Abstract
Background
Dose-dense chemotherapy has become a mainstay regimen in the adjuvant setting for women with high-risk breast cancer. We performed a systematic review and meta-analysis of the existing data from randomized controlled trials regarding the efficacy and toxicity of the dose-dense chemotherapy approach in nonmetastatic breast cancer.
Methods
Randomized controlled trials that compared a dose-dense chemotherapy protocol with a standard chemotherapy schedule in the neoadjuvant or adjuvant setting in adult women older than 18 years with breast cancer were identified by searching The Cochrane Cancer Network register of trials, The Cochrane Library, and LILACS and MEDLINE databases (from January 1966 to January 2010). Hazard ratios (HRs) of death and recurrence and relative risks of adverse events were estimated and pooled. All statistical tests were two-sided.
Results
Ten trials met the inclusion criteria and were classified into two categories based on trial methodology. Three trials enrolling 3337 patients compared dose-dense chemotherapy with a conventional chemotherapy schedule (similar agents). Patients who received dose-dense chemotherapy had better overall survival (HR of death = 0.84, 95% confidence interval [CI] = 0.72 to 0.98, P = .03) and better disease-free survival (HR of recurrence or death = 0.83, 95% CI = 0.73 to 0.94, P = .005) than those on the conventional schedule. No benefit was observed in patients with hormone receptor–positive tumors. Seven trials enrolling 8652 patients compared dose-dense chemotherapy with regimens that use standard intervals but with different agents and/or dosages in the treatment arms. Similar results were obtained for these trials with respect to overall survival (HR of death = 0.85, 95% CI = 0.75 to 0.96, P = .01) and disease-free survival (HR of recurrence or death = 0.81, 95% CI = 0.73 to 0.88, P < .001). The rate of nonhematological adverse events was higher in the dose-dense chemotherapy arms than in the conventional chemotherapy arms.
Conclusion
Dose-dense chemotherapy results in better overall and disease-free survival, particularly in women with hormone receptor–negative breast cancer. However, additional data from randomized controlled trials are needed before dose-dense chemotherapy can be considered as the standard of care.
CONTEXT AND CAVEATS
Prior knowledge
Dose-dense chemotherapy has become a mainstay regimen in the adjuvant setting for women with high-risk breast cancer.
Study design
Systematic review and meta-analysis of 10 randomized controlled trials that compared dose-dense chemotherapy with a standard chemotherapy schedule in women with nonmetastatic breast cancer.
Contribution
Dose-dense chemotherapy results in better overall and disease-free survival, particularly in women with hormone receptor–negative breast cancer. The rate of nonhematological adverse events was higher in the dose-dense chemotherapy arms than in the conventional chemotherapy arms.
Implications
The lack of obvious benefit in patients with hormone receptor–positive breast cancer indicates the need for further prospective randomized trials of the classical conserved design in this patient population.
Limitations
There was substantial statistical heterogeneity among the trials. The small number of included trials makes the outcomes more likely to have been influenced by a potential publication bias.
From the Editors
The implementation of adjuvant therapy, hormonal therapy, and chemotherapy has made a major impact on disease-free survival and overall survival in premenopausal and postmenopausal women with early-stage breast cancer (1). Unfortunately, many breast cancer patients who are diagnosed and treated properly will suffer from recurrence and ultimately die of this disease. Since the introduction of anthracycline-based regimens for the treatment of breast cancer and the demonstration of their superiority to other combination chemotherapy (1), a variety of approaches have evolved regarding adjuvant polychemotherapy, including the addition of novel drugs such as taxanes and targeted agents and the establishment of dose-intensive (2) and dose-dense chemotherapy regimens (3,4) that were based on mathematical models of human breast cancer growth (5).
Administration of dose-dense chemotherapy became possible with the introduction of granulocyte colony-stimulating factor, which allowed the chemotherapy courses to be condensed without causing unacceptable toxicity. A dose-dense chemotherapy approach using concurrent doxorubicin and cyclophosphamide followed by paclitaxel was assessed in the pivotal Cancer and Leukemia Group B 9741 trial, a phase III prospective randomized trial of adjuvant treatment of women with node-positive early-stage breast cancer (6). This trial showed a statistically significant improvement in disease-free survival and overall survival for the dose-dense chemotherapy arm and has become the cornerstone for the current therapeutic treatment of early-stage breast cancer in many centers.
The Italian Gruppo Oncologico Nord Ovest-Mammella InterGruppo trial used a similar approach to test a non-taxane–based regimen (7). Although the point estimates for event-free survival and overall survival favored the dose-dense regimen, they were not statistically significant (7). A third trial in the neoadjuvant setting failed to show statistically significant improvement in pathological response rates, disease-free survival, or overall survival for the dose-dense regimen (8). Several other randomized trials have examined the concept of dose-dense chemotherapy (9–18); however, these studies are difficult to interpret because the treatment groups differed in terms of dose density, drug regimens, number of cycles, and the application of sequential strategies.
Given this paucity of data, we performed a systematic review and meta-analysis of the evidence from randomized trials for the efficacy and toxicity of the dose-dense chemotherapy approach in the treatment of early-stage and locally advanced breast cancer.
