Abstract
Objectives
Electrostatic forces mediate the initial interaction between cationic colistin and Gram-negative bacterial cells. Lipopolysaccharide (LPS) loss mediates colistin resistance in some A. baumannii strains. Our aim was to determine the surface charge of colistin-susceptible and –resistant A. baumannii as a function of growth phase and in response to polymyxin treatment.
Methods
The zeta potential of A. baumannii ATCC 19606 and 10 clinical multidrug-resistant strains (MICs 0.5–2 mg/L) was assessed. Colistin-resistant derivatives (MIC >128 mg/L) of wild-type strains were selected in the presence of 10 mg/L colistin, including the LPS-deficient lpxA mutant, ATCC 19606R. To determine the contribution of LPS to surface charge, two complemented ATCC 19606R derivatives were examined, namely ATCC 19606R + lpxA (containing an intact lpxA gene) and ATCC 19606R + V (containing empty vector). Investigations were conducted as a function of growth phase and polymyxin treatment (1, 4 and 8 mg/L).
Results
Wild-type cells exhibited a greater negative charge (−60.5 ± 2.36 to −26.2 ± 2.56 mV) thancolistin-resistant cells (−49.2 ± 3.09 to −19.1 ± 2.80 mV) at mid-log phase (ANOVA, P < 0.05). Opposing growth-phase trends were observed for both phenotypes: wild-type cells displayed reduced negative charge and colistin-resistant cells displayed increased negative charge at stationary compared with mid-logarithmic phase. Polymyxin exposure resulted in a concentration-dependent increase in zeta potential. Examination of ATCC 19606R and complemented strains supported the importance of LPS in determining surface charge, suggesting a potential mechanism of colistin resistance.
Conclusions
Zeta potential differences between A. baumannii phenotypes probably reflect compositional outer-membrane variations that impact the electrostatic component of colistin activity.
Keywords: physicochemical properties, Gram-negative, polymyxin
Introduction
The increasing prevalence of multidrug-resistant (MDR) bacterial pathogens globally, coupled with a steady decline in the development of new antimicrobial agents1 has the world facing a possible return to the ‘pre-antibiotic’ era.2 The approval of several new antibiotics active against Gram-positive bacteria has provided some respite for those infections,3 but unfortunately Gram-negative pathogens, such as Acinetobacter baumannii, are proving more problematic with predictions that the availability of a new antibiotic against these infections is still at least a decade away.4
The complex Gram-negative cell envelope presents an effective permeability barrier to the passage of many antibiotics.5 This barrier can be largely attributed to anionic lipopolysaccharide (LPS) molecules that principally comprise the outer leaflet of the bi-layer membrane. The formation of a dense, negatively charged surface layer6 is favourable to the action of polymyxins. Polymyxin antibiotics structurally comprise a cyclic heptapeptide ring, attached to a tripeptide side chain, which is covalently linked to a fatty acyl tail.7 At physiological pH, protonation of five primary amine groups together with the hydrophobic tail deliver a cationic amphipathic character that has been suggested to enable self-promoted uptake of the antibiotic through the outer membrane.8 Initial electrostatic binding to negatively charged phosphates present on the lipid A portion of LPS displaces divalent cations that play a stabilizing role in bridging neighbouring LPS molecules.6 Insertion of the N-terminal fatty-acyl tail of polymyxins into the external lipid sheet is thus enabled, resulting in membrane disruption.8 Polymyxin B and colistin (polymyxin E) have a rapid bactericidal effect against many MDR Gram-negative species.9 In an era of diminishing therapeutic options, these polymyxins have been revived as a last line of defence against MDR Gram-negative infections.
The Gram-negative pathogen A. baumannii has created considerable challenges for clinicians due to a high level of intrinsic antimicrobial resistance, as well as a remarkable ability to acquire numerous genetic mutations leading to the production of the MDR phenotype.10 This dilemma is accentuated by the emergence of extremely drug-resistant strains, which are resistant to all antibiotics, including colistin.11,12 Furthermore, the phenomenon of colistin heteroresistance describes strains that appear susceptible on the basis of MICs, but harbour highly colistin-resistant subpopulations.13,14 These subpopulations may be amplified upon colistin exposure, potentially resulting in therapeutic failure.14 Studies of polymyxin resistance in other Gram-negative species have highlighted LPS modifications involving alterations to lipid A phosphate groups by the addition of sugar moieties under the control of the two-component regulatory systems, PhoPQ and PmrAB.15–17 These changes result in a reduction in the net negative charge of lipid A, effectively diminishing the electrostatic polymyxin–bacterium interaction. Importantly, we have recently shown that colistin resistance in the A. baumannii reference strain, ATCC 19606, can be mediated by complete loss of LPS. Sequence analysis of paired colistin-susceptible and –resistant ATCC 19606 strains revealed that spontaneous mutations in the key lipid A biosynthesis genes, lpxA, lpxC and lpxD, resulted in the arrest of lipid A biosynthesis and therefore LPS assembly.18 While the genetic and molecular basis of polymyxin resistance in other A. baumannii strains remains to be fully established, it is likely that modification of the bacterial surface charge is involved.
