Abstract
Background
Use of androgen suppression therapy (AST) in prostate cancer increased more than threefold from 1991 to 1999. The 2003 Medicare Modernization Act reduced reimbursements for AST by 64% between 2004 and 2005, but the effect of this large reduction on use of AST is unknown.
Methods
A cohort of 72 818 men diagnosed with prostate cancer in 1992–2005 was identified from the Surveillance, Epidemiology, and End Results database. From Medicare claims data, indicated AST was defined as 3 months or more of AST in the first year in men with metastatic disease (n = 8030). Non-indicated AST was defined as AST given without other therapies such as radical prostatectomy or radiation in men with low-risk disease (n = 64 788). The unadjusted annual proportion of men receiving AST was plotted against the median Medicare AST reimbursement. A multivariable model was used to estimate the odds of AST use in men with low-risk and metastatic disease, with the predictor of interest being the calendar year of the payment change. Covariates in the model included age in 5-year categories, clinical tumor stage (T1–T4), World Health Organization grade (1–3, unknown), Charlson comorbidity (0, 1, 2, ≥3), race, education, income, and tumor registry site, all as categorical variables. The models included variations in the definition of AST use (≥1, ≥3, and ≥6 months of AST). All statistical tests were two-sided.
Results
AST use in the low-risk group peaked at 10.2% in 2003, then declined to 7.1% in 2004 and 6.1% in 2005. After adjusting for tumor and demographic covariates, the odds of receiving non-indicated primary AST decreased statistically significantly in 2004 (odds ratio [OR] = 0.70, 95% confidence interval = 0.61 to 0.80) and 2005 (OR = 0.61, 95% confidence interval = 0.53 to 0.71) compared with 2003. AST use in the metastatic disease group was stable at 60% during the payment change, and the adjusted odds ratio of receiving AST in this group was unchanged in 2004–2005.
Conclusions
In this example of hormone therapy for prostate cancer, decreased physician reimbursement was associated with a reduction in overtreatment without a reduction in needed services.
CONTEXTS AND CAVEATS
Prior knowledge
The federal government reduced reimbursements for androgen suppression therapy (AST) by 64% between 2004 and 2005 as part of the 2003 Medicare Modernization Act, but whether this reduction resulted in decreased use of AST for prostate cancer is unknown.
Study design
Data on 72 818 patients with prostate cancer were collected from the Surveillance, Epidemiology, and End Results (SEER) database and compared with Medicare claims records.
Contribution
Use of AST for 64 788 men with low-risk disease declined in 2004–2005, but remained stable for 8030 men with metastatic disease.
Implications
Decreases in physician reimbursement for AST did not affect indicated treatment for high-risk patients. Reduced reimbursement rates may reduce overtreatment in men with low-risk disease.
Limitations
Only records for Medicare patients were used, so the results may not be valid for younger men. Changes in the way SEER classified tumor grade in 2003 may have resulted in misclassification of disease categories. Prostate-specific antigen values were not included in the analysis because SEER did not report these values until 2004.
From the Editors
Physician financial incentives stemming from a fee-for-service payment system may induce overtreatment. Such overtreatment negatively affects health-care quality and may be an important factor in the rising costs of health care (1–3). In 1963, Arrow (4) first detailed the conflict of interest and effect on health-care utilization that could arise when the physician acts as both the agent (patient adviser) and seller of health care.
Large variations in care utilization exist, but demarcating overtreatment or undertreatment is difficult because in many disease models the appropriate level of treatment is unclear or too complex to be presented in a simple model (5). However, in examples in which evidence-based guidelines exist for a therapy with high reimbursement, one may explore the hypothesis that financial incentives affect treatment utilization. In particular, overtreatment may occur when financial incentives induce physicians to expand the use of a therapy beyond the group in which it has proven benefit. In an effort to explore this hypothesis, we examined whether utilization rates of androgen suppression therapy (AST) for prostate cancer in both indicated and non-indicated cases changed coincident with a large rapid reduction in the reimbursement for this treatment.
AST, delivered as a depot injection of luteinizing hormone–releasing hormone, is indicated for management of prostate cancer in two clinical situations, as palliative therapy in metastatic prostate cancer (6) or in combination with external beam radiotherapy in the treatment of locally advanced prostate cancer (7), for which it has been shown to improve survival. Seventy percent of men with metastatic prostate cancer in the United States receive AST within 4 months of diagnosis (8). AST use is associated with multiple adverse effects (9–13), is costly, and has not been shown to improve survival or other patient-centered outcomes in men with low-risk cancer; thus, current guidelines recommend watchful waiting or local therapy such as radiotherapy or radical prostatectomy and not AST use in men with low-risk prostate cancer (14,15). AST given as the sole treatment in low-risk prostate cancer (commonly known as “primary AST”) is the most extreme example of non-indicated use.