Methods
Search Strategy
Relevant randomized clinical trials were identified by searching the most recent update of The Cochrane Central Register of Controlled Trials (January 2010), The Cochrane Library (January 2010 issue), and LILACS (Latin American and Caribbean Health Sciences) and MEDLINE databases (January 1, 1966, to January 1, 2010). The terms “adjuvant,” “neoadjuvant,” and “dose-dense chemotherapy” (and similar terms) and “breast cancer” and similar terms were cross-searched by using the following search algorithm: ((dose-dense chemotherapy OR dense OR acceler* OR weekly OR bi-weekly OR biweekly) AND (Breast neoplasm MeSH OR ((breast OR mammary OR mamario OR seno) AND (carcinoma OR malignan* OR neoplasm OR tumor)))) AND (randomized controlled trial [pt]OR controlled clinical trial [pt]OR randomized controlled trial [mh]OR double-blind method [mh] OR single-blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial”) [tw] OR singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw] AND (mask* [tw] OR blind* [tw]))) OR (placebos [mh] OR placebo* [tw] or random* [tw] PR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospective* [tw] OR volunteer* [tw] NOT (animals [mh] NOT humans [mh]). We also searched for relevant abstracts in the annual conference proceedings up to December 2009 for the American Society of Clinical Oncology, European Society for Medical Oncology, and the San Antonio Breast Cancer Symposium. References of selected articles were scanned for any other relevant trials, and the original trialists were contacted about possible unpublished trials.
Selection Criteria
We included in the systematic review all randomized controlled trials that compared a dose-dense chemotherapy protocol with a standard schedule of chemotherapy in the neoadjuvant or adjuvant setting in adult women older than 18 years with breast cancer. Trials were included regardless of publication status and language. Two trials in a foreign language were assessed by a native speaker (neither was included in the meta-analysis). We defined two types of trials: Trials that evaluated similar doses of agents in both treatment arms but with condensed schedule in one arm, which we designated conserved dose-dense chemotherapy trials, and trials that compared a condensed schedule with a standard one but used different agents or doses in the two arms were designated modified dose-dense chemotherapy trials. Trials in which overall survival was the primary outcome measure but was not reported were included if all other inclusion criteria were met. Authors of these trials were contacted and asked to provide data for the primary outcome measure if those data have been collected.
We excluded trials that assessed dose-dense chemotherapy in malignancies other than breast cancer or that evaluated dose-dense chemotherapy combined with radiotherapy, trials in which the definition of dose-dense chemotherapy differed from the original definition (4), trials that included high-dose chemotherapy arms with peripheral stem cell support, and randomized controlled phase II trials that evaluated toxicity only.
Two authors (LB and IBA) independently inspected each reference title identified by the search and applied the inclusion criteria. For possibly relevant articles and in cases of disagreement between the two reviewers, the full article was obtained and inspected independently by the two reviewers.
Data Extraction and Quality Assessment
Trials that fulfilled the systematic review inclusion criteria were assessed for methodological quality by two authors (LB and IBA). Information about randomization and allocation concealment, sample size, exclusions after randomization, and the length of follow-up were recorded as is considered acceptable for Cochrane reviews (19). The same two authors independently extracted the data from publications of included trials. The data extraction was discussed, decisions were documented, and, if necessary, the authors of the trials were contacted for clarification. Authors of included trials were contacted for all data relevant to the primary and secondary outcomes of this study and quality variables.
Outcome Measures
The primary outcome was overall survival, which was defined as time from randomization to death. Secondary outcomes were disease-free survival (defined as the time from randomization to earliest occurrence of relapse or death from any cause), event-free survival (defined as the length of time from the end of treatment to the earliest occurrence of a severe side effect of treatment, cancer recurrence or progression, or death from treatment side effects or from the cancer itself) (www.nature.com/nrc/journal/v3/n7/glossary/nrc1125_glossary.html), and toxicity (defined as grade 3 or 4 hematological and nonhematological adverse events). For the analysis of toxicity, we calculated the mean number of events per chemotherapy cycle by summing all events reported and dividing by the number of cycles delivered in each trial.
Statistical Analysis
Hazard ratios (HRs) and variances for time to event outcomes were estimated as described by Parmar et al. (20) and pooled according to the inverse of variance method with the use of Review Manager software (version 4.2 for Windows; The Cochrane Collaboration, Oxford, UK). A hazard ratio less than 1 favored dose-dense chemotherapy. Pooled relative risks (RRs) and 95% confidence intervals (CIs) for dichotomous data were estimated using the Mantel–Haenszel method (21). We assessed heterogeneity of the trials’ results by inspecting graphical presentations and by calculating a χ2 test of heterogeneity and the I2 statistic of inconsistency. We also report the Z statistic for the overall effect (Review Manager). Statistically significant heterogeneity was defined as a χ2 P value less than .1 or an I2 statistic greater than 50% (www.nature.com/nrc/journal/v3/n7/glossary/nrc1125_glossary.html). We used a fixed-effect model to pool relative risks for toxicity except in the event of statistically significant heterogeneity, in which case a random-effects model was used (ie, the inverse of variance method and the DerSimonian and Laird method) (17).
Subgroup analyses were performed to assess potential contributions to the main outcomes. A funnel plot estimating the precision of trials (plots of logarithm of the hazard ratio for efficacy against the sample size) was examined for asymmetry to estimate publication bias. Publication bias was also estimated by the formal Begg–Mazumdar rank correlation test and the Egger test (18,22).
Sensitivity analyses were performed to assess the robustness of the findings to different aspects of the trials methodology, including allocation concealment (adequate or unclear), exclusions after randomization (reported or not reported), sample size (≤100 vs >100 patients; cutoff was adopted from routine studies in which fewer than 100 patients is considered a “small” study), and length of follow-up. All statistical tests were two-sided. P values less than 0.5 were considered statistically significant.