Zeta potential measurements have been applied in bacteriological studies to address the hypothesis that the susceptibility of a bacterium to peptide antibiotics may be closely related to the charge exhibited on the cell surface.19–23 No such studies have been conducted on A. baumannii. Furthermore, while the ongoing expression of membrane proteins, lipids and extracellular polymers throughout the bacterial growth cycle has been shown to affect surface physicochemical properties of Escherichia coli,24,25 the influence of these potential modifications on the surface charge of A. baumannii cells and the resultant electrostatic interaction with colistin has yet to be addressed. Using the zeta potential as a measure of bacterial surface charge, the aims of this study were to compare the surface charge of colistin-susceptible and -resistant A. baumannii cells, to investigate the differences in the surface charge with respect to bacterial growth phase and finally, to determine the influence of colistin exposure on the surface charge of A. baumannii at different growth phases.
Materials and methods
Chemicals
Stock solutions (1 mg/mL) of colistin (20 374 U/mg colistin sulphate; Zhejiang Shenghua Biok Biology Co. Ltd, China) and polymyxin B nonapeptide (PBN, Lot No. 088K4054; Sigma-Aldrich, Castle Hill, Australia) were prepared in Milli-Q™ water (Millipore, North Ryde, Australia) and filtered through 0.22 µm syringe filters (Sartorius, Melbourne, Australia). Both solutions were stored at 4°C for up to 1 month; conditions under which these antibiotics have proved to be stable.26
Bacterial strains
Thirteen strains of A. baumannii were employed in this study; these included six colistin-heteroresistant clinical MDR strains (clinical strains FADDI-AB016, FADDI-AB027, FADDI-AB032, FADDI-AB048, FADDI-AB050 and FADDI-AB052) and reference strain ATCC 19606 (from the ATCC, Manassas, VA, USA), as well as four colistin-susceptible clinical MDR strains (FADDI-AB013, FADDI-AB014, FADDI-AB035 and FADDI-AB040). The MICs of colistin for these strains ranged from 0.5 to 2 mg/L. From each of these wild-type strains, paired colistin-resistant derivatives were obtained;13 these were designated as ‘R’ strains and were able to grow on nutrient agar plates containing 10 mg/L colistin (see below) (Medium Preparation Unit, Melbourne, Australia). The paired colistin-resistant strains had MICs of >128 mg/L. Additionally, the zeta potential of two complemented strains derived from ATCC 19606R was measured to examine the effect of LPS loss on the surface charge of A. baumannii: colistin-susceptible ATCC 19606R + lpxA, which contained an introduced intact lpxA gene (MIC 1 mg/L), and ATCC 19606R + V, a colistin-resistant control strain that was deficient in the lpxA gene but contained an empty vector (MIC >128 mg/L).18 All strains were stored in tryptone soya broth (Oxoid, Adelaide, Australia) with 20% glycerol at −80°C.
Wild-type strains were subcultured onto nutrient agar plates before the experiments. Cation-adjusted Mueller–Hinton broth (Oxoid, Adelaide, Australia) was employed for overnight cultures, and subsequently used to prepare mid-logarithmic cells according to OD600 (0.4–0.6) by subculture into fresh broth (1 in 100 dilution). Stationary-phase cultures were grown overnight. All broth cultures were incubated at 37°C in a shaking water bath at 100 rpm. Growth of paired colistin-resistant strains was conducted in the same manner as for their parent wild-type strains; the initial subculture and overnight broth culture were grown in the presence of 10 mg/L colistin to maintain selective pressure. Mid-logarithmic and stationary-phase colistin-resistant cultures were grown in the absence of colistin to avoid potential structural modifications induced by the antibiotic. Identical sample preparation protocols were also employed for strains ATCC 19606R + lpxA and ATCC 19606R + V, with the addition that these complemented strains were grown in the presence of 100 mg/L ampicillin to maintain selection for the plasmids.18
Sample preparation for measurement of zeta potential
To achieve adequate cleansing of the bacterial surface prior to zeta potential measurements, cells were harvested from broth culture by centrifugation at 3000 g for 5 min at 25°C, and washed twice with Milli-Q™ water as described previously.27 For all investigations, washed cells were resuspended in the test solution to prepare bacterial suspensions containing ∼1 × 109 cfu/mL, following which a 10-fold dilution was performed in the same medium immediately prior to zeta potential measurement. The resulting suspension was used to fill clear disposable zeta cells (ATA Scientific, Australia) immediately prior to zeta potential measurement.
The influence of ionic strength and pH on the zeta potential of bacterial cells has been reported,28–31 thus demonstrating the importance of determining the effect of these conditions on the zeta potential of A. baumannii. For this purpose, cultures were washed and suspended with potassium chloride (KCl) solutions over a range of pH values (5.0–8.0), and low (0.01–0.3 mM) and high (10–300 mM) ionic strengths. Adjustment of pH was achieved by drop-wise addition of HCl (10% v/v) or NaOH (10% w/v) solution. Ionic strength investigations were conducted at pH 5.5, while pH investigations were conducted in 0.01 mM KCl solution.