AST use increased more than threefold between 1991 and 1999 across patient age, disease stage, and grade, irrespective of whether the treatment was considered clinically indicated or not (16). By 2001, Medicare expenditures for AST had increased to more than one billion dollars. Faced with rising costs of AST, the federal government reduced AST reimbursement by 64% between 2004 and 2005 as part of the 2003 Medicare Modernization Act (17). Rates of AST use dropped by 14% in 2005 (18). Because both indicated and non-indicated AST use had been high and reimbursement was reduced dramatically, this policy change served as an ideal natural experiment to test the effect of financial incentives on the provision of indicated vs non-indicated care. Because payment for other chemotherapeutic agents was also reduced under the Medicare Modernization Act, this investigation is highly relevant to the current health-care reform debate as it pertains to cancer care.
We investigated the effect of a large reduction in Medicare physician reimbursement in 2004–2005 on indicated vs non-indicated AST use as a model of the effects of financial incentives on overtreatment. We hypothesized that the payment change would not affect indicated AST use in metastatic disease, whereas non-indicated use (primary AST in low-risk disease) would decline following the reduction in physician reimbursement. To evaluate this hypothesis, we measured rates of AST utilization in men diagnosed with prostate cancer between 1992 and 2005, stratified by treatment indication.
Methods
Data Sources
Data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry were linked to Medicare enrollment records and utilization data (SEER-Medicare). SEER contains patient and tumor characteristics as well as treatment information through 6 months after cancer diagnosis. The 11 geographic regions making up the SEER registry in 1992–1999 accounted for about 14% of the United States population and the 14 regions in 2000–2005 accounted for 25% of the population. An elderly subset (≥65 years of age) of the SEER population may be followed long-term by linking SEER information to Medicare claims data.
Cohort Identification
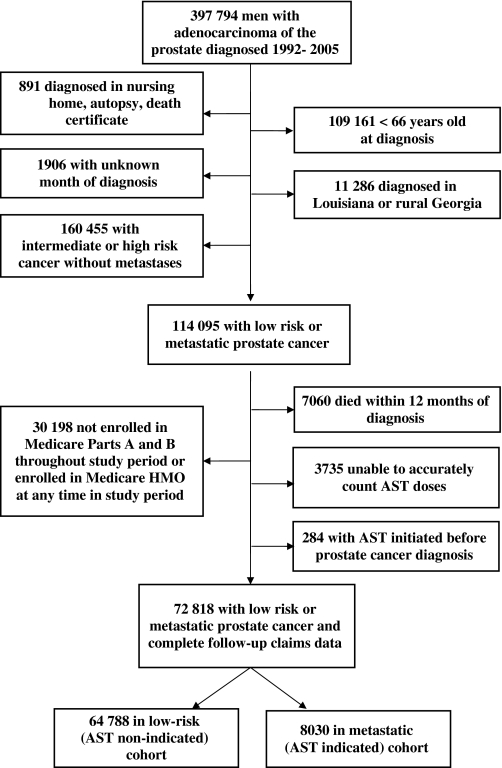
From the SEER database, we identified 397 794 men diagnosed with adenocarcinoma of the prostate in 1992–2005 and excluded 891 who were diagnosed on death certificate, at autopsy, or in a nursing home, and 109 161 diagnosed at ages younger than 66 years (Figure 1). We also excluded 1906 with an unknown month of diagnosis and 11 286 who were diagnosed in rural Georgia (sparse population and rare cases) or Louisiana (missing 2005 data due to Hurricane Katrina). Men with nonmetastatic intermediate or high-risk prostate cancer were excluded (n = 160 455), leaving a sample (n = 114 095) of men with either metastatic disease (AST indicated cohort) or low-risk localized disease (AST non-indicated cohort) at diagnosis. Men who died within 12 months of cancer diagnosis (n = 7060) were excluded because they would have died before the endpoint could be measured. Also excluded were those men unlikely to have complete claims data (ie, not enrolled in fee-for-service Medicare throughout the study period, n = 30 198), those for whom we were not able to count the AST doses (n = 3735), and those who started AST before diagnosis of prostate cancer (n = 284). This process yielded a final cohort of 72 818 patients, 8030 men in the AST indicated cohort and 64 788 in the AST non-indicated cohort. Three registries were added to SEER in 2000 (Greater California, Kentucky, and New Jersey). Unless otherwise specified, all analyses included these added registries.