Results
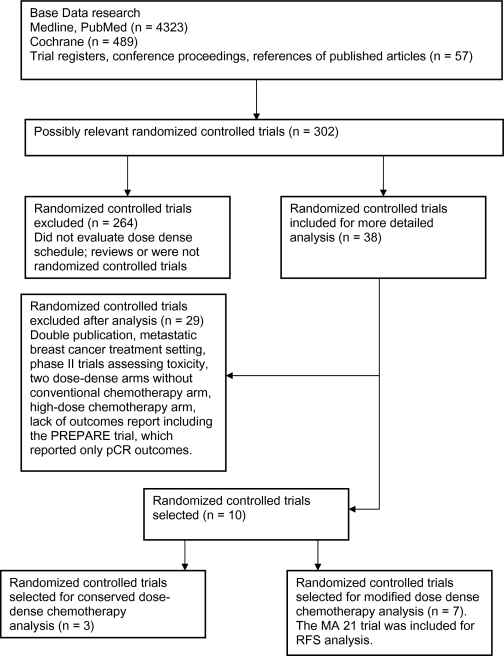
We identified 4869 potentially relevant articles in the primary literature search (Figure 1), of which 11 were randomized phase III trials that met the inclusion criteria and were divided into two groups based on the trial methodology. The first group—the conserved dose-dense chemotherapy trials—included three trials (Table 1), and the second group—the modified dose-dense chemotherapy trials—included seven trials (Table 2). The PREPARE trial (24) was included in the systematic review but not in the meta-analysis because no survival outcomes were provided. Chemotherapy protocols of the included trials are listed in Table 3.
Figure 1.
Randomized controlled trials search and selection. pCR = pathological complete response; RFS = relapse-free survival.
Table 1.
Conserved dose-dense chemotherapy trials*
| First author, year (reference) | Study location | Treatment setting | Treatment protocol† | Treatment interval,‡ d | Number of patients | Follow-up, mo | Median age, y | Stage | ER status, % | PR status, % | Number of events |
||
| Tumor size | Nodal status, % | DFS | OS | ||||||||||
| Venturini, 2005 (7) | Italy | Adjuvant | ECF | 14 | 604 | 316 | 50 | <2 cm: 49; 2.1– 5.0 cm: 45; 5 cm: 5 | Neg: 36; Pos: 64 | Pos: 52; Neg: 41 | Pos: 39; Neg: 48 | 168 | 104 |
| ECF | 21 | 610 | <70§ | 191 | 118 | ||||||||
| Citron, 2003 (6) | United States | Adjuvant | ATC | 14 | 493 | 78 | 50 | <2 cm: 20; >2 cm: 28 | 1–3 pos: 29; 4–9 pos: 14; 10 pos: 6 | Pos: 32.1; Neg: 17 | ND | 230 | 168 |
| AC then T | 14 | 484 | |||||||||||
| ATC | 21 | 495 | <2 cm: 18; >2 cm: 29 | 1–3 pos: 29; 4–9 pos: 14; 10 pos: 6 | Pos: 32.3; Neg: 6.5 | ND | 278 | 202 | |||||
| AC then T | 21 | 501 | |||||||||||
| Baldini, 2003 (8) | Italy | Neoadjuvant, adjuvant | CEF × 3 then surgery radiation then CMF CEF intercalated × 6 | 14 | 77 | 60 | 52 | IB–IC: 25; IIIA: 20; IIIB: 50║ | Pos: 18.6; Neg: 8.6 | Pos: 12.6; Neg: 22.6 | 29 | 22 | |
| CEF × 3 then surgery radiation then CMF CEF intercalated × 6 | 21 | 73 | 50 | Pos: 22; Neg: 8.6 | Pos: 17.3; Neg: 24 | 37 | 24 | ||||||
A = adriamycin; C = cyclophosphamide; DFS = disease-free survival; E = epirubicin; ER = estrogen receptor; F = fluorouracil; M = methotrexate; Neg = negative; ND = no data; OS = overall survival; Pos = positive; PR = progesterone receptor; T = paclitaxel.
For trials protocols, see Table 3.
Dose-dense arms are those with 14-day interval between cycles.
As reported.
The staging system used in this trial was not reported but is presumed to be the American Joint Committee on Cancer, Cancer Staging Manual, Sixth Edition
Table 2.
