Abstract
Introduction:
Clinical and preclinical studies suggest that regulation of nicotinic acetylcholine receptors (nAChR) maybe involved in the etiology of withdrawal symptoms.
Methods:
We evaluated heteromeric nAChR regulation via [3H]epibatidine binding following cessation of chronic nicotine or varenicline treatment. Animals were concurrently tested in the marble-burying test to evaluate treatment-related effects.
Results:
We found that both nicotine (18 mg/kg/day, free base) and varenicline (1.8 mg/kg/day) chronically administered for 14 days upregulated nAChRs significantly in the cortex, hippocampus, striatum, and thalamus. The duration of upregulation (up to 72 hr) was both drug and region specific. In addition to nAChR upregulation, chronic administration of both nicotine and varenicline had anxiolytic-like effects in the marble-burying test. This effect was maintained for 48 hr following cessation of varenicline but was absent 24 hr following cessation from nicotine. Additionally, marble-burying behavior positively correlated to the regulation of cortical nAChRs following cessation of either treatment.
Conclusions:
Varenicline has been shown to be an efficacious smoking cessation aid, with a proposed mechanism of action that includes modulation of dopamine release in reward areas of the brain. Our studies show that varenicline elicits both anxiolytic effects in the marble-burying test as well as region- and time-specific receptor upregulation. These findings suggest receptor upregulation as a mechanism for its efficacy as a smoking cessation therapy.
Introduction
Smoking is the largest preventable cause of death and disease in the United States, with about 46 million U.S. adults currently smoking (Centers for Disease Control and Prevention, 2007). Furthermore, repeated quit attempts are common in smoking individuals, and a recent study found that less than 10% of quit attempts resulted in continuous abstinence for 1 year (Gonzales et al., 2006). Reasons cited for this impaired ability to remain abstinent include prolonged withdrawal symptoms comprised of both attentional (Rukstalis, Jepson, Patterson, & Lerman, 2005) and affective (Cook, Spring, McChargue, & Hedeker, 2004; Pomerleau et al., 2005) elements. With the advent of newer pharmacotherapies like Chantix (varenicline), successful quit attempts have significantly increased. Varenicline, a nicotinic acetylcholine receptor (nAChR) partial agonist, is reported to increase continuous abstinence rates to ∼50% following 12 weeks of treatment, which is significantly better than bupropion (∼30%) or placebo (∼18%; Cahill, Stead, & Lancaster, 2009; Gonzales et al., 2006; Nides et al., 2008). The rationale for varenicline's success is thought to be due to its dual actions on nicotinic signaling in the brain: (a) It acts as a partial agonist to elicit moderate amounts of dopamine from nerve terminals and (b) it has higher affinity at the receptor than nicotine and thus blocks nicotine's rewarding effects following an acute relapse (Rollema et al., 2007). However, the benefit of varenicline treatment is reduced by half at 1 year postquit (Gonzales et al., 2006), suggesting that other mechanisms may be important in maintaining long-term abstinence rates.
While varenicline is proposed to work via modulation of dopamine release in the mesolimbic reward pathways, a potential alternative to the dopamine reward hypothesis of smoking relapse is related to the direct effects of nicotine on select subtypes of nAChRs. Chronic administration of nicotine results in a robust upregulation of receptors, predominantly the α4β2* nAChR subtype (Flores, Rogers, Pabreza, Wolfe, & Kellar, 1992). Though the mechanism is not fully understood, this upregulation has been demonstrated in cell culture (Xiao et al., 2006), rodents (Marks, Burch, & Collins, 1983; Schwartz & Kellar, 1983), monkeys (Kassiou et al., 2001), and humans (Perry, Davila-Garcia, Stockmeier, & Kellar, 1999) and serves as a hallmark of chronic nicotine treatment. Although many studies have examined nicotine withdrawal symptoms, few studies have drawn parallels between the upregulation of nAChRs via chronic nicotine and withdrawal phenotypes. A recent single photon emission computed tomography (SPECT) study using a ligand specific to β2-containing receptors has suggested that increased relapse rate is directly related to nicotinic receptor availability (Cosgrove et al., 2009). Furthermore, the study demonstrated that these receptors remain elevated in the clinical population up to 12 weeks. Few preclinical studies have established a withdrawal time course for receptors to return to baseline following chronic nicotine treatment (Collins, Luo, Selvaag, & Marks, 1994; Hulihan-Giblin, Lumpkin, & Kellar, 1990; Pietila, Lahde, Attila, Ahtee, & Nordberg, 1998), and none have correlated this time course with any behavioral outcomes. Furthermore, studies examining varenicline's effects on nAChR regulation are completely lacking.