Figure 1.
Flowchart describing initial dataset and exclusions leading to final cohort.
Characterization of Low-Risk and Metastatic Cohorts
SEER classifies men as having metastatic disease if metastases are found within 6 months of diagnosis. All men with metastases were considered to have an indication for AST, regardless of disease grade or stage and regardless of other therapies given. Characterization of the low-risk cohort required consideration of some changes in the way SEER has reported prostate cancer information over time. Prostate-specific antigen (PSA) value was not reported by SEER until 2004–2005, and so it was not used as a risk stratification criterion. Tumor size was limited to unilateral T2 tumors (T2a or T2a/b depending on the year of diagnosis, because the staging definition changed over time). World Health Organization (WHO) grade 1–2 corresponded to Gleason score 2–7 in 1992–2002 but to Gleason score 2–6 in 2003–2005. Starting in 2004, SEER began reporting actual Gleason score in addition to WHO grade; thus, we could reclassify Gleason 7 tumors with Gleason 2–6 tumors for 2004–2005 but not for 2003. However, this reclassification would create instability in the cohort classification between 2003 and 2004, which was our time of interest (the first payment reduction was in January 2004). Therefore, in our primary analysis, the low-risk group was defined as having tumors of stage T2a/b or smaller (unilateral tumor) and WHO grade 1–2 (Gleason score 2–7 for 1992–2002 and 2–6 for 2003–2005), but we looked for a change in our univariate outcome with the alternate definition (Gleason score 2–7 for 1992–2002, 2–6 for 2003, and 2–7 for 2004–2005).
Characterization of Demographic Variables and Covariates
Comorbidity was classified using a modification of the Charlson comorbidity index for use with Medicare data (19,20). Specifically, International Classification of Diseases, Ninth Revision codes consistent with comorbidities of interest were examined for 1 year before cancer diagnosis. Race was determined from SEER files. Education and income levels were assigned by zip code using US Census information from 2000. Differences in demographic and clinical variables between the low-risk and metastatic groups of patients were evaluated with the χ2 test. Time trends in demographic and clinical characteristics among the low-risk patients were compared with the χ2 test for heterogeneity, excluding the registries added in 2000.
Characterization of AST Therapy
AST therapy was identified from Medicare claims data as previously described by Shahinian et al. (16). Briefly, Medicare physician inpatient and outpatient claims were searched to identify use of AST (Healthcare Common Procedure Coding System codes J9202, J9217, J9219, and J3315). Receipt of a 1-month depot resulted in a patient being classified as having received AST for that month. Longer length depots (3, 4, 6, and 12 months) were operationalized as 3, 4, 6, and 12 1-month depot injections as appropriate.
Indicated AST was defined as receiving at least 3 months of AST within 1 year of diagnosis in men with metastatic disease. Primary AST was defined as receiving at least 3 months of AST without any local therapy directed at the prostate (radical prostatectomy, external beam radiotherapy, intensity-modulated radiotherapy, brachytherapy, high-dose rate brachytherapy, cryoablation, or thermotherapy) within 1 year of diagnosis in men with low-risk disease. Focusing on primary AST avoided uncertainty in our outcome assessment, which would have been caused by changes in the evidence supporting the use of AST with radical prostatectomy or radiotherapy during our study period; throughout the study period, primary AST in low-risk disease was not supported by evidence or guidelines (14). Utilization of surgical castration within the first year after diagnosis was identified through Medicare claims for simple orchiectomy (Current Procedural Terminology code, CPT 54520). Throughout this article, AST refers only to chemical castration.
Unadjusted Prevalence of AST Use
The crude percent of patients in the metastatic and low-risk cohorts who received at least 3 months of AST in the year of cancer diagnosis were tabulated for the years 1992–2005. For each year, the mean Medicare reimbursement per monthly dose of AST was calculated from the claims identified in our cohort. To better understand nonclinical contributors to the patterns of AST use, data were additionally examined through stratification by race, income, and education. We also examined the outcome when varying the definition of low-risk disease (as described above). We limited this unadjusted analysis to those registries continuously reporting data to SEER from 1992 to 2005 but investigated the outcome if we included those registries added in 2000. Any change in the rate of primary AST may reflect a change in the preference for primary local therapies (eg, radical prostatectomy). To investigate for such an effect, we also calculated the rate of primary AST in the cohort of patients not undergoing any local therapy. Because we did not see variation in use by low-risk definition, by the number of registries included, or by including vs excluding those patients treated with local therapy, the multivariable models were based on the a priori preferred definition of low-risk disease (stage T2a/b or smaller and WHO grade 1–2, which represented Gleason score 2–7 for 1992–2002 and 2–6 for 2003–2005) and included all registries and the cohort of all low-risk patients, regardless of local therapy.