Modified dose-dense chemotherapy trials*
| First author, year (reference) | Study location | Treatment setting | Treatment protocol† | Treatment interval,‡ d | Number of patients | Follow-up, mo | Median age (range), y | Stage§ |
ER status, % | PR status, % | DFS events | OS events | |
| Tumor size, % | Nodal status, % | ||||||||||||
| Von Minkcwitz, 2005 (14) | Germany | Neoadjuvant | A DOC | 14 | 455 | 60 | 52 (24–77) | T1: 0.7; T2: 84; T3: 15.2 | Pos: 18.8 | Pos: 32.5 | Pos: 31 | 112 | 57 |
| AC DOC | 21 | 458 | 51 (24–74) | Pos: 25 | Pos: 34 | Pos: 28 | 113 | 48 | |||||
| Linden, 2007 (11) | United States | Adjuvant | A then C | Days 1 and 2 21 (A), 14 (C) | 1524 | 86 | 47 (22.8–76.9) | T1: 15.6; T2: 30.1; T3: 3.4 | N1: 26.5 | Pos: 24; Neg: 24.4 | Pos: 23; Neg: 24.4 | 358 | 237 |
| AC | 21 | 1590 | 47.5 (21.9–76.6) | T1: 17.3; T2: 29.8; T3: 3.5 | N1: 25.4 | Pos: 24; Neg: 26 | Pos: 23; Neg: 27 | 407 | 274 | ||||
| Kummel, 2006 (15) | Germany | Adjuvant | ET then CMF | 14 | 116 | 38.7 | 52.9 (NR) | T1: 28; T2: 58; T3: 18 | N1: 90; N2: 10 | Pos: 34 Neg: 11 | ND | 33 | 15 |
| EC then CMF | 21 | 115 | Pos: 39 Neg: 9 | 38 | 22¶ | ||||||||
| Therasse, 2003 (13) | Europe | Adjuvant | EC | 14 | 224 | 66 | 49 (29–79) | T0–2: 2; T3: 8; T4: 90 | N0–1: 28.7; N2: 18.4; N3: 1.4 | Pos: 21; Neg: 8; Pos: 21 | ND | ND | 109 |
| C: d1–14; E: d1, 8; F: d1, 8 | 21 | 224 | 49 (16–72) | N0–1: 26.7; N2: 20.4; N3: 1.4 | Pos: 18 Neg: 11.4 UNK: 19.5 | 108 | |||||||
| Moebus, 2003 (12) | Germany | Adjuvant | ETC | 14 | 590 | 60 | 50 (NR) | Median (SD) 3 cm (1.7) | N4–9: 59; N>10: 41 | ER pos PR pos : 39 | 94¶ | 43 | |
| EC then T | 21 | 584 | ER pos PR pos: 37 | 127¶ | 60 | ||||||||
| Uncht, 2009 (16) | Germany | Neoadjuvant | E then T surgery CMF | 14 | 333 | 55 | 49 (27–68) | T2: 27.3; T3: 13.1; T4: 9 | Pos: 26 | ER pos PR pos: 28.5 | 92 | 52 | |
| ET surgery CMF | 21 | 335 | 51 (26–66) | T2: 25; T3: 15.2; T4: 8.6 | Pos: 27 | ER pos PR pos: 31 | 123 | 71 | |||||
| Burnell, 2009 (23) | Canada | Adjuvant | EC then T ‖ | 14 (EC), 21 (T) | 701 | 30.4 | NR (40–49) | T1: 34.2; T2: 53.8; T3: 9.7 | N0: 28.2; N1–3: 43.2; N4–10: 22.3 | ER pos: 58.9 | 73¶ | NR | |
| AC then T | 21 | 702 | NR (40–49) | T1: 36.3; T2: 54.6; T3: 8.1 | N0: 27.8; N1–3: 43.6; N4–10: 22.5 | ER pos: 58.8 | 105¶ | ||||||
| Burnell, 2009 (23) | Canada | Adjuvant | EC then T ‖ | 14 (EC), 21 (T) | 701 | 30.4 | NR (40–49) | T1: 34.2; T2: 54.8; T3: 9.7 | N0: 28.2; N1–3: 43.2; N4–10: 22.3 | ER pos: 58.9 | 73¶ | NR | |
| CEF | 28 | 701 | NR (40–49) | T1: 34.2; T2: 55.8; T3: 8.8 | N0: 28; N1–3: 43.2; N4–10: 22.1 | ER pos: 59.6 | 70¶ | ||||||
| Untch, 2008 (24) | Germany | Neoadjuvant | E then T then CMF | 15 (E and T), 28 (CMF) | 363 | 38.4 | 48 (23–65) | T1–3: 86.2; T4: 8.5 | N0: 37.2; Pos: 50.1 | ER pos: 41.6 | 132# | 39# | |
| EC then T | 22 | 370 | 49 (26–65) | T1–3: 90.8 T4: 7.3 | N0: 38.4; Pos: 50 | ER pos: 43 | 141# | 48# | |||||
A = adriamycin; C = cyclophosphamide; DFS = disease-free survival; DOC = docetaxel; E = epirubicin; ER = estrogen receptor; F = fluorouracil; M = methotrexate; ND = no data; Neg = negative; NR = not reported; OS = overall survival; Pos = positive; PR = progesterone receptor; T = paclitaxel; UNK = unknown.
For trials protocols see Table 3.
Dose-dense arms are those with 14 days interval between cycles.
Classified according to American Joint Committee on Cancer, Cancer Staging Manual, Sixth Edition, unless otherwise indicated.
Dose-dense arm.
Relapse-free survival events.
No hazard ratio or P value reported.
Table 3.