To examine the effect of polymyxin treatment on A. baumannii cells, colistin or PBN (1 mg/mL) was added to 5 mL of bacterial culture (∼1 × 109 cfu/mL) to achieve final concentrations of 1, 4 and 8 mg/L. PBN (an inactive derivative of polymyxin B produced by proteolytic cleavage of the terminal diaminobutyric acid residue and N-fatty acyl chain) was used as a comparator for colistin. Bacterial broth cultures were then incubated in a shaking water bath (37°C, 100 rpm) for 20 min and prepared for zeta potential analysis as described above.
Zeta potential measurement
The electrophoretic mobility (EPM) of bacterial cells was measured with a zeta potential analyser at 150 V (Zetasizer Nano ZS™; Malvern Instruments Ltd, Malvern, UK) before being converted to zeta potentials using the Helmholtz–Smoluchowski theory.32 Measurements were performed at 25°C in either Milli-Q™ water or KCl solutions of desired pH and ionic strength. Prior to sample analysis, polarization of the electrodes using the test solution was carried out in the absence of cells under identical conditions to establish consistent sample conductance readings.33 EPM measurements were performed in triplicate on three separately prepared samples on at least two separate days to determine reproducibility of results. Between each measurement, electrodes were rinsed with copious amounts of ethanol and Milli-Q™ water, followed by the test bacterial suspension. Statistical analyses were conducted using the Student's t-test and analysis of variance (ANOVA) using GraphPad Prism V5.0 software (GraphPad Software, San Diego, CA, USA).
Results
Influence of ionic strength and pH on zeta potential of A. baumannii
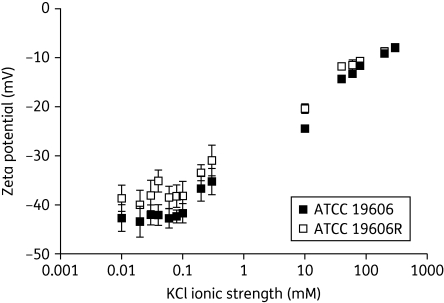
The influence of ionic strength on the zeta potential and background conductivity of paired colistin-susceptible and -resistant A. baumannii ATCC 19606 cells is illustrated in Figure 1. Similar results were acquired for FADDI-AB016 and FADDI-AB016R strains (data not shown). At ionic strengths of <0.1 mM, the zeta potential was independent of ionic strength (Figure 1), and the colistin-resistant cells displayed less-negative zeta potentials in comparison with the colistin-susceptible cells. Above ionic strengths of 0.1 mM, the zeta potential of both phenotypes increased with ionic strength and the difference observed between the zeta potentials measured for colistin-susceptible and -resistant cells became almost negligible. A linear relationship between background conductivity and ionic strength was acquired across the entire range of ionic strengths tested (0.01–300 mM) in the presence of bacteria (data not shown). Analysis of variance revealed that alterations in pH across the range 5–8 did not significantly influence the zeta potential of colistin-susceptible and –resistant A. baumannii cells (P > 0.05; Figure 2). The change in the pH of Milli-Q™ water over time was examined in the present study, revealing a stable pH of ∼5.8 over the course of 8 h at room temperature (data not shown). As this pH value of 5.8 is close to the pH of 6 included in the above-mentioned study (Figure 2), post-hoc analysis of the zeta potentials at pH 6 versus the most physiologically relevant pH of 7 was conducted, revealing no significant difference (t-test, P > 0.05). From these data, the decision was made to perform subsequent measurements in Milli-Q™ water, where the ionic strength is negligible, thereby allowing for a valid comparison between the zeta potential of colistin-susceptible and -resistant A. baumannii.
Figure 1.
Zeta potential (mean ± SD) of A. baumannii ATCC 19606, and paired colistin-resistant strain ATCC 19606R, at mid-logarithmic phase as a function of KCl ionic strength at pH 5.5.
Figure 2.
Zeta potential (mean ± SD) of paired colistin-susceptible and -resistant A. baumannii strains measured in 0.01 mM KCl as a function of pH.
Zeta potential of colistin-susceptible versus -resistant A. baumannii
Figure 3 shows the zeta potential of paired colistin-susceptible and -resistant A. baumannii cells at mid-logarithmic phase measured in Milli-Q™ water. A negative zeta potential was obtained for all tested strains. Colistin-susceptible A. baumannii strains exhibited zeta potentials ranging from −60.5 ± 2.36 to −26.2 ± 2.56 mV. The zeta potential values of colistin-resistant cells were significantly less negative overall than those of their colistin-susceptible parent strains, ranging from −49.2 ± 3.09 to −19.1 ± 2.80 (Figure 3; ANOVA, P < 0.05); post-hoc analysis revealed that the zeta potential of colistin-susceptible and -resistant cells was not statistically different for the FADDI-AB014 paired strains, nor for the complemented derivative strains (ATCC 19606R + lpxA versus ATCC 19606R + V strains), containing the lpxA gene and vector only, respectively (t-test, P > 0.05). Additionally, there was no statistically significant difference between the zeta potentials of the colistin-susceptible strains ATCC 19606 and ATCC 19606R + lpxA, or the colistin-resistant strains ATCC 19606R and ATCC 19606R + V (t-test, P < 0.05).