Multivariable Logistic Regression Analysis
Separate logistic regression models were constructed for metastatic and low-risk cohorts. In both cohorts, the outcome of interest was receipt of at least 3 months of AST within 12 months of diagnosis. The primary predictor of interest was calendar year with the odds of AST in each calendar year referenced against 2003 (the year preceding the payment change). Covariates in the model included age in 5-year categories, clinical T stage (1–4), WHO grade (1–3, unknown), Charlson comorbidity (0, 1, 2, ≥3), race, education, income, and tumor registry site, all as categorical variables. The models used variations in the definition of AST use (≥1, ≥3, and ≥6 months of AST).
Results
Patient Characteristics
By definition, the metastatic and low-risk groups differed by grade and stage of disease (P < .001, Table 1). Due to the large study population, all other demographic variables were also statistically significantly different between the groups (P < .001 for all), but some of these differences were clinically small. Men with metastatic disease were much older and slightly less likely to be of non-Hispanic white ethnicity. Men with metastatic disease had less formal education and had lower median income. The comorbidity index was only slightly different between low-risk and metastatic cohorts. We examined time trends in patient characteristics in the low-risk group by diagnosis year. Across the 14 years of study, there was a consistent trend toward younger age, lower stage of disease, and higher grade of disease with time (P < .001; Table 2).
Table 1.
Patient characteristics stratified by low-risk vs metastatic prostate cancer*
| Characteristic | Low-risk prostate cancer (N = 64 788), No. (%) | Metastatic prostate cancer (N = 8030), No. (%) |
| Age, y | ||
| 66–69 | 17 622 (27.2) | 1812 (22.6) |
| 70–74 | 22 224 (34.3) | 2248 (28.0 |
| 75–79 | 15 634 (24.1) | 1861 (23.2) |
| 80–84 | 6764 (10.4) | 1297 (16.2) |
| ≥85 | 2544 (3.9) | 812 (10.1) |
| Clinical tumor stage† | ||
| 1 | 46 333 (71.5) | 479 (6.0) |
| 2 | 18 455 (28.5) | 1295 (16.1) |
| 3 | 0 | 530 (6.6) |
| 4 | 0 | 5377 (67.0) |
| Unknown | 0 | 349 (4.3) |
| WHO grade‡ | ||
| 1 | 8564 (13.2) | 161 (2.0) |
| 2 | 56 224 (86.8) | 2590 (32.3) |
| 3 | 0 | 4298 (53.5) |
| Undifferentiated | 0 | 106 (1.3) |
| Unknown | 0 | 875 (10.9) |
| Charlson comorbidity index | ||
| 0 | 49 566 (76.5) | 6360 (79.2) |
| 1 | 10 391 (16.0) | 1079 (13.4) |
| 2 | 3060 (4.7) | 378 (4.7) |
| ≥3 | 1771 (2.7) | 213 (2.7) |
| Race | ||
| Non-Hispanic white | 51 578 (79.6) | 5932 (73.9) |
| Non-Hispanic black | 5253 (8.1) | 959 (11.9) |
| Hispanic | 5008 (7.7) | 608 (7.6) |
| Asian | 2719 (4.2) | 495 (6.2) |
| Other/unknown | 230 (0.4) | 36 (0.4) |
| Median income§ | ||
| 0–$35 654 | 15 033 (23.2) | 2290 (28.5) |
| $35 655–46 118 | 15 499 (23.9) | 2005 (25.0) |
| $46 119–60 409 | 15 630 (24.1) | 1866 (23.2) |
| ≥$60 410 | 16 003 (24.7) | 1499 (18.7) |
| Unknown | 2623 (4.0) | 370 (4.6) |
| Percent of adults with less than high school education§ | ||
| 0–18.5 | 15 755 (24.3) | 1712 (21.3) |
| 18.6–25.2 | 15 579 (24.0) | 1938 (24.1) |
| 25.3–32.5 | 15 420 (23.8) | 2013 (25.1) |
| 32.5–100 | 15 411 (23.8) | 1997 (24.9) |
| Unknown | 2623 (4.0) | 370 (4.6) |
| Rural status | ||
| Nonrural | 59 603 (92.0) | 7275 (90.6) |
| Rural | 5183 (8.0) | 755 (9.4) |
Includes patients reported by all SEER registries, including those added in 2000. As expected, there were statistically significant differences between the low-risk and metastatic groups in all clinical and demographic variables (ie, P < .001 for all comparisons, using two-sided χ2 test), so P values are not included in the table. SEER = Surveillance, Epidemiology, and End Results database; WHO = World Health Organization.