Trials chemotherapy protocols*
| Study | Conventional arm | Dose-dense arm |
| Venturini, 2005 (7) | F: 600 mg/m2 + E: 60 mg/m2 + C: 600 mg/m2, 4 cycles | F: 600 mg/m2 + E: 60 mg/m2 + C: 600 mg/m2, 4 cycles |
| Citron, 2003 (6) | A: 60 mg/m2 4 cycles then T: 175 mg/m2 4 cycles then C: 600 mg/m2, 4 cycles | A: 60 mg/m2 ,4 cycles then T: 175 mg/m2, 4 cycles then C: 600 mg/m2, 4 cycles |
| A: 60 mg/m2 + C: 600 mg/m2 4 cycles then T: 175 mg/m2, 4 cycles | A: 60 mg/m2 + C: 600 mg/m2, 4 cycles then T: 175 mg/m2, 4 cycles | |
| Baldini, 2003 (8) | C: 600 mg/m2 + E: 60 mg/m2 + F: 600 mg/m2, 3 cycles then local therapy then CEF (same) intercalated by C: 600 mg/m2 + M: 60 mg/m2 + F: 600 mg/m2 for a total of 6 cycles | C: 600 mg/m2, E: 60 mg/m2, F: 600 mg/m2, 3 cycles then local therapy then CEF (same) intercalated by C: 600 mg/m2 + M: 60 mg/m2 + F: 600 mg/m2 for a total of 6 cycles |
| Von Minckwitz, 2005 (14) | D: 60 mg/m2 + C: 600 mg/m2 4 cycles , DOC: 75 mg/m2, 4 cycles | D: 50 mg/m2 + DOC: 75 mg/m2, 4 cycles |
| Linden, 2007 (11) | A: 54 mg/m2 + C: 1.2 g/m2, 6 cycles | A: 40.5 mg/m2 days 1 and 2, 4 cycles; C: 2.4 gm/m2, 3 cycles |
| Kümmel, 2006 (15) | E: 90 mg/m2 + C: 600 mg/m2, 4 cycles; C: 600 mg/m2 + M: 40 mg/m2 + F: 600 mg/m2, 3 cycles | E: 90 mg/m2 + T: 175 mg/m2, 4 cycles; C: 600 mg/m2 + M: 40 mg/m2 + F: 600 mg/m2, 3 cycles |
| Therasse, 2003 (13) | E: 120 mg/m2 + C: 830 mg/m2, 6 cycles | C: 75 mg/m2 orally days 1–14 + E: 60 mg/m2 days 1 and 8 + F: 500 mg/m2 days 1 and 8, 6 cycles |
| Möbus, 2003 (12) | E: 90 mg/m2 + C: 600 mg/m2, 4 cycles followed by T: 175 mg/m2, 4 cycles | E: 150 mg/m2 + T: 225 mg/m2 + C: 2500 mg/m2, 3 cycles |
| Untch, 2009 (16) | E: 90 mg/m2 + T: 175 mg/m2 4 cycles, surgery then C: 500 mg/m2 + M: 40 mg/m2 + F: 600 mg/m2 days 1 and 8 every 28 days, 3 cycles | E: 150 mg/m2, 3 cycles then T: 250 mg/m2, 3 cycles, surgery then C: 500 mg/m2 + M: 40 mg/m2 + F: 600 mg/m2 days 1 and 8 every 28 days, 3 cycles |
| Burnell, 2009 (23) | C: 75 mg/m2 orally × 14 days + E: 60 mg/m2 days 1 and 8 + F: 500 mg/m2 days 1 and 8 every 28 days, 6 cycles | E: 120 mg/m2 + C: 830 mg/m2, 6 cycles every 14 days, then T: 175 mg/m2 every 21 days, 4 cycles |
| D: 60 mg/m2 + C: 600 mg/m2, 4 cycles then P: 175 mg/m2, 4 cycles | ||
| Untch, 2008 (24) | E: 90 mg/m2 + C: 600 mg/m2 every 22 days 4 cycles then T: 175 mg/m2 every 22 days, 4 cycles | E: 150 mg/m2 every 15 days, 3 cycles then T: 225 mg/m2 every 15 days, 3 cycles then C: 500 mg/m2 + M: 40 mg/m2 + F: 600 mg/m2 days 1 and 8 every 28 days, 3 cycles |
A = adriamycin; C = cyclophosphamide; DOC = docetaxel; E = epirubicin; F = fluorouracil; M = methotrexate; T = paclitaxel.
Meta-analysis of Conserved Dose-Dense Chemotherapy Trials
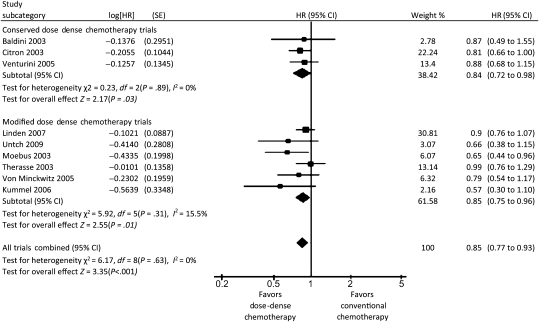
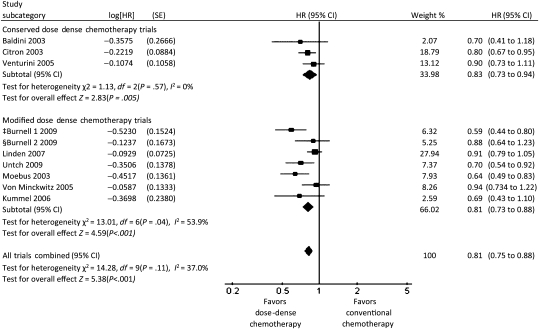
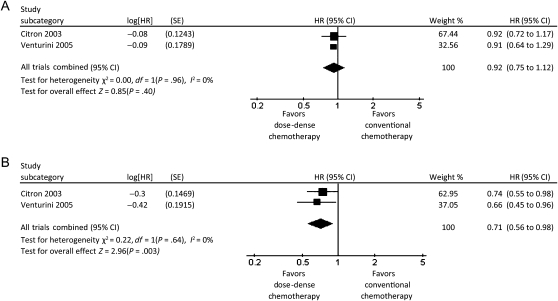
A total of 3337 patients were randomly assigned in the three trials that we classified as conserved dose-dense chemotherapy trials (6–8). In a meta-analysis of these three trials, patients in the dose-dense chemotherapy arms had better overall survival than patients in the conventional chemotherapy arms (HR of death = 0.84; 95% CI = 0.72 to 0.98, P = .03), and there was no heterogeneity among these trials with respect to overall survival (I2 = 0%) (Figure 2). Dose-dense chemotherapy had a similar benefit with respect to disease-free survival (HR of relapse or death = 0.83; 95% CI = 0.73 to 0.94, P = .005), with no heterogeneity (I2 = 0%) (Figure 3). In a sensitivity analysis based on estrogen and progesterone receptor status using data from the two trials that examined disease-free survival (6,7), dose-dense chemotherapy had a statistically significant benefit with respect to disease-free survival only in receptor-negative patients (HR of relapse or death = 0.71; 95% CI = 0.56 to 0.89; I2 = 0%) (Figure 4, A and B).