Figure 3.
Zeta potential (mean ± SD) of mid-logarithmic paired colistin-susceptible and –resistant A. baumannii cells. ‘ATCC 19606R derivatives’ refers to colistin-susceptible ATCC 19606R + lpxA and colistin-resistant ATCC 19606R + V, which were both derived from ATCC 19606R.
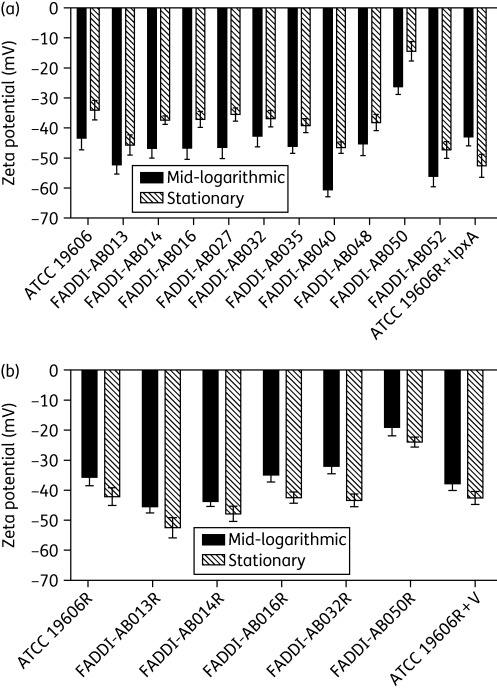
Influence of growth phase on the zeta potential of A. baumannii
Figure 4 illustrates the zeta potential of colistin-susceptible (Figure 4a) and -resistant (Figure 4b) A. baumannii cells, at both mid-logarithmic and stationary phases. All measurements were conducted in Milli-Q™ water. Analysis of variance revealed that overall, colistin-susceptible strains exhibited a significantly less negative surface charge at stationary phase in comparison with mid-logarithmic cells (P < 0.05); ATCC 19606R + lpxA exhibited the opposite trend to all other colistin-susceptible strains in that a greater negative surface charge was detected at stationary phase. Similarly, a greater negative surface charge was exhibited by all colistin-resistant strains, including ATCC 19606R + V, at stationary phase in comparison with mid-logarithmic phase (ANOVA, P < 0.05).
Figure 4.
Zeta potential (mean ± SD) of colistin-susceptible (a) and -resistant (b) A. baumannii cells, including ATCC 19606R + lpxA and ATCC 19606R + V, at mid-logarithmic and stationary growth phases.
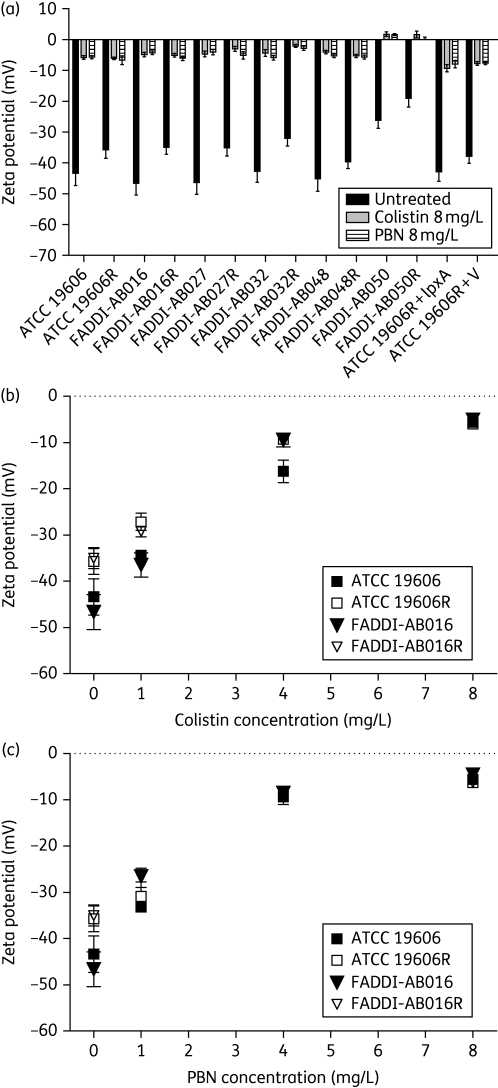
Effect of colistin treatment on the zeta potential of A. baumannii
The contribution of colistin and PBN to the background conductivity of the bacterial suspension was found to be negligible at the highest tested concentration of 8 mg/L, with an increase of no greater than 0.004 mS/cm (data not shown). Figure 5(a) illustrates a substantial increase in zeta potential (i.e. less negative) upon colistin and PBN exposure (8 mg/L) for both colistin-susceptible and -resistant cells at mid-logarithmic phase, including strains ATCC 19606R + lpxA and ATCC 19606R + V. Colistin treatment of stationary-phase A. baumannii yielded similar observations to those observed with mid-logarithmic cells (data not shown). In all experiments, the surface charge neutralizing effect imparted by colistin and PBN was of similar magnitude. Concentration-dependent changes to A. baumannii zeta potential were demonstrated following treatment of two paired colistin-susceptible and -resistant strains (ATCC 19606 and FADDI-AB016) with colistin (Figure 5b) and PBN (Figure 5c) at concentrations of 1, 4 and 8 mg/L.