While its effects on receptor regulation are unknown, varenicline can enhance both positive affect and cognitive function during smoking cessation in clinical studies (Patterson et al., 2009) and can augment the effects of antidepressants in depressed smokers (Philip, Carpenter, Tyrka, Whiteley, & Price, 2009) as well as in a preclinical model of antidepressant efficacy (Rollema, Guanowsky, et al., 2009). Depression and anxiety often accompany nicotine withdrawal (Dani & Harris, 2005). However, the impact of varenicline on anxiety-related behaviors has not been well studied. Furthermore, the effects of nicotinic compounds on various preclinical tests of anxiety have been mixed as studies examining the effects of nicotine in the elevated zero maze, the elevated plus maze, and the mirror chamber have yielded diverse and conflicting results (Picciotto, Brunzell, & Caldarone, 2002). One potential paradigm used to evaluate putative anxiolytic compounds that is sensitive to nicotine as well as a variety of anxiolytic compounds is the marble-burying test (Broekkamp, Rijk, Joly-Gelouin, & Lloyd, 1986; Ichimaru, Egawa, & Sawa, 1995; Njung’e & Handley, 1991; Turner, Castellano, & Blendy, 2010). Therefore, in the present study, we examined the effects of chronic nicotine and varenicline on marble burying in a comprehensive time course of withdrawal in parallel with nicotinic receptor binding.
Materials and methods
Animals
Male 129SvJ;C57Bl/6J F1 hybrid mice (6–12 weeks of age, 25–35 g) were bred, group housed, and maintained on a 12-h light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. All experimental testing sessions were conducted between 9:00 and 11:00 a.m., with animals randomly assigned to treatment conditions and tested in counterbalanced order.
Drugs
Doses of nicotine tartrate (Sigma-Aldrich, St. Louis, MO) and varenicline tartrate (provided by Pfizer Global Research and Development, Groton, CT) are reported as free-base weight.
Osmotic Minipumps
Nicotine tartrate and varenicline tartrate were dissolved in sterile 0.9% saline solution and infused through subcutaneous osmotic minipumps for 14 days (Model 2002; Alzet, Palo Alto, CA). Mice were anesthetized with an isoflurane/oxygen vapor mixture (1%–3%), and osmotic minipumps were inserted subcutaneously using aseptic surgery techniques. Minipumps were placed parallel to the spine at shoulder level with the flow moderator directed away from the wound. The wound was closed with 7-mm stainless steel wound clips (Reflex, Cellpoint Scientific, Gaithersburg, MD). Two weeks following minipump implantation, withdrawal was induced by surgical removal of the osmotic minipumps (saline, nicotine, and varenicline) at specific timepoints prior to testing (24 hr, 48 hr, 72 hr, or 7 days).
Marble-Burying Test
After 1-hr acclimation, mice (n = 6 per group) were placed individually in small cages (26 × 20 × 14 cm) in which 20 marbles had been equally distributed on top of mouse bedding (5-cm deep), and a lid was placed on top of the cage. Mice were left undisturbed for 15 min, after which time a blind observer counted the number of buried marbles (i.e., those covered by bedding three quarters or more).
Receptor Binding
Brain regions examined were constrained by a minimal tissue amount required for homogenate-binding assays. Tissues were harvested from animals immediately following behavioral testing. The samples were homogenized in 50 mM Tris–HCl (Sigma-Aldrich) buffer, pH 7.4 at 24°C, and centrifuged twice at 35,000 × g for 10 min in fresh buffer. The membrane pellets were resuspended in fresh buffer and added to tubes containing a saturating concentration (2 nM) of [3H]epibatidine ([3H]EB; PerkinElmer, Boston, MA), an excellent ligand because of its extremely low nonspecific binding and its high-affinity binding to all heteromeric nAChRs. Incubations were performed in Tris buffer at pH 7.4 for 2 hr at 24°C with [3H]EB. Bound receptors were separated from free ligand by vacuum filtration over GF/C glass fiber filters (Brandel, Gaithersburg, MD) that were pretreated with 0.5% polyethyleneimine (Sigma-Aldrich). The filters were then counted in a liquid scintillation counter. Nonspecific binding was determined in the presence of 300 μM nicotine, and specific binding was defined as the difference between total binding and nonspecific binding.