Clinical tumor stage from Clinical Extension variable in SEER.
WHO grade from SEER. WHO grade 1–2 correlates with Gleason score 2–7 tumors in 1992–2002 and Gleason score 2–6 tumors in 2003–2005.
Determined by average zip code level information according to 2000 United States census data.
Table 2.
Patient characteristics by year for low-risk group*
| Characteristic | 1992–1995 (N = 13 172), No. (%) | 1996–1999 (N = 14 541), No. (%) | 2000–2002 (N = 10 513), No. (%) | 2003–2005 (N = 8502), No. (%) |
| Age, y | ||||
| 66–69 | 3183 (24.2) | 3998 (27.5) | 2743 (26.1) | 2491 (29.3) |
| 70–74 | 4782 (36.3) | 5040 (34.7) | 3499 (33.3) | 2727 (32.1) |
| 75–79 | 3158 (24.0) | 3450 (23.7) | 2717 (25.8) | 2023 (23.8) |
| 80–84 | 1464 (11.1) | 1469 (10.1) | 1137 (10.8) | 930 (10.9) |
| ≥85 | 585 (4.4) | 584 (4.0) | 417 (4.0) | 331 (3.9) |
| Clinical tumor stage† | ||||
| 1 | 7728 (58.7) | 10 120 (69.6) | 7619 (72.5) | 6736 (79.2) |
| 2 | 5444 (41.3) | 4421 (30.4) | 2894 (27.5) | 1766 (20.8) |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 0 |
| WHO grade‡ | ||||
| 1 | 4401 (33.4) | 2261 (15.5) | 619 (5.9) | 251 (3.0) |
| 2 | 8771 (66.6) | 12 280 (84.5) | 9894 (94.1) | 8251 (97.0) |
| 3 | 0 | 0 | 0 | 0 |
| Undifferentiated | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 0 |
| Charlson comorbidity index | ||||
| 0 | 10 249 (77.8) | 11 287 (77.6) | 8000 (76.1) | 6466 (76.1) |
| 1 | 2055 (15.6) | 2254 (15.5) | 1738 (16.5) | 1353 (15.9) |
| 2 | 539 (4.1) | 624 (4.3) | 513 (4.9) | 438 (5.2) |
| ≥3 | 329 (2.5 | 376 (2.6 | 262 (2.5) | 245 (2.9) |
| Race | ||||
| Non-Hispanic white | 10 806 (82.0) | 11 661 (80.2) | 8133 (77.4) | 6395 (75.2) |
| Non-Hispanic black | 1106 (8.4) | 1321 (9.1) | 941 (9.0) | 710 (8.4) |
| Hispanic | 686 (5.2) | 909 (6.3) | 808 (7.7) | 838 (9.9) |
| Asian | 531 (4.0) | 614 (4.2) | 591 (5.6) | 527 (6.2) |
| Other/unknown | 43 (0.3) | 36 (0.2) | 40 (0.4) | 32 (0.4) |
| Median income§ | ||||
| 0–$35 654 | 3059 (23.2) | 3173 (21.8) | 2157 (20.5) | 1667 (19.6) |
| $35 655–46 118 | 3587 (27.2) | 3714 (25.5) | 2641 (25.1) | 1992 (23.4) |
| $46 119–60 409 | 3152 (23.9) | 3530 (24.3) | 2533 (24.1) | 2156 (25.4) |
| ≥$60 410 | 2849 (21.6) | 3566 (24.5) | 2782 (26.5) | 2300 (27.1) |
| Unknown | 525 (4.0) | 558 (3.8) | 400 (3.8) | 387 (4.6) |
| Percent of adults with less than high school education§ | ||||
| 0–18.5 | 3458 (26.3) | 3945 (27.1) | 2943 (28.0) | 2433 (28.6) |
| 18.6–25.2 | 3008 (22.8) | 3356 (23.1) | 2566 (24.4) | 2132 (25.1) |
| 25.3–32.5 | 3106 (23.6) | 3364 (23.1) | 2450 (23.3) | 1979 (23.3) |
| 32.5–100 | 3075 (23.3) | 3318 (22.8) | 2154 (20.5) | 1571 (18.5) |
| Unknown | 525 (4.0) | 558 (3.8) | 400 (3.8) | 387 (4.6) |
| Rural status | ||||
| Nonrural | 4401 (33.4) | 2261 (15.5) | 619 (5.9) | 251 (3.0) |
| Rural | 8771 (66.6) | 12 280 (84.5) | 9894 (94.1) | 8251 (97.0) |
Excluding registries added in 2000. There were statistically significant differences in all clinical and demographic variables across years (ie, P < .001 for all comparisons by χ2 test for heterogeneity), so P values are not shown in table. SEER = Surveillance, Epidemiology, and End Results database; WHO = World Health Organization.