Figure 5.
(a) The increase in mean zeta potential for a series of paired A. baumannii strains at mid-logarithmic phase following treatment with 8 mg/L colistin and PBN. (b and c) The effect of increasing concentrations of colistin and PBN, respectively, on the zeta potential of paired colistin-susceptible and -resistant A. baumannii ATCC 19606 and FADDI-AB016 at mid-logarithmic phase. Data shown represent the mean ± SD.
Discussion
Ionizable acidic and basic moieties present on phospholipids, lipoproteins and LPS, which comprise the outer glycolipid sheet of the asymmetrical outer membrane, collectively dictate the net surface charge on Gram-negative bacteria.34 LPS represents the primary component of the outer leaflet, and consists of an outer O-polysaccharide side chain connected to a core oligosaccharide, which is attached to lipid A; it is the lipid A portion that is incorporated within the membrane, forming an anchor upon which LPS can build. Anionic character is mainly afforded by carboxyl and phosphate moieties concentrated on lipid A as well as sugars within the core oligosaccharide.35 Various forces work in concert to stabilize divalent cations that bind LPS molecules within the membrane, resulting in the formation of a dense negatively charged barrier, which poses a major hindrance to the passage of many hydrophobic antibiotics.36 For polymyxins, which are cationic at physiological pH, antibacterial activity is initiated by electrostatic attraction to the anionic surface of Gram-negative bacterial cells.8,37
Bacterial surface charge has often been described by the zeta potential, which represents the potential at the shear plane of the electrical double layer encompassing a cell in solution. This charge is highly dependent on both the composition of the cell surface and the nature of the surrounding medium. Consideration of the background conductivity (salt concentration) and pH of the electrolyte in which cells are suspended is thus necessary, as these factors govern the adsorption of ions onto bacterial cells, and influence the degree of ionization of charged moieties on the cell surface.29 The corresponding rise in zeta potential of A. baumannii cells towards neutrality observed with increasing ionic strength (Figure 1) may be explained by shielding of the actual surface charge by increased amounts of cations within the proximity of the negatively charged bacterial surface. The zeta potential of both colistin-susceptible and -resistant A. baumannii cells behaved independently of pH across the range 5–8 (ANOVA, P > 0.05; Figure 2). This has been observed for other Gram-negative species,28–31 and represents a condition in which carboxyl, phosphate and amino functionalities on bacterial cell surfaces would be expected to be mostly ionized. In the present study, zeta potential measurements were performed immediately following sample preparation in Milli-Q™ water, which maintained a stable pH of ∼5.8 over 8 h. Thus, the zeta potential measurements were performed at a physiologically relevant pH, and potentially confounding influences arising from variations in pH and ionic strength were minimized.
The negative zeta potentials observed in this study for A. baumannii cells at mid-logarithmic phase were of a magnitude comparable to those observed for other Gram-negative species under similar conditions.28,30,31,38 The less-negative zeta potential exhibited by colistin-resistant cells in comparison with colistin-susceptible cells at mid-logarithmic phase (Figure 3), is most plausibly explained by alterations in the composition and structure of the outer membrane. This finding may also explain the greater tendency for colistin-resistant A. baumannii cells to aggregate in small chains or clusters27 in comparison with their wild-type parent cells, and correlates with the understanding that the stability of colloid aggregate systems is enhanced with particles exhibiting a lower magnitude of charge owing to decreased electrostatic repulsion.28 Charge shielding esterification of phosphates present on lipid A with 4-amino-4-deoxy-l-arabinose or 2-aminoethanol, has been noted in polymyxin-resistant strains of E. coli,39 Pseudomonas aeruginosa,15 Salmonella typhimurium40 and Yersinia pestis.16 We have recently demonstrated an additional mechanism of colistin resistance in A. baumannii that involves the complete loss of LPS from the outer membrane, due to mutations of any of the key lipid A biosynthesis genes, lpxA, lpxC or lpxD.18 In accordance with this finding, the zeta potential of the LPS-deficient ATCC 19606R complemented with lpxA (i.e. A. baumannii ATCC 19606R + lpxA) was found to mirror that of wild-type ATCC 19606 (colistin-susceptible) (t-test, P < 0.05); similarly, comparable results were obtained for ATCC 19606R + V (containing empty vector) and ATCC 19606R (colistin–resistant) (t-test, P < 0.05). The modification15,16,39,40 or complete loss of lipid A would be expected to lead to a less negatively charged Gram-negative outer membrane, as was observed with A. baumannii in this study (Figure 3). The residual negative charge on colistin-resistant cells (Figure 3) may be attributed to negatively charged functionalities present on phospholipids and proteins.41
Bacterial growth is known to influence the conformation and composition of outer-membrane lipids42 and LPS,43 which is likely to be reflected by alterations to surface charge. The reported effect of growth phase on bacterial surface charge varies depending on the species; while zeta potential changes have been noted for E. coli and Lactobacillus rhamnosus,44–46 growth-phase effects have not been demonstrated for P. aeruginosa or other lactic acid bacterial strains.38,47 Interest in examining the zeta potential of A. baumannii at different growth phases stemmed from findings that revealed morphological changes27 and reduced colistin susceptibility of A. baumannii at stationary phase in comparison with mid-logarithmic phase.48 In the present study, we report a less-negative zeta potential for the colistin-susceptible cells at stationary phase in comparison with mid-logarithmic phase (Figure 4a). This would theoretically reduce the initial electrostatic attraction between colistin and bacterial cells, and may thus be expected to decrease antibacterial activity as has been observed for colistin-susceptible A. baumannii strains.48 For colistin-resistant strains (including ATCC 19606R + V) and colistin-susceptible ATCC 19606R + lpxA, the opposite trend was noted whereby cells exhibited a greater negative charge at stationary phase (Figure 4); reasons for this phenomenon are unknown and warrant further investigation.