Data Analysis
Using the GraphPad Prism 5.0 software package (GraphPad Software, San Diego, CA), statistical analyses of the differences between groups were assessed using two-way analysis of variance followed by Bonferroni's multiple comparison tests. To better assess the time course and significant correlations between nAChR-binding densities and the number of marbles buried in the marble-burying test, we used group data in the generation of plotted points for a Pearson correlation analysis with 95% CI.
Results
Both nicotine and varenicline treatment results in long-lasting upregulation of nicotinic receptors in the cortex, striatum, hippocampus, and thalamus. Although a hallmark of chronic nicotine administration is the upregulation of heteromeric nicotinic receptors, no previous studies have examined the effect of chronic varenicline, an α4β2 nAChR partial agonist, on nAChR regulation in vivo. Furthermore, few studies have examined the time course for return of receptors to baseline levels following cessation of chronic treatment. Therefore, to determine if heteromeric nAChRs were upregulated following chronic administration of nicotine and varenicline and to evaluate the time course of upregulated nAChRs to return to baseline, we treated mice with either nicotine or varenicline for 14 days and then examined receptor levels with [3H]EB, which binds with very high affinity to all heteromeric nAChR subtypes in brain.
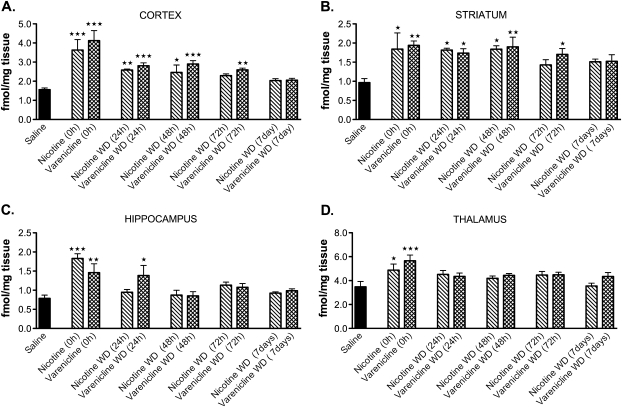
As shown in Figure 1A–D, both chronic nicotine (18 mg/kg/day, free base) and chronic varenicline (1.8 mg/kg/day) administration resulted in significant upregulation of nAChRs in the cortex, striatum, hippocampus, and thalamus. Drug-specific effects were observed in the cortex (A), striatum (B), and hippocampus (C), where the upregulation induced by varenicline treatment was significantly longer lasting compared with chronic nicotine treatment. Additionally, a region-specific effect was observed. Overall upregulation of nAChRs following cessation of treatment remained significantly elevated for up to 72 hr in the cortex (A) and striatum (B), while the hippocampus (C) and thalamus (D) rapidly downregulated nAChRs following termination of treatment.
Figure 1.
Effects of chronic treatment of nicotine and varenicline on nicotinic receptor regulation. Homogenate-binding experiments with a saturating concentration of [3H]epibatidine ([3H]EB, 2 nM) were performed on cortical (A), striatal (B), hippocampal (C), and thalamic (D) tissues from chronically treated animals. (A) Cortical homogenates from mice that received chronic treatment with nicotine (18 mg/kg/day) or varenicline (1.8 mg/kg/day) had significantly higher levels of [3H]EB binding relative to saline-treated controls, which persisted for up to 72 hr. (B) Striatal homogenates from mice that received chronic treatment with nicotine (18 mg/kg/day) or varenicline (1.8 mg/kg/day) had significantly higher levels of [3H]EB binding relative to saline-treated controls, which persisted for up to 72 hr. (C) Hippocampal homogenates from mice that received chronic treatment with nicotine (18 mg/kg/day) or varenicline (1.8 mg/kg/day) had significantly higher levels of [3H]EB binding relative to saline-treated controls, which persisted for up to 24 hr. (D) Thalamic homogenates from mice that received chronic treatment with nicotine (18 mg/kg/day) or varenicline (1.8 mg/kg/day) had significantly higher levels of [3H]EB binding relative to saline-treated controls (***p = .001; **p = .01; *p = .5; N = 6 per group). WD = withdrawal.