Clinical tumor stage from Clinical Extension variable in SEER.
WHO grade from SEER. WHO grade 1–2 correlates with Gleason score 2–7 tumors in 1992–2002 and Gleason score 2–6 tumors in 2003–2005.
Determined by average zip code level information according to 2000 United States census data.
Unadjusted Prevalence of AST Use
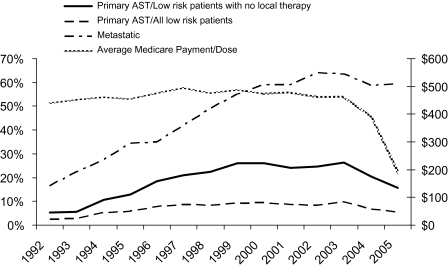
Use of AST in the metastatic group peaked at 64.0% in 2003 and decreased to 58.5% in 2005 (Figure 2) (64.0% in 2003 and 63.1% in 2005 when including registries added in 2000). Use of primary AST in the low-risk group was fairly stable between 1999 and 2003, peaking at 10.2% in 2003. Primary AST then declined to 7.1% in 2004 and 6.1% in 2005. These trends were consistent when the registries added in 2000 were included (2003: 10.6%, 2004: 7.5%, 2005: 6.8%). These trends were consistent when varying the definition of low-risk disease and when including the registries added in 2000. The frequency of primary AST among the cohort of low-risk patients limited to those not undergoing any local therapy peaked at 26.0% in 2003 and declined to 16.2% in 2005. The trends in primary AST generally parallel those in mean Medicare reimbursement for AST, which stabilized at $446 from 1999 to 2003 and declined by 57% to $190 per 1-month dose in 2005. Whereas stratified analyses showed some variation in percent utilization by demographic and socioeconomic factors, no change in the variation was seen specific to the years 2004–2005 (results not shown). Orchiectomy was rare in low-risk disease and was stable between 2000 and 2005 at 9%–10% in men with metastases.
Figure 2.
Proportion of all metastatic (indicated) and low-risk (non-indicated) prostate cancer patients receiving androgen suppression therapy (AST). The proportion receiving non-indicated AST is presented in two ways: with the cohort being all low-risk patients and, alternatively, only those low-risk patients who received no local therapy (ie, radical prostatectomy or radiotherapy). Plotted against the right hand y-axis is the average Medicare payment for each 1 month of AST. Data are shown over the years 1992–2005 and is limited to those registries continuously reporting data to SEER throughout the study period (ie, excludes those added in 2000).
Multivariable Logistic Regression Analysis
Year of diagnosis was not a statistically significant predictor of AST use in cases of metastatic disease (2005 vs 2003: odds ratio [OR] = 0.90, 95% confidence interval [CI] = 0.68 to 1.18; Table 3). After adjusting for tumor and demographic covariates, the odds of receiving non-indicated primary AST decreased statistically significantly in 2004 (OR = 0.70, 95% CI = 0.61 to 0.80) and 2005 (OR = 0.61, 95% CI = 0.53 to 0.71) compared with 2003. The odds of receiving primary AST was slightly higher in 2003 than in 2001 or 2002, likely representing the effect of the change in the SEER grading of prostate cancer in 2003. Whereas other covariates were also statistically significant in the model, these indicated a global change in the odds of receiving AST, not specific to the time of the payment change. No formal test for interaction was done because our stratified univariate analyses had indicated no effect modification with any of the covariates. Model results were consistent when the definition of AST use was lowered to 1 month or more of AST and increased to 6 months or more in the first year after diagnosis.
Table 3.