To examine the relationship between surface charge and polymyxin activity, colistin and PBN treatment of paired A. baumannii strains was conducted. Measurements were performed in Milli-Q™ water in which the background conductivity was ∼0.0015 mS/cm. At this low range, the zeta potential behaved independently of the electrolyte concentration (Figure 1). Hence, as increases in background conductivity detected following polymyxin addition in Milli-Q™ water were negligible (<0.004 mS/cm), comparisons between untreated and treated A. baumannii cells were possible. Structurally, colistin and polymyxin B differ in a single amino acid residue. PBN is a polymyxin B derivative produced by proteolytic cleavage of the terminal diaminobutyric amino acid residue and N-fatty acyl tail.49 These deletions are accompanied by a loss of antibacterial activity, owing to the inability of the fatty acyl chain to penetrate the lipid barrier.37,49 However, retention of the cationic character of PBN at physiological pH enables it to maintain electrostatic contacts with the outer membrane, thus sensitizing Gram-negative bacteria to hydrophobic antibiotics.50 In keeping with this suggestion, colistin and PBN were both found to neutralize the charge on colistin-susceptible and -resistant cells to a similar extent at mid-logarithmic (Figure 5) and stationary growth phases (data not shown). This finding substantiates the importance of electrostatic forces in initiating polymyxin action; an observation that has also been reported for other cationic antimicrobial peptides.19,23,51–53 It should be noted that these zeta potential alterations not only reflect the specific binding of colistin and PBN to LPS on colistin-susceptible cells, but also involve non-specific binding to other negatively charged moieties, such as proteins and phospholipids present on the surface of both phenotypes. Indeed, as ATCC 19606R produces no LPS, colistin binding to the surface of these cells must result entirely from binding to other surface molecules. Interestingly, while the amount of colistin required to neutralize the surface charge of both the ATCC 19606 parent strain and the LPS-deficient strain, ATCC 19606R, was very similar (Figure 5b), the colistin-resistant derivative is >100-fold more resistant to killing by colistin. This suggests that electrostatic binding of colistin is necessary but not sufficient for bacterial killing.
Commercially available instrumentation to determine the zeta potential employs micro-electrophoresis or electrophoretic light scattering methods. Calculations are based on the particle EPM using the Helmholtz–Smoluchowski equation,32 which is a model developed for solid spherical particles. Complexities are seen with the approximation of bacterial charge using this theory, as the soft polyelectrolyte layer surrounding the cell is ion permeable.54 Alternative models have been derived for soft particles;55 however, deficiencies have similarly been identified in their application to biological samples. Consequently, the zeta potential popularly remains as an accepted predictor of bacterial surface charge. In view of that, the relative measurements obtained in this investigation provide a valid comparison between colistin-susceptible and -resistant A. baumannii at different growth phases, and in response to colistin treatment.
In summary, our study is the first to reveal a relationship between colistin susceptibility and net bacterial surface charge, in which a less negative charge was detected for colistin-resistant cells. The effect of growth phase on the zeta potential of colistin-susceptible and -resistant cells was also observed. These differences may be a manifestation of variations in the composition of the external leaflet of the outer membrane. Comparison between ATCC 19606 and ATCC 19606R complemented strains supported the involvement of LPS in surface charge, reflecting a potential mechanism of colistin resistance. In addition, treatment with both colistin and PBN neutralized the charge on both colistin-susceptible and -resistant A. baumannii cells. Overall, our study has reinforced the importance of the electrostatic interaction as a key component involved in the initiation of colistin antibacterial activity. Further investigations into the molecular basis for the interaction between colistin and A. baumannii cells will provide important information for the development of novel antibiotics.
Funding
This study was supported by a project grant awarded to R. L. N., J. D. B., J. L., B. A. and M. H. by the Australian National Health and Medical Research Council (NHMRC). R. L. N. and J. L. are supported by research grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01A1070896 and R01AI079330). J. L. is an Australian NHMRC R. Douglas Wright Research Fellow.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Acknowledgements
We thank Professor John Turnidge (SA Pathology, Women's and Children's Hospital, Adelaide) and Associate Professor Denis Spelman (Department of Microbiology and Infectious Diseases Unit, Alfred Hospital, Melbourne) for providing clinical strains.