Chronic nicotine and varenicline have anxiolytic-like effects in the marble-burying test and duration of this effect correlates to regulation of the α4β2* nAChR subtype. We previously found both acute nicotine and acute varenicline to have anxiolytic effects in the marble-burying paradigm (Turner et al., 2010). However, the effects of chronic nicotine or varenicline administration in this test are unknown. Therefore, to evaluate if changes in anxiety behaviors correlated with receptor upregulation, chronically treated animals were tested in the marble-burying paradigm prior to receptor-binding studies with [3H]EB.
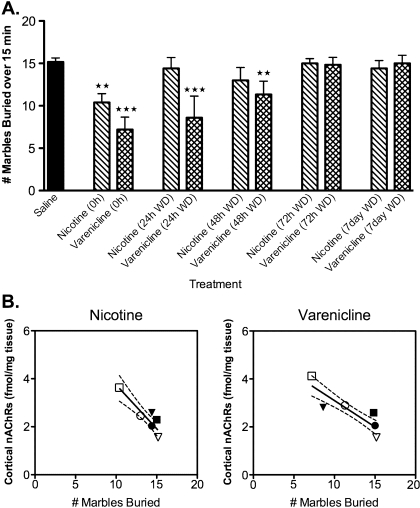
As shown in Figure 2A, both chronic nicotine (18 mg/kg/day) and chronic varenicline (1.8 mg/kg/day) resulted in a reduced number of marbles being buried, indicating an anxiolytic-like response. In nicotine-treated animals, this effect was only observed during drug administration and behavior returned to baseline by 24 hr. However, in varenicline-treated animals, an anxiolytic-like effect was observed up to 48 hr following cessation of treatment.
Figure 2.
Chronic nicotine and varenicline had anxiolytic effects in the marble-burying test, which correlated to nAChR levels in the cortex. (A) Animals chronically treated with either nicotine (18 mg/kg/day) or varenicline (1.8 mg/kg/day) buried fewer marbles than saline controls. In contrast to the nicotine treated group, this effect persisted for 48 hr in the varenicline-treated animals (***p = .001; **p = .01). (B) Treatment with either nicotine (18 mg/kg/day) or varenicline (1.8 mg/kg/day) resulted in a significant correlation between density of cortical nAChRs and the number of marbles buried in all treatment groups (p < .02). Group data for the respective treatment groups (nicotine or varenicline) are represented in the correlational graphs. In each of the panels, the hollow square (□) represents the 0-hr timepoint, filled triangle (▾) represents the 24-hr withdrawal (WD) timepoint, hollow circle (○) represents the 48-hr WD timepoint, filled square (▪) represents the 72-hr WD timepoint, filled circle (•) represents the 7-day WD timepoint, and hollow triangle (△) represents the grouped saline data. Dashed lines indicate the 95% CI (N = 5–8 per group).
One strength of the experimental design was the ability to directly correlate behaviors observed in the marble-burying test with nAChR levels as evaluated by [3H]EB binding. Of interest, in the cortex, [3H]EB binding was significantly correlated with marble-burying behavior in both the nicotine and the varenicline treatment groups across all timepoints, where higher levels of nAChRs corresponded to fewer marbles buried (Figure 2B). No significant correlation was observed between the density of nicotinic receptors in the striatum, hippocampus, or thalamus with the number of marbles buried (data not shown).
Discussion
Our findings indicate that both chronic nicotine and chronic varenicline induce significant nAChR upregulation in cortex, striatum, hippocampus, and thalamus. These effects were longer lasting in the varenicline treatment group than in the nicotine treatment group. This may be explained by differences in nicotine and varenicline pharmacokinetics and metabolism in the mouse. Previous work has shown that nicotine is rapidly metabolized, resulting in a very short half-life in the mouse of 6–7 min (Matta et al., 2007). In contrast, varenicline is not effectively metabolized and exhibits a relatively long half-life in the mouse of 1.4 hr (Obach et al., 2006). Differences in receptor affinities for nicotine and varenicline may also account for their differences in the timecourse of nAChR regulation. The Ki of varenicline for α4β2 nAChRs (0.4 nM) is ∼10× higher than that of nicotine (6.0 nM; Rollema, Hajos, et al., 2009). However, we used chronic doses that reflect this difference in affinity (18 mg/kg/day nicotine vs. 1.8 mg/kg/day varenicline).