Multivariable logistic regression models predicting the odds of receiving androgen suppression therapy (AST) in non-indicated and indicated settings
| Non-indicated cohort |
Indicated cohort |
|||
| Variable | OR (95% CI)* | P | OR (95% CI)* | P |
| Year of diagnosis | ||||
| 1992 | 0.23 (0.18 to 0.30) | <.001 | 0.11 (0.08 to 0.14) | <.001 |
| 1993 | 0.26 (0.21 to 0.34) | <.001 | 0.17 (0.13 to 0.22) | <.001 |
| 1994 | 0.55 (0.45 to 0.66) | <.001 | 0.23 (0.18 to 0.30) | <.001 |
| 1995 | 0.62 (0.52 to 0.74) | <.001 | 0.33 (0.25 to 0.43) | <.001 |
| 1996 | 0.86 (0.73 to 1.01) | .064 | 0.32 (0.24 to 0.43) | <.001 |
| 1997 | 0.95 (0.81 to 1.11) | .521 | 0.43 (0.32 to 0.57) | <.001 |
| 1998 | 0.91 (0.78 to 1.07) | .248 | 0.62 (0.46 to 0.83) | .002 |
| 1999 | 0.99 (0.85 to 1.16) | .939 | 0.77 (0.57 to 1.04) | .085 |
| 2000 | 0.92 (0.82 to 1.05) | .211 | 0.94 (0.73 to 1.22) | .662 |
| 2001 | 0.84 (0.74 to 0.95) | .006 | 0.88 (0.68 to 1.14) | .331 |
| 2002 | 0.85 (0.75 to 0.96) | .011 | 0.97 (0.75 to 1.26) | .829 |
| 2003 | 1.00 (referent) | 1.00 (referent) | ||
| 2004 | 0.697 (0.61 to 0.80) | <.001 | 0.90 (0.68 to 1.18) | .449 |
| 2005 | 0.613 (0.53 to 0.71) | <.001 | 0.85 (0.64 to 1.12) | .256 |
| Age, y | ||||
| 66–69 | 1.00 (referent) | 1.00 (referent) | ||
| 70–74 | 1.78 (1.60 to 1.99) | <.001 | 1.16 (1.01 to 1.34) | .036 |
| 75–79 | 3.98 (3.57 to 4.42) | <.001 | 1.40 (1.21 to 1.62) | <.001 |
| 80–84 | 9.41 (8.42 to 10.52) | <.001 | 1.29 (1.09 to 1.52) | .003 |
| >85 | 12.28 (10.76 to 14.01) | <.001 | 1.29 (1.06 to 1.56) | .010 |
| Clinical tumor stage† | ||||
| 1 | 1.00 (referent) | 1.00 (referent) | ||
| 2 | 1.09 (1.02 to 1.17) | .016 | 1.44 (1.15 to 1.81) | .002 |
| 3 | n/a | 1.24 (0.94 to 1.66) | .134 | |
| 4 | n/a | 1.20 (0.96 to 1.49) | .105 | |
| Unknown | n/a | 1.56 (1.14 to 2.12) | .005 | |
| WHO grade‡ | ||||
| 1 | 1.00 (referent) | 1.00 (referent) | ||
| 2 | 1.47 (1.32 to 1.63) | <.001 | 1.13 (0.75 to 1.68) | .563 |
| 3 | n/a | 1.43 (0.96 to 2.12) | .081 | |
| Undifferentiated | n/a | 1.64 (0.92 to 2.91) | .093 | |
| Unknown | n/a | 1.47 (0.96 to 2.24) | .074 | |
| Charlson comorbidity index | ||||
| 0 | 1.00 (referent) | 1.00 (referent) | ||
| 1 | 1.30 (1.21 to 1.41) | <.001 | 1.50 (1.30 to 1.74) | <.001 |
| 2 | 1.40 (1.24 to 1.58) | <.001 | 1.28 (1.01 to 1.61) | .039 |
| ≥3 | 1.72 (1.49 to 1.99) | <.001 | 1.20 (0.89 to 1.63) | .230 |
| Race | ||||
| Non-Hispanic white | 1.00 (referent) | 1.00 (referent) | ||
| Non-Hispanic black | 1.02 (0.90 to 1.16) | .720 | 0.48 (0.40 to 0.58) | <.001 |
| Hispanic | 1.63 (1.47 to 1.81) | <.001 | 0.86 (0.71 to 1.05) | .129 |
| Asian | 1.38 (1.18 to 1.61) | <.001 | 0.94 (0.73 to 1.21) | .623 |
| Unknown | 1.24 (0.77 to 2.02) | .380 | 0.89 (0.43 to 1.87) | .765 |
| Median income§ | ||||
| 0–$36 697 | 1.00 (referent) | 1.00 (referent) | ||
| $36 698–47 468 | 1.00 (0.92 to 1.10) | .932 | 1.08 (0.94 to 1.25) | .287 |
| $47 469–61 190 | 0.86 (0.78 to 0.95) | .003 | 1.20 (1.03 to 1.41) | .023 |
| ≥$61 191 | 0.83 (0.74 to 0.93) | .002 | 1.21 (1.00 to 1.47) | .049 |
| Unknown | 1.02 (0.84 to 1.23) | .874 | 0.83 (0.63 to 1.11) | .213 |
| Percent of adults with less than high school education§ | ||||
| 0–18.5 | 1.00 (referent) | 1.00 (referent) | ||
| 18.6–25.2 | 1.08 (0.98 to 1.19) | .102 | 1.07 (0.92 to 1.25) | .376 |
| 25.2–32.5 | 1.19 (1.07 to 1.33) | .002 | 0.95 (0.79 to 1.14) | .587 |
| 32.5–100 | 1.23 (1.08 to 1.40) | .002 | 0.88 (0.71 to 1.08) | .224 |
| Rural status | ||||
| Nonrural | 1.00 (referent) | 1.00 (referent) | ||
| Rural | 1.31 (1.15 to 1.49) | <.001 | 0.83 (0.68 to 1.03) | .091 |