References
- 1.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Paterson DL, Lipman J. Returning to the pre-antibiotic era in the critically ill: the XDR problem. Crit Care. 2007;35:1789–91. doi: 10.1097/01.CCM.0000269352.39174.A4. [DOI] [PubMed] [Google Scholar]
- 3.Steenbergen JN, Alder J, Thorne GM, et al. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother. 2005;55:283–8. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 4.Payne DJ, Gwynn MN, Holmes DJ, et al. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storm DR, Rosenthal KS, Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–63. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 8.Hancock REW, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–23. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 10.Jain R, Danziger LH. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann Pharmacother. 2004;38:1449–59. doi: 10.1345/aph.1D592. [DOI] [PubMed] [Google Scholar]
- 11.Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001–2004) Clin Microbiol Infect. 2006;12:315–21. doi: 10.1111/j.1469-0691.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 12.Ko KS, Suh JY, Kwon KT, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60:1163–7. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Rayner CR, Nation RL, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–50. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother. 2008;52:351–2. doi: 10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooderham WJ, Hancock RE. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa. FEMS Microbiol Rev. 2009;33:279–94. doi: 10.1111/j.1574-6976.2008.00135.x. [DOI] [PubMed] [Google Scholar]
- 16.Winfield MD, Latifi T, Groisman EA. Transcriptional regulation of the 4-amino-4-deoxy-L-arabinose biosynthetic genes in Yersinia pestis. J Biol Chem. 2005;280:14765–72. doi: 10.1074/jbc.M413900200. [DOI] [PubMed] [Google Scholar]
- 17.Barrow K, Kwon DH. Alterations in two-component regulatory systems of phoPQ and pmrAB are associated with polymyxin B resistance in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53:5150–4. doi: 10.1128/AAC.00893-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffatt JH, Harper M, Harrison P, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.00834-10. doi:10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuoka S, Howe J, Andra J, et al. Physico-chemical and biophysical study of the interaction of hexa- and heptaacyl lipid A from Erwinia carotovora with magainin 2-derived antimicrobial peptides. Biochim Biophys Acta. 2008;1778:2051–7. doi: 10.1016/j.bbamem.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Ouhara K, Komatsuzawa H, Yamada S, et al. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–96. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 21.Willumeit R, Kumpugdee M, Funari SS, et al. Structural rearrangement of model membranes by the peptide antibiotic NK-2. Biochim Biophys Acta. 2005;1669:125–34. doi: 10.1016/j.bbamem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Ouhara K, Komatsuzawa H, Kawai T, et al. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. J Antimicrob Chemother. 2008;61:1266–9. doi: 10.1093/jac/dkn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengoechea JA, Lindner B, Seydel U, et al. Yersinia pseudotuberculosis and Yersinia pestis are more resistant to bactericidal cationic peptides than Yersinia enterocolitica. Microbiology. 1998;144:1509–15. doi: 10.1099/00221287-144-6-1509. [DOI] [PubMed] [Google Scholar]
- 24.Walker SL, Hill JE, Redman JA, et al. Influence of growth phase on adhesion kinetics of Escherichia coli D21g. Appl Environ Microbiol. 2005;71:3093–9. doi: 10.1128/AEM.71.6.3093-3099.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker SL, Redman JA, Elimelech M. Influence of growth phase on bacterial deposition: interaction mechanisms in packed-bed column and radial stagnation point flow systems. Appl Environ Microbiol. 2005;39:6405–11. doi: 10.1021/es050077t. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Milne RW, Nation RL, et al. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003;47:1364–70. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soon RL, Nation RL, Hartley PG, et al. Atomic force microscopy investigation of the morphology and topography of colistin- heteroresistant Acinetobacter baumannii strains as a function of growth phase and in response to colistin treatment. Antimicrob Agents Chemother. 2009;53:4979–86. doi: 10.1128/AAC.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klodzinska E, Szumski M, Dziubakiewicz E, et al. Effect of zeta potential value on bacterial behavior during electrophoretic separation. Electrophoresis. 2010;31:1590–6. doi: 10.1002/elps.200900559. [DOI] [PubMed] [Google Scholar]
- 29.Hong Y, Brown DG. Electrostatic behavior of the charge-regulated bacterial cell surface. Langmuir. 2008;24:5003–9. doi: 10.1021/la703564q. [DOI] [PubMed] [Google Scholar]
- 30.Schinner T, Letzner A, Liedtke S, et al. Transport of selected bacterial pathogens in agricultural soil and quartz sand. Water Res. 2010;44:1182–92. doi: 10.1016/j.watres.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Kim HN, Bradford SA, Walker SL. Escherichia coli O157:H7 transport in saturated porous media: role of solution chemistry and surface macromolecules. Environ Sci Technol. 2009;43:4340–7. doi: 10.1021/es8026055. [DOI] [PubMed] [Google Scholar]
