Abstract
Despite extensive research, the pathogenesis of neurodegenerative Alzheimer's disease (AD) still eludes our comprehension. This is largely due to complex and dynamic cross-talks that occur among multiple cell types throughout the aging process. We present a mathematical model that helps define critical components of AD pathogenesis based on differential rate equations that represent the known cross-talks involving microglia, astroglia, neurons, and amyloid-β (Aβ). We demonstrate that the inflammatory activation of microglia serves as a key node for progressive neurodegeneration. Our analysis reveals that targeting microglia may hold potential promise in the prevention and treatment of AD.
Introduction
Alzheimer's disease (AD) is one of the most prevalent neurodegenerative disorders associated with aging, causing dementia and related severe public health concerns [1]. Despite extensive research effort and progress, the pathogenesis of AD remains incompletely understood, partly due to highly complex and intertwined intercellular cross-talks taking place throughout the aging process [2]. Consequently, despite limited treatment options to manage and slow the progression of AD, no effective cure is available.
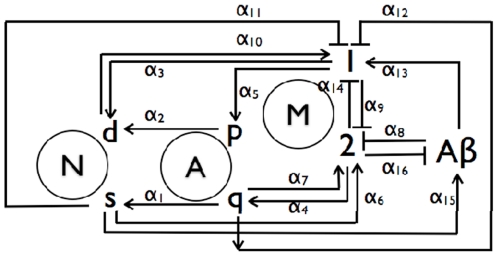
Although the deposition of amyloid-β (Aβ) peptides and formation of senile plaques in the brain is the cardinal morphological feature identifying the clinical phenotype of AD [3], [4], increasing clinical and basic studies suggest that inflammatory activation of microglia may play an equally important role during the initiation and progression of the disease [5]. Microglia are resident innate immune macrophages within brain tissues, capable of expressing pro-inflammatory mediators and reactive oxygen species when activated by inflammatory signals including amyloid-β (Aβ) [6]. In healthy brains, together with quiescent astroglia (Aq), resting microglia may adopt an anti-inflammatory state (M2) and in turn foster neuron survival (Ns) and prevent astroglia proliferation (Ap) [7], [8]. As inflammatory signals (e.g. Aβ) gradually build, microglia may adopt an activated pro-inflammatory state (M1), leading to Ap proliferation and neuron death (Nd) [9], [10], [11]. Neuronal debris, amyloid-β (Aβ), and/or proliferating astroglia (Ap) may in turn further exacerbate the inflammatory phenotype of M1 macroglia [12], [13]. The multiple positive and negative feedbacks among these cells are thus crucial for neurodegeneration that eventually alters the neuronal structure and function during the pathogenesis of AD (Figure 1).
Figure 1. Schematic of the AD mechanism that incorporates feedback influences from surviving and dead neurons, Ns and Nd, quiescent and proliferating astroglia Aq and Ap, reactive and normal microglia, M1 and M2, and Aβ.
The rates associated with the pathways are included in Table 1.
Due to its multi-cellular components and complex nature, conventional experimental approaches have failed to identify critical underlying causes for AD, contributing to the lack of an effective therapeutic treatment. Mathematical models can serve as powerful tools to understand the molecular and cellular processes that control complex diseases [14], [15]. Indeed, there have been several attempts to model the process of senile plaque formation [16], [17], [18], [19]. Specifically, these approaches focused on a nucleation step that is coupled with rates for the irreversible binding of Aβ monomers to the fibril ends, the lateral aggregation of filaments into fibrils, and fibril elongation through end-to-end association. Other modeling efforts examined the signaling cascade responsible for microglia migration and activation in response to an initial inflammation-provoking stimulus involving Aβ [16], [20].
However, no systematic modeling approaches have been reported to examine the network cross-talks among microglia, neuron, and astroglia, and the corresponding pathological consequence. Here, we evaluate the dynamic network involving multiple cross-talks among distinct states of microglia, astroglia, and neurons through a mathematical model. Our approach has led to an intriguing insight suggesting that microglia activation in addition to a threshold for Aβ may be the critical initiator for the pathogenesis of AD.