Both chronic nicotine and chronic varenicline produced effects in the marble-burying test in mice. Similar to the binding data, varenicline's effect on marble burying was longer lasting than that of nicotine. By 24 hr following cessation of drug administration, nicotine was no longer anxiolytic, while the varenicline-treated groups continued to display anxiolytic effects up to 48 hr following cessation of treatment. Unlike previous studies examining symptoms of nicotine withdrawal (Fowler, Arends, & Kenny, 2008; Jackson, McIntosh, Brunzell, Sanjakdar, & Damaj, 2009; Stoker, Semenova, & Markou, 2008), no anxiogenic withdrawal effect was apparent in the marble-burying test. This lack of withdrawal phenotype in the marble-burying test may lie with the test itself. A recent study reported that marble burying does not directly correlate with other tests of anxiety (open-field and light–dark box) and may more accurately reflect measures of perseverative behavior (Thomas et al., 2009). While the Thomas et al. study examined anxiety-like behavior in the marble-burying test, it did not correlate anxiolytic efficacy in multiple tests of anxiety. However, the present study compared anxiolytic efficacy of nicotinic compounds, and in this regard, the marble-burying test has been shown to be responsive to clinically relevant medications for anxiety disorders, both anxiolytics such as chlordiazepoxide and antidepressants such as fluoxetine (Broekkamp et al., 1986; Ichimaru et al., 1995; Nicolas, Kolb, & Prinssen, 2006; Njung’e & Handley, 1991). Furthermore, in recent studies, we have shown that acute doses of nicotine and varenicline have anxiolytic-like effects in the marble-burying test and the novelty-induced hypophagia test (a test sensitive to both anxiolytics as well as antidepressants; Turner et al., 2010).
In both the nicotine- and varenicline-treated groups, the anxiolytic effects in the marble-burying task could be significantly correlated to nicotinic receptor levels in the cortex but not to nAChR levels in the other brain regions. While the cortex may play a role in mediating marble-burying behavior, this correlation may also reflect a difference in nAChR subtypes within this brain region. Heteromeric nAChRs in the cortex are almost exclusively made up of the α4β2 subtype, which is the subtype most readily upregulated following chronic administration of nicotine (Gaimarri et al., 2007). Thus, regulation of this subtype may underlie the affective effects of both nicotine and varenicline. This is supported by the observation that α4* or β2* knockout mice do not express anxiogenic phenotypes following nicotine withdrawal (Fowler et al., 2008).
Many smokers experience anxiety symptoms during acute abstinence, which may contribute to relapse (Dani & Harris, 2005). Not surprisingly, adherence to treatment for tobacco dependence has been shown to predict abstinence rates (Hays, Leischow, Lawrence, & Lee, 2010). There are many factors underlying poor adherence to treatment regimens, one of which is affective state (DiMatteo, Lepper, & Croghan, 2000). The significance of nAChR upregulation in the etiology of smoking behavior is not well understood. The present data demonstrate a striking temporal correlation between nAChR upregulation and anxiolytic-like response to chronic nicotine and varenicline. Determining if these outcomes are causally related will be important. Furthermore, understanding how varenicline extends receptor upregulation compared with nicotine may lead to the development of more effective therapies for smoking cessation.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (P50 CA 143187) and by the National Institute on Drug Abuse at the National Institutes of Health (1-F32-DA026236-01A1).
Declaration of Interests
JRT, LMC, and JAB have no competing interests.
Acknowledgments
We thank Dr. Hans Rollema (Pfizer, Groton, CT) for providing a sample and pharmacological details of varenicline.