- 32.Hiemenz PC. Electrophoresis and Other Electrokinetic Phenomena. New York: Marcel Dekker; 1977. [Google Scholar]
- 33.de Kerchove AJ, Elimelech M. Relevance of electrokinetic theory for “soft” particles to bacterial cells: implications for bacterial adhesion. Langmuir. 2005;21:6462–72. doi: 10.1021/la047049t. [DOI] [PubMed] [Google Scholar]
- 34.Wise DL, Trantolo DJ, Altobelli DE, et al. Human Biomaterials Applications. Totowa, NJ: Humana Press; 1996. [Google Scholar]
- 35.Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–70. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 36.Peterson AA, Hancock RE, McGroarty EJ. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol. 1985;164:1256–61. doi: 10.1128/jb.164.3.1256-1261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velkov T, Thompson PE, Nation RL, et al. Structure-activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruinsma GM, Rustema-Abbing M, van der Mei HC, et al. Effects of cell surface damage on surface properties and adhesion of Pseudomonas aeruginosa. J Microbiol Methods. 2001;45:95–101. doi: 10.1016/s0167-7012(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 39.Gatzeva-Topalova PZ, May AP, Sousa MC. Crystal structure of Escherichia coli ArnA (PmrI) decarboxylase domain. A key enzyme for lipid A modification with 4-amino-4-deoxy-L-arabinose and polymyxin resistance. Biochemistry. 2004;43:13370–9. doi: 10.1021/bi048551f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trent MS, Ribeiro AA, Lin S, et al. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J Biol Chem. 2001;276:43122–31. doi: 10.1074/jbc.M106961200. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Reyes M, Rodriguez-Falcon M, Chiva C, et al. The cost of resistance to colistin in Acinetobacter baumannii: a proteomic perspective. Proteomics. 2009;9:1632–45. doi: 10.1002/pmic.200800434. [DOI] [PubMed] [Google Scholar]
- 42.El-Khani MA, Stretton RJ. Effect of growth medium on the lipid composition of log and stationary phase cultures of Salmonella typhimurium. Microbios. 1981;31:161–9. [PubMed] [Google Scholar]
- 43.Bravo D, Silva C, Carter JA, et al. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J Med Microbiol. 2008;57:938–46. doi: 10.1099/jmm.0.47848-0. [DOI] [PubMed] [Google Scholar]
- 44.Deepika G, Green RJ, Frazier RA, et al. Effect of growth time on the surface and adhesion properties of Lactobacillus rhamnosus GG. J Appl Microbiol. 2009;107:1230–40. doi: 10.1111/j.1365-2672.2009.04306.x. [DOI] [PubMed] [Google Scholar]
- 45.Eboigbodin K, Newton J, Routh A, et al. Bacterial quorum sensing and cell surface electrokinetic properties. Appl Microbiol Biotechnol. 2006;73:669–75. doi: 10.1007/s00253-006-0505-4. [DOI] [PubMed] [Google Scholar]
- 46.Bolster CH, Cook KL, Marcus IM, et al. Correlating transport behavior with cell properties for eight porcine Escherichia coli isolates. Environ Sci Technol. 2010;44:5008–14. doi: 10.1021/es1010253. [DOI] [PubMed] [Google Scholar]
- 47.Boonaert CJP, Rouxhet PG. Surface of lactic acid bacteria: relationships between chemical composition and physicochemical properties. Appl Environ Microbiol. 2000;66:2548–54. doi: 10.1128/aem.66.6.2548-2554.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poudyal A, Owen RJ, Bulitta JB, et al. High initial inocula and stationary growth phase substantially attenuate killing of Klebsiella pneumoniae and Acinetobacter baumannii by colistin. Abstracts of the Forty-eighth Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2008; Washington, DC, USA: American Society for Microbiology; p. 28. Abstract A-1673. [Google Scholar]
- 49.Duwe AK, Rupar A, Horsman GB, et al. In vitro cytotoxicity and antibiotic activity of polymyxin B nonapeptide. Antimicrob Agents Chemother. 1986;30:340–1. doi: 10.1128/aac.30.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dillen K, Bridts C, Van der Veken P, et al. Adhesion of PLGA or Eudragit/PLGA nanoparticles to Staphylococcus and Pseudomonas. Int J Pharm. 2008;349:234–40. doi: 10.1016/j.ijpharm.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 52.Velasco J, Bengoechea JA, Brandenburg K, et al. Brucella abortus and its closest phylogenetic relative, Ochrobactrum spp., differ in outer membrane permeability and cationic peptide resistance. Infect Immun. 2000;68:3210–8. doi: 10.1128/iai.68.6.3210-3218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alves CS, Melo MN, Franquelim HG, et al. Escherichia coli cell surface perturbation and disruption induced by antimicrobial peptides, BP100 and pepR. J Biol Chem. 2010;285:27536–44. doi: 10.1074/jbc.M110.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohshima H. Electrophoresis of soft particles. Adv Colloid Interface Sci. 1995;62:189–235. doi: 10.1016/j.cis.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Ohshima H, Kondo T. Approximate analytic expression for the electrophoretic mobility of colloidal particles with surface-charge layers. J Colloid Interface Sci. 1989;130:281–2. doi: 10.1006/jcis.2001.7608. [DOI] [PubMed] [Google Scholar]