Methods
Mathematical Method
We propose a sixteen pathway AD mechanism involving seven species that is shown schematically in Fig. 1. The paths have rates αi that implicitly represent the influences of intercellular signaling along them. The mechanism is based on an assumption of constant risk of neuronal death, i.e., a single event randomly initiates cell death independently of the state of any other neuron at any instant [21]. The spatiotemporal influence of diffusion is neglected since local cell events are assumed to occur on a slower timescale than signal dispersion through chemotaxis.
The seven rate equations for the cell populations and the number of Aβ molecules in an arbitrary local volume can be written through seven coupled rate equations, namely,
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
These relate the change in each cell population or the number of Aβ molecules at any instant to the values of all species at that time. For instance, Eq. (1) relates the rate of change in Ns to the Aq, Ap, and M1 populations with the pathway weights α1, α2, and α3, respectively. Whereas Aq increases the rate of change of Ns, Ap and M1 decrease it. Equation (5) for the rate of change of the M2 population is the most complex, since it involves nine pathway weights, and five cell populations and Aβ. The conversion of Ns into Nd is irreversible, whereas those of Aq and M2 into Ap and M1 are reversible.
The rates for each αi are specified, as shown in presented in Table 1 for each pathway. Since the literature points to the path Ns → Aβ being dominant, we assume that it is also the fastest. Its rate is set at 1/year, i.e., each year every Ns cell stimulates the formation of a sustaining an Aβ molecule. Likewise, since neuronal survival decreases significantly once disease progresses, we assume that the overall path M2 → Aq → Ns is slow so that the associated rates α1 and α4 are also relatively the smallest. The other rates are similarly specified in terms of their relative abilities to facilitate or inhibit the formation of a cell or Aβ molecule according to the particular pathway. Next, we specify the initial composition of the volume under consideration. These initial conditions for the seven species are presented in Table 2.
Table 1. Mathematical parameters describing the functional interactions among various cell types.
| Rate | 1/year | Pathway | S(Ns) | S(Nd) | S(M1) | S(M2) | Sensitivity |
| α1 | 10−5 | Aq → Ns | 50000 | −50000 | −6000 | 6000 | Strong |
| α2 | 10−3 | Ap → Nd | −500 | 500 | −60 | 60 | Weak |
| α3 | 10−2 | M1 → Nd | −200 | 200 | −35 | 35 | Weak |
| α4 | 10−4 | M2 → Aq | 500 | −500 | 150 | −150 | Weak |
| α5 | 10−2 | M1 → Ap | −3 | 3 | 1 | −1 | Weak |
| α6 | 10−2 | Ns → M2 | 500 | −500 | −6000 | 6000 | Weak |
| α7 | 10−4 | Aq → M2 | 5000 | −5000 | −50000 | 50000 | Strong |
| α8 | 10−2 | Aβ ⊥ M2 | −400 | 400 | 5000 | −5000 | Moderate |
| α9 | 10−2 | M1 ⊥ M2 | −30 | 30 | 250 | −250 | Weak |
| α10 | 10−2 | Nd → M1 | −8 | 8 | 90 | −90 | Weak |
| α11 | 10−2 | Ns ⊥ M1 | 500 | −500 | −6000 | 6000 | Moderate |
| α12 | 10−4 | Aq ⊥ M1 | 5000 | −5000 | −50000 | 50000 | Strong |
| α13 | 10−2 | Aβ → M1 | −400 | 400 | 5000 | −5000 | Moderate |
| α14 | 10−4 | M2 ⊥ M1 | 5000 | −5000 | −50000 | 50000 | Strong |
| α15 | 1 | Ns → Aβ | −10 | 10 | 100 | −100 | Weak |
| α16 | 10−2 | M2 ⊥ Aβ | 100 | −100 | −1000 | 1000 | Weak |
| αr | 1 | M2 ⊥ Aβ | 8 | −8 | −100 | 100 | Weak |
The rates αi associated with the pathways of the AD mechanism, and the sensitivities of the Ns and Nd populations to variations in the values of αi. The values for the sensitivity coefficients S(Nj, j = s, d) = dNj/dαi are determined after 20 years for ±2.5% perturbations in each αi value. A cell population is more sensitive to a change in a rate that produces a larger value of |S(Nj)|. Positive S(Nj) imply that a rate contributes to an increase in Nj while a negative value entails a corresponding population decrease.