The outcome is the odds of receiving 3 months of primary AST in low-risk disease or 3 months of AST in metastatic disease, respectively. The a priori predictor of interest is the year of diagnosis, particularly 2004 and 2005 (after the payment change, marked in bold) relative to 2003 (before the payment change). Other variables also affect the odds of receiving AST; however, year of cancer diagnosis remains a statistically significant predictor after controlling for these other factors. Models are based on data from all SEER registries, including those added in 2000. SEER registry was also included as a covariate in the model but results are not presented because of the large number of registries. CI = confidence interval; n/a = not applicable; OR = odds ratio; SEER = Surveillance, Epidemiology, and End Results database; WHO = World Health Organization.
Clinical tumor stage from Clinical Extension variable in SEER.
WHO grade from SEER. WHO grade 1–2 correlates with Gleason score 2–7 tumors in 1992–2002 and Gleason score 2–6 tumors in 2003–2005.
Determined by average zip code level information according to 2000 United States census data.
Discussion
In a cohort of men newly diagnosed with low risk prostate cancer between 1992–2005, a 64% reduction in reimbursement for AST was associated with a 40% relative reduction in the prevalence of non-indicated AST (10.2% vs. 6.1%) and a 39% reduction in the adjusted odds of receiving non-indicated AST. There was no statistically significant change for indicated AST in the same time period among men with metastatic disease.The effect for non-indicated AST was stable over alternative definitions of low-risk localized disease and variations in the number of SEER registries included in the analysis. The decline in primary AST could not be attributed to a growth in the preference for competing treatment choices such as radical prostatectomy or radiotherapy because the effect was still seen when eliminating such patients from the low-risk cohort, which is relevant because minimally invasive radical prostatectomy (21) and intensity modulated radiotherapy increased in popularity during the same period.
Weight et al. (18) measured the total number of annual Medicare prescriptions for AST from 2001 to 2005 and found a 14% decline in 2005. Their study suggested several alternative explanations beyond financial incentives for an observed decline in Medicare prescriptions for AST. First, there may have been a shift in the presentation of prostate cancer toward lower risk disease. We controlled for such a shift through stratification by tumor characteristics and indications for AST use. Second, there may have been a trend toward using longer acting agents, leading to a reduction in the number of prescriptions per year. We controlled for the possibility of such a trend by operationalizing longer acting agents as several doses of a 1-month agent. Third, there may have been a shift toward intermittent AST, which is a strategy of delivering AST for a limited period, beginning only when the PSA starts to rise and typically ending several months later, after PSA levels fall (22). By limiting our investigation to the receipt of at least 3 months of AST in the first year, we minimized the possibility of this confounding. Specifically, among non-indicated AST prescriptions, receipt of 1–2 months of AST without receiving a third month was rare (<5% in 2000 and 2005). Last, Weight et al. (18) hypothesized that the decline in prescription rates might represent a decrease in the use of neoadjuvant AST. We controlled for such a decrease by limiting our non-indicated cases to primary AST monotherapy.
The growing body of evidence of harms attributable to AST may be argued to have resulted in the observed decline in AST use. However, post hoc analyses and administrative database studies documenting high-risk complications of AST such as osteoporotic fractures, metabolic syndrome, and cardiovascular morbidity were mostly published after 2005 (9,10,12). If physicians were increasingly aware of the unintended consequences of AST before the publication of these results, this could have accounted for the decline in AST utilization in 2004–2005. Similar analyses of trends in communities less prone to financial incentives such as the US Veterans Affairs or Canadian health system may shed light on this matter. However, trends in other disease