Figure 2.
Forest plot of hazard ratios (HRs) comparing overall survival for patients who received dose-dense chemotherapy vs those who received conventional chemotherapy in the conserved dose-dense chemotherapy trials, in the modified dose-dense chemotherapy trials, and for all trials combined. Hazard ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represent the estimated overall effect based on the meta-analysis fixed effect of all trials.
Figure 3.
Forest plot of hazard ratios (HRs) comparing disease-free survival for patients who received dose-dense chemotherapy vs those who received conventional chemotherapy in the conserved dose-dense chemotherapy trials, in the modified dose-dense chemotherapy trials, and for all trials combined. Hazard ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represents the estimated overall effect based on the meta-analysis fixed effect of all trials. ‡EC then T (dose-dense arm) vs AC then T. §EC then T (dose-dense arm) vs CEF.
Figure 4.
Forest plot of hazard ratios (HRs) comparing disease-free survival for estrogen receptor–positive and estrogen receptor–negative patients who received dose-dense chemotherapy vs those who received conventional chemotherapy in the conserved dose-dense chemotherapy trials. A) Estrogen receptor–positive patients. B) Estrogen receptor–negative patients. Hazard ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represents the estimated overall effect based on the meta-analysis fixed effect of all trials.
Meta-analysis of Modified Dose-Dense Chemotherapy Trials
A total of 8652 patients were randomly assigned to seven trials classified as modified dose-dense chemotherapy trials (11–16,23) that evaluated efficacy and toxicity of dose-dense chemotherapy in the adjuvant and neoadjuvant settings. In a meta-analysis of six of these trials (11–16), patients in the dose-dense chemotherapy arms had statistically significantly better overall survival than patients in the conventional chemotherapy arms (HR of death = 0.85; 95% CI = 0.75 to 0.96); heterogeneity in overall survival among the trials was low (I2 = 15.5%) (Figure 2). Dose-dense chemotherapy had a similar benefit with respect to disease-free survival in these six trials (HR of relapse or death = 0.81; 95% CI = 0.73 to 0.88) although the heterogeneity was high (I2 = 53.9%) (Figure 3). An analysis by receptor status was not performed because of the lack of these data. In the MA21 trial (23), relapse-free survival did not differ between the treatment arms in the estrogen receptor–positive population.
Meta-analysis of Efficacy for All Dose-Dense Chemotherapy Trials
A meta-analysis of all dose-dense chemotherapy trials (6–8,11–16,23) revealed that patients who received dose-dense chemotherapy had statistically significantly better overall survival (HR of death = 0.85; 95% CI = 0.77 to 0.93) (Figure 2) and disease-free survival (HR of relapse or death = 0.81; 95% CI = 0.75 to 0.88) (Figure 3) compared with patients who received conventional chemotherapy. No heterogeneity was observed among the trials for overall survival (I2 = 0%), and low heterogeneity was observed for disease-free survival (I2 = 37%).
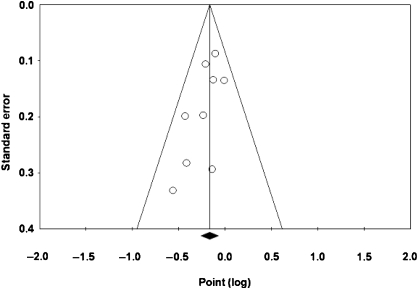
A funnel plot of the primary outcome revealed slight asymmetry, which may indicate a small study effect (ie, publication bias) (Figure 5). However, neither the Egger linear regression test (P = .066) nor the Begg–Mazumdar rank correlation test (P = .14) showed a statistically significant association between the study effects and the study size.
Figure 5.
Funnel plot of overall survival in all dose-dense chemotherapy trials for the visual detection of systematic publication bias and small study effect. Each circle represents treatment effect expressed as the logarithm of the hazard ratio of overall survival in each trial plotted against standard error as a measure of study size. The diamond and the vertical line represent the pooled estimate from the meta-analysis.
Meta-analysis of Adverse Events
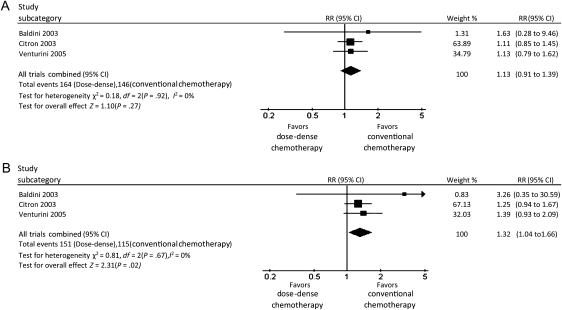
In the three conserved dose-dense chemotherapy trials, there was no difference in the number of grade 3 or 4 adverse events between the dose-dense and conventional chemotherapy arms (RR = 1.13; 95% CI = 0.91 to 1.39, I2 = 0%) (Figure 6, A). Delivery of dose-dense chemotherapy requires the use of growth factor support, which may prevent grade 3 and 4 neutropenia. We therefore performed an analysis that excluded bone marrow–related toxicity. The number of grade 3 or 4 nonhematological adverse events was higher in the dose-dense chemotherapy arm than in the conventional chemotherapy arm (RR = 1.32; 95% CI = 1.04 to 1.66, I2 = 0%) (Figure 6, B). We obtained nonconclusive results regarding adverse events in the modified dose-dense chemotherapy trials because of high heterogeneity (I2 = 95.8%). Thus, we used a random-effect model, which showed non-statistically significant higher toxicity in the dose-dense chemotherapy arm compared with the conventional chemotherapy arm (RR = 1.36; 95% CI = 0.87 to 2.13; P = .18).