Table 2. Sensitivities of the cell types to the initial conditions.
| Ns(0) | Nd(0) | Aq(0) | Ap(0) | M1(0) | M2(0) | Aβ (0) | |
| Value | 104 | 102 | 105 | 103 | 103 | 105 | 103 |
| S(Ns) | 1 | ≈0 | ≈0 | ≈0 | −0.2 | 0 | ≈0 |
| S(Nd) | ≈0 | 1 | ≈0 | ≈0 | 0.2 | ≈0 | ≈0 |
| S(Aq) | ≈0 | ≈0 | 1 | ≈0 | −0.2 | ≈0 | ≈0 |
| S(Ap) | ≈0 | ≈0 | ≈0 | 1 | 0.2 | ≈0 | ≈0 |
| S(M1) | ≈0 | 0.2 | ≈0 | ≈0 | 1.2 | ≈0 | ≈0 |
| S(M2) | ≈0 | −0.2 | ≈0 | ≈0 | −0.2 | 1 | ≈0 |
| S(Aβ) | 1 | ≈0 | ≈0 | ≈0 | −0.2 | ≈0 | ≈0 |
Initial values X(0) of the initial cell populations and the number of molecules of Aβ in an arbitrary local volume and orders of magnitude for the sensitivity coefficients S(Nj, j = s,d) = dNj/d(X(0)) determined after 20 years for tenfold perturbations, i.e., 10× and 10−1×, in these initial values.
Results
Our objective is to be able to describe neuropathogenesis during AD in terms of the Ns and Nd populations. Hence, we first determine the sensitivities of these cells to changes in the rates αi, using the usual definition of the sensitivity coefficient,
| (8) |
The sensitivity coefficients for Ns, Nd, M1 and M2 cells, presented in Table 1, are determined after 20 years for ±2.5% perturbations in each αi value. A cell population is more sensitive to a change in a rate that produces a larger value of |S(Nj)|. Positive values for S(Nj) imply that a rate contributes to an increase in Nj while a negative value implies that its influence leads to a corresponding population decrease. The sensitivity analysis shows that the Ns and Nd populations are most sensitive to the path Aq → Ns, which increases neuronal survival and decreases neuron death. Important paths that inhibit neuropathogenesis include Aq → M2, Aq ⊥ M1 and M2 ⊥ M1, while those that enhance disease involve M1 → Nd, Aβ ⊥ M2 and Aβ → M1.
A similar analysis that perturbs the initial cell populations and the number of Aβ molecules tenfold is presented in Table 2. It shows that, in comparison to the other species, the M1 population is most sensitive to these substantial perturbations in the initial amount of any species while Nd is only sensitive to the initial amounts of M1 and M2. This implies an important role for microglia during AD progression. The sensitivity coefficients S(M1) and S(M2), also presented in Table 1, show that, as for Ns and Nd, the dominant paths that inhibit neuropathogenesis by affirming M2 and decreasing M1 are also Aq → M2, Aq ⊥ M1 and M2 ⊥ M1. Once again, paths 7 and 14 involving Aβ, i.e., Aβ ⊥ M2 and Aβ → M1 promote AD progression. The model suggests that interventions aimed at decreasing α8 and α13, which involve M1, M2 and Aβ and contribute to AD progression, are the ones more likely to diminish neuropathogenesis. This intuitive result emphasizes that decreasing the number of reactive microglia and ensuring a sufficient population of quiescent astroglia is important in treating AD.
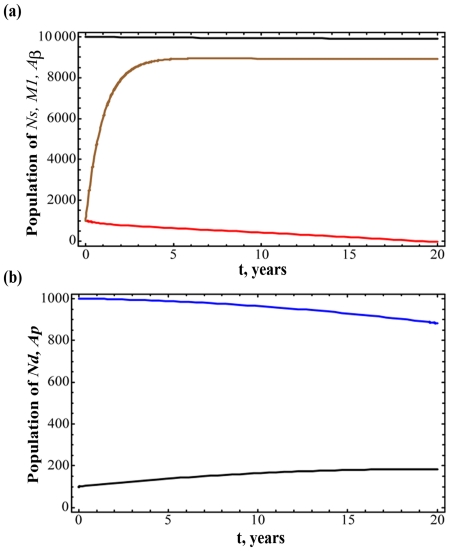
The temporal variation in various species for the rates in Table 1 is illustrated in Fig. 2. Figure 2(a) presents the Ns, M1 and Aβ populations over 20 years, and Fig. 2(b) the corresponding values for Nd and Ap. Most notable is the influence of the removal rate αr, which stabilizes the number of Aβ molecules after three years. Following that period, there is only a gradual increase in Nd that is coupled with a corresponding decline in Ns. Consequently, the microglia populations are also relatively stable. Therefore, the rates in Table 1 should be considered as being representative of a healthy population.
