2007;403:323–34. doi: 10.1042/BJ20061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.