References
- Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. European Journal Pharmacology. 1986;126:223–229. doi: 10.1016/0014-2999(86)90051-8. doi:10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead L, Lancaster T. A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Safety. 2009;32:119–135. doi: 10.2165/00002018-200932020-00005. doi:5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults–United States, 2006. Morbidity Mortality Weekly Report. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Collins AC, Luo Y, Selvaag S, Marks MJ. Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. Journal of Pharmacology and Experimental Therapeutics. 1994;271:125–133. [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. doi:10.1080/1462220031000165684946RJ5WGKFDMEANTP. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T. Beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of General Psychiatry. 2009;66:666–676. doi: 10.1001/archgenpsychiatry.2009.41. doi:66/6/666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nature Neuroscience. 2005;8:1465–1470. doi: 10.1038/nn1580. doi:nn1580. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. doi:ioi90679. [DOI] [PubMed] [Google Scholar]
- Flores C, Rogers S, Pabreza L, Wolfe B, Kellar K. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Molecular Pharmacology. 1992;41:31–37. [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: Evidence from genetically modified mice. Behavioral Pharmacology. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. doi:10.1097/FBP.0b013e32830c360e00008877-200809000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaimarri A, Moretti M, Riganti L, Zanardi A, Clementi F, Gotti C. Regulation of neuronal nicotinic receptor traffic and expression. Brain Research Reviews. 2007;55:134–143. doi: 10.1016/j.brainresrev.2007.02.005. doi:S0165-0173(07)00029-X. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. doi:296/1/47. [DOI] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: Association with smoking abstinence and predictors of adherence. Nicotine and Tobacco Research. 2010;12:574–581. doi: 10.1093/ntr/ntq047. doi:ntq047. [DOI] [PubMed] [Google Scholar]
- Hulihan-Giblin BA, Lumpkin MD, Kellar KJ. Effects of chronic administration of nicotine on prolactin release in the rat: Inactivation of prolactin response by repeated injections of nicotine. Journal of Pharmacology and Experimental Therapeutics. 1990;252:21–25. [PubMed] [Google Scholar]
- Ichimaru Y, Egawa T, Sawa A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Japanese Journal of Pharmacology. 1995;68:65–70. doi: 10.1254/jjp.68.65. doi:10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. Journal of Pharmacology and Experimental Therapeutics. 2009;331:547–554. doi: 10.1124/jpet.109.155457. doi:jpet.109.155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiou M, Eberl S, Meikle SR, Birrell A, Constable C, Fulham MJ, et al. In vivo imaging of nicotinic receptor upregulation following chronic (-)-nicotine treatment in baboon using SPECT. Nuclear Medicine and Biology. 2001;28:165–175. doi: 10.1016/s0969-8051(00)00206-7. doi: S0969-8051(00)00206-7. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1983;226:817–825. [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berlin) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. doi:10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Nicolas L, Kolb Y, Prinssen E. A combined marble burying-locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. European Journal of Pharmacology. 2006;547:106–115. doi: 10.1016/j.ejphar.2006.07.015. doi:10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nides M, Glover ED, Reus VI, Christen AG, Make BJ, Billing CB., Jr. Varenicline versus bupropion SR or placebo for smoking cessation: A pooled analysis. American Journal of Health Behavior. 2008;32:664–675. doi: 10.5555/ajhb.2008.32.6.664. doi:10.5555/ajhb.2008.32.6.664. [DOI] [PubMed] [Google Scholar]
- Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacology Biochemistry and Behavior. 1991;38:63–67. doi: 10.1016/0091-3057(91)90590-x. doi:0091-3057(91)90590-X. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metabolism and Disposition. 2006;34:121–130. doi: 10.1124/dmd.105.006767. doi:dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser A, Loughead J, Perkins K, Gur R, et al. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. doi:10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: Membrane binding and autoradiography studies. Journal of Pharmacology and Experimental Therapeutics. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Philip N, Carpenter L, Tyrka A, Whiteley L, Price L. Varenicline augmentation in depressed smokers: An 8-week, open-label study. Journal of Clinical Psychiatry. 2009;70:1026–1031. doi: 10.4088/jcp.08m04441. doi:ej08m04441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pietila K, Lahde T, Attila M, Ahtee L, Nordberg A. Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn Schmiedeberg's Archives of Pharmacology. 1998;357:176–182. doi: 10.1007/pl00005152. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. doi: T7882681U7106256. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends in Pharmacological Sciences. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. doi:S0165-6147(07)00127-7. [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. European Journal of Pharmacology. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. doi:S0014-2999(09)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochemical Pharmacology. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. doi:S0006-2952(09)00445-6. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. Journal of Substance Abuse Treatment. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. doi:S0740-5472(05)00042-5. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Kellar K. Nicotinic cholinergic receptor binding sites in the brain: Regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Semenova S, Markou A. Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology. 2008;54:1223–1232. doi: 10.1016/j.neuropharm.2008.03.013. doi: S0028-3908(08)00083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor L, Paylor R. Marble burying reflects a repetitive and perservative behavior more than novelty-induced anxiety. Psychopharmacology (Berlin) 2009 doi: 10.1007/s00213-009-1466-y. doi:10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-a have differential effects on affective behavior. Journal of Pharmacology and Experimental Therapeutics. 2010;334:665–672. doi: 10.1124/jpet.110.166280. doi:jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, et al. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. doi:10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]