Figure 2. Dynamic simulation of various cell populations during the progression of Alzheimer's disease.
The (a) Ns (black), M1 (red) and Aβ (blue), and (b) Nd (black) and Ap (blue) populations over 20 years for the rates reported in Table 1. The removal rate αr stabilizes the net number of Aβ molecules after three years so that there is only a gradual increase in Nd and corresponding decline in Ns thereafter. The microglia populations are also consequently relatively stable.
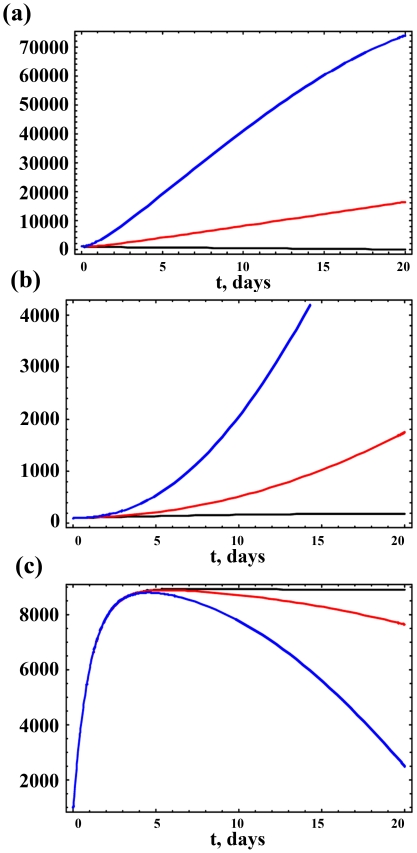
We examine the influence of varying αr on neuropathogeneis in Fig. 3, which presents the M1, Ap, Aβ and Nd populations over 20 years for three values of αr. As αr decreases, there is an increasing neuronal death. Thus, all four populations, which are associated with AD progression, increase. While microglia play an important role in AD, Fig. 3 shows how the local Aβ concentration plays a critical role in initiating and promoting AD.
Figure 3. Dynamic variations of cell populations given distinct Aβ removal rate.
Variations in the (a) M1, (b) Ap, (c) Aβ, and (d) Nd populations over 20 years for three values of αr = 1× (black), 10−1× (red), and 10−2× (blue) the value reported in Table 1 for the Aβ removal rate. As αr decreases, there is an increase in neuropathogenesis so that all four populations increase.
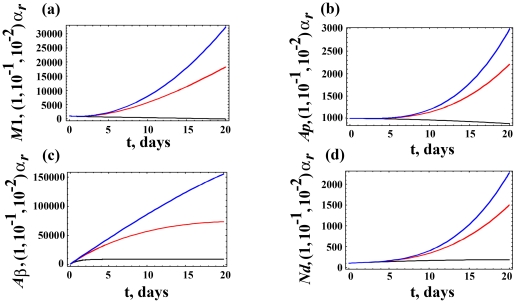
We investigate this further by varying α8 and α13. Figure 4 presents results for the M1, Nd and Aβ populations over 20 years for three values of α13. As α13 increases, the M1, Ap and Nd populations also increase, leading to an associated decrease in neuronal survival, as illustrated through Eqs. (1) and (2) of the mathematical model. A tenfold increase in α13 leads to a near doubling in Nd after 20 years. As Ns decreases so does Aβ, but the smaller protein concentration is still sufficient to promote neuropathogenesis among the smaller Ns population. Identical results are obtained for similar variations in the rate α8 for Aβ ⊥ M2, since the sensitivity coefficients for each of M1 and Nd towards paths 8 and 13 are identical.
Figure 4. Dynamic variations of cell populations given distinct impacts of Aβ on M1 macrophages (the value of α13).
Variations in the (a) M1, (b) Nd and (c) Aβ populations over 20 years for three values of α13 = 1× (black), 10× (red), and 50× (blue) the value reported in Table 1 for the path Aβ → M1. As α13 increases, M1 and Nd also increase and, consequently, there is an associated decrease in neuronal survival. This is also illustrated through Eqs. (1) and (2) of the mathematical model.