Figure 6.
Forest plots of relative risks (RRs) of adverse events for patients who received dose-dense chemotherapy vs those who received conventional chemotherapy in the conserved dose-dense chemotherapy trials. A) All grade 3–4 adverse events. B) Grade 3–4 adverse events except leukopenia. Relative risks for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval (CI). The diamonds represents the estimated overall effect based on the meta-analysis fixed effect of all trials.
Discussion
This systematic review and meta-analysis shows that dose-dense chemotherapy for the treatment of women with high-risk breast cancer improves both disease-free and overall survival compared with conventional chemotherapy. The dose-dense chemotherapy approach resulted in better overall survival and better disease-free survival in trials that evaluated similar doses of agents in both treatment arms but with a condensed schedule in one arm as well as in trials that compared a condensed schedule with standard one but used different agents or doses in the two arms. The results for all trials combined were statistically significant, with no statistical heterogeneity among the trials.
The other important finding of this systematic review is the paucity of randomized controlled trials with an adequate study design, that is, trials that evaluate the same agents and doses in the conventional arm as in the investigational arm. Most of the trials included in the meta-analysis did not preserve this last characteristic, which is why we classified the trials according to their design.
Two recent trials have addressed the dose-dense chemotherapy approach. The recently published Canadian MA21 trial (23), which included three treatment arms that were anthracycline based, may provide key answers about the efficacy of the dose-dense approach with and without the use of taxane therapy. The PREPARE trial (24), which evaluated the effect of preoperative dose-dense and dose-intensified chemotherapy with anthracycline and taxane with or without darbepoetin alfa in breast cancer patients, was not included in the meta-analysis due to lack of outcome data.
Our meta-analysis of the efficacy of dose-dense chemotherapy according to hormone receptor status revealed that hormone receptor–positive patients did not benefit from dose-dense chemotherapy. This result was based on data from the conserved dose-dense chemotherapy trials; data from the modified dose-dense chemotherapy trials were not available. Nevertheless, data from the MA21 trial further support this finding. In the MA21 trial, relapse-free survival did not differ between the treatment arms in estrogen receptor–positive patients. Restricting the use of dose-dense chemotherapy to hormone receptor–negative patients may be justified, both in terms of the costs and the potential adverse events (25).
We also found that there was no increase in overall treatment-related adverse events associated with the dose-dense approach. Because a dose-dense chemotherapy schedule requires granulocyte colony-stimulating factor support, we performed an analysis of adverse events that excluded bone marrow–related events. In the conserved dose-dense chemotherapy trials, the number of nonhematological adverse events for the dose-dense chemotherapy arms was higher compared with that in the conventional chemotherapy arm. However, because of high heterogeneity among the modified dose-dense chemotherapy trials, the increase in adverse events for dose-dense vs conventional chemotherapy was not statistically significant.
The dose-dense chemotherapy approach uses prophylactic growth factor support to facilitate the safe delivery of dose-dense chemotherapy (26,27). The economic burden of this issue, as well as possible unrecognized growth factor–related toxicities, such as pulmonary toxicity, should be considered (28).
Several limitations of this analysis must be acknowledged. First, only three randomized controlled trials used the same agents and doses in the conventional chemotherapy arm as in the investigational (ie, dose-dense chemotherapy) arm. The fact that most of the included trials did not evaluate the same agents and dose in both arms resulted in major statistical heterogeneity among the trials. Second, the slight asymmetry of the funnel plot of the primary outcome suggests small study effects. Although results of the Egger and Begg–Mazumdar tests did not support the possibility of publication bias, the small number of included trials makes it difficult to distinguish a chance finding from true asymmetry. Also, the small number of included trials makes the outcomes more likely to have been influenced by a potential publication bias. We attempted to avoid such bias by searching for and including in our meta-analysis conference proceedings, databases of ongoing trials, and unpublished data.
Studies are needed to better define the patient population that will benefit the most from dose-dense chemotherapy. The concept of metronomic chemotherapy as evaluated, for example, by the Southwest Oncology Group (29) is a variation of the dose-dense strategy whereby chemotherapy is administered as relatively low and minimally toxic doses at frequent and regular intervals (30). Assessing the efficacy and feasibility of this milder chemotherapeutic approach might identify ways to integrate biological performance with the best treatment. This analysis indicates that dose-dense chemotherapy has a clear benefit for patients with hormone receptor–negative breast cancer. The lack of obvious benefit in patients with hormone receptor–positive breast cancer shown by this analysis indicates the need for further prospective randomized trials of the classical conserved design in this patient population.
Funding
The authors received no external funding for this study.
Footnotes
L. Bonilla, I. Ben-Aharon, L. Leibovici, and S. M. Stemmer contributed equally to this study. The authors take full responsibility for the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
References
- 1.Early Breast Cancer Trialists Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Hryniuk WM. Average relative dose intensity and the impact on design of clinical trials. Semin Oncol. 1987;14(1):65. [PubMed] [Google Scholar]
- 3.Biganzoli L, Piccart MJ. The bigger the better? Or what we know and what we still need to learn about anthracycline dose per course, dose density and cumulative dose in the treatment of breast cancer. Ann Oncol. 1997;8(12):1177–1182. doi: 10.1023/a:1008295432012. [DOI] [PubMed] [Google Scholar]
- 4.Hudis C, Seidman A, Baselga J, et al. Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: feasibility and efficacy. J Clin Oncol. 1999;17(1):93–100. doi: 10.1200/JCO.1999.17.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Norton La: A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48(24, pt 1):7067. [PubMed] [Google Scholar]
- 6.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of intergroup trial C9741. Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 7.Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst. 2005;97(23):1724–1733. doi: 10.1093/jnci/dji398. [DOI] [PubMed] [Google Scholar]
- 8.Baldini E, Gardin G, Giannessi PG, et al. Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: a randomized phase III trial in locally advanced breast cancer. Ann Oncol. 2003;14(2):227–232. doi: 10.1093/annonc/mdg069. [DOI] [PubMed] [Google Scholar]
- 9.Untch M, Thomssen C, Steffen K, et al. Five year results of a randomized multicenter dose intense (DI-EC) study with epirubicin (E) and cyclophosphamide (C) in high risk breast cancer patients: a treatment of short duration with comparable efficacy to conventional chemotherapy. Breast Cancer Res Treat. 2002;76(suppl 1):S158. Abstract 641. [Google Scholar]
- 10.Seidman AD, Hudis CA, Albanel J, et al. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol. 1998;16(10):3353–3361. doi: 10.1200/JCO.1998.16.10.3353. [DOI] [PubMed] [Google Scholar]
- 11.Linden HM, Haskell CM, Green SJ, et al. Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313) J Clin Oncol. 2007;25(6):656–661. doi: 10.1200/JCO.2006.07.0847. [DOI] [PubMed] [Google Scholar]
- 12.Möbus VJ, Untch M, Du Bois A, et al. Dose-dense sequential chemotherapy with epirubicin(E), paclitaxel (T) and cyclophosphamide (C) (ETC) is superior to conventional dosed chemotherapy in high-risk breast cancer patients (≥ 4 +LN). First results of an AGO-trial. Proc Am Soc Clin Oncol. 2004;22(suppl July 15):S14. Abstract 513. [Google Scholar]
- 13.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with dose intensified epirubicin and cyclophosphamide plus filgrastim as neoadjuvant treatment in locally advanced breast cancer. An EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21(5):843–850. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 14.Von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23(12):2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 15.Kümmel S, Krocker J, Kohls A, et al. Randomised trial: survival benefit and safety of adjuvant dose-dense chemotherapy for node-positive breast cancer. Br J Cancer. 2006;94(9):1237–1244. doi: 10.1038/sj.bjc.6603085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Untch M, Möbus V, Kuhn W, et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol. 2009;27(18):2938–2945. doi: 10.1200/JCO.2008.20.3133. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. The Cochrane Collaboration; 2008. www.cochrane-handbook.org. Accessed September 30, 2008. [Google Scholar]
- 20.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 23.Burnell M, Levine M, Chapman JA, et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol. 2010;28(1):77–82. doi: 10.1200/JCO.2009.22.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Untch M, Fasching PA, Bauerfeind I, et al. PREPARE trial. A randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF with a standard dosed epirubicin/cyclophosphamide followed by paclitaxel ± darbapoetin alfa in primary breast cancer: a preplanned interim analysis of efficacy at surgery. J Clin Oncol. 2008;26(suppl May 20) doi: 10.1093/annonc/mdq709. Abstract 517. [DOI] [PubMed] [Google Scholar]
- 25.Kümmel S, Rezai M, Kimmig R, Schmid P. Dose-dense chemotherapy for primary breast cancer. Curr Opin Obstet Gynecol. 2007;19(1):75–81. doi: 10.1097/GCO.0b013e328011f99a. [DOI] [PubMed] [Google Scholar]
- 26.Doorduijn JK, Buijt I, Van der Holt B, et al. Economic evaluation of prophylactic granulocyte colony stimulating factor during chemotherapy in elderly patients with aggressive non-Hodgkin’s lymphoma. Haematologica. 2004;89(9):1109–1117. [PubMed] [Google Scholar]
- 27.Timmer-Bonte JN, Adang EM, Smit HJ, et al. Cost-effectiveness of adding granulocyte colony-stimulating factor to primary prophylaxis with antibiotics in small-cell lung cancer. J Clin Oncol. 2006;24(19):2991–2997. doi: 10.1200/JCO.2005.04.3281. [DOI] [PubMed] [Google Scholar]
- 28.Yerushalmi R, Kramer MR, Rizel S, et al. Decline in pulmonary function in patients with breast cancer receiving dose-dense chemotherapy: a prospective study. Ann Oncol. 2009;20(3):437–440. doi: 10.1093/annonc/mdn652. [DOI] [PubMed] [Google Scholar]
- 29.Ellis GK, Green SJ, Russell CA, et al. SWOG 0012, A randomized phase III comparison of standard doxorubicin and cyclophosphamide followed by weekly paclitaxel versus weekly doxorubicin and daily oral cyclophosphamide plus G-CSF followed by weekly paclitaxel as neoadjuvant therapy for inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24(12s) suppl 18 doi: 10.1200/JCO.2009.27.6543. LBA537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McArthur HL, Hudis CA. Dose-dense therapy in the treatment of early-stage breast cancer: an overview of the data. Clin Breast Cancer. 2007;(suppl 1):S6–S10. doi: 10.3816/cbc.2007.s.007. [DOI] [PubMed] [Google Scholar]