Discussion
We present a mathematical model for neuropathogenesis during AD that involves neurons, normal and reactive glial cells, and Aβ. It uses neuronal death as a surrogate for senile plaque formation. By monitoring neuronal health, we are able to identify intuitive strategies for interventions. In particular, the model suggests that the most effective intervention is one that improves the inhibition of reactive microglia and Aβ by normal microglia, and ensuring a sufficient population of quiescent astroglia. Overall, neuropathogenesis proceeds through the production of reactive microglia.
Our analysis is consistent with experimental data that indicate that inflammation may be an early initiator for AD, long before the apparent senile plaque formation [22], [23]. It further reinforces the notion that additional studies should be directed at examining earlier inflammatory signals and alterations involving microglia as a key node so as to better define AD initiation and understand mechanisms for effective prevention and treatment of the disease.
We realize that our mathematical analysis is an initial attempt to examine AD and may not fully account for the associate intertwined cellular communication pathways. Nevertheless, it serves as a hypothesis provoking and building process that should encourage integrated analyses of AD pathogenesis. Future experimental data examining the cross-talks among microglia, astroglia, and neurons will allow us to better refine our model and implement realistic parameters in the rate equations.
Acknowledgments
The authors would like to thank members of the Li laboratory for critical discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Internal funding from Virginia Tech supported this work. Virginia Tech had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 2.Citron M. Alzheimer's disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Neuroscience - Alzheimer's disease: Genotypes, phenotype, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 4.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 6.Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol. 2010;41:115–128. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang Y, Campbell L, Zheng B, Fan L, Cai Z, et al. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166:464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 10.Park KW, Baik HH, Jin BK. Interleukin-4-induced oxidative stress via microglial NADPH oxidase contributes to the death of hippocampal neurons in vivo. Curr Aging Sci. 2008;1:192–201. doi: 10.2174/1874609810801030192. [DOI] [PubMed] [Google Scholar]
- 11.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 12.Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiol Dis. 2010;37:503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein-Keshet L. Mathematical Models in Biology: Society for Industrial and Applied Mathematics 2005.
- 15.Ganguly R, Puri IK. Mathematical model for the cancer stem cell hypothesis. Cell proliferation. 2006;39:3–14. doi: 10.1111/j.1365-2184.2006.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelstein-Keshet L, Spiros A. Exploring the Formation of Alzheimer's Disease Senile Plaques in Silico. Journal of Theoretical Biology. 2002;216:301–326. doi: 10.1006/jtbi.2002.2540. [DOI] [PubMed] [Google Scholar]
- 17.Lomakin A, Teplow DB, Kirschner DA, Benedek GB. Kinetic Theory of Fibrillogenesis of Amyloid Œ≤ -protein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7942–7947. doi: 10.1073/pnas.94.15.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallitto MM, Murphy RM. A Mathematical Model of the Kinetics of [beta]-Amyloid Fibril Growth from the Denatured State. Biophysical Journal. 2001;81:1805–1822. doi: 10.1016/S0006-3495(01)75831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C-C, Nayak A, Sethuraman A, Belfort G, McRae GJ. A Three-Stage Kinetic Model of Amyloid Fibrillation. Biophysical Journal. 2007;92:3448–3458. doi: 10.1529/biophysj.106.098608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luca M, Chavez-Ross A, Edelstein-Keshet L, Mogilner A. Chemotactic signaling, microglia, and Alzheimer's disease senile plaques: Is there a connection? Bulletin of Mathematical Biology. 2003;65:693–730. doi: 10.1016/S0092-8240(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 21.Clarke G, Collins RA, Leavitt BR, Andrews DF, Hayden MR, et al. A one-hit model of cell death in inherited neuronal degenerations. Nature. 2000;406:195–199. doi: 10.1038/35018098. [DOI] [PubMed] [Google Scholar]
- 22.Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, et al. Inflammation occurs early during the Abeta deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Cuello AC, Ferretti MT, Leon WC, Iulita MF, Melis T, et al. Early-stage inflammation and experimental therapy in transgenic models of the Alzheimer-like amyloid pathology. Neurodegener Dis. 2010;7:96–98. doi: 10.1159/000285514. [DOI] [PubMed] [Google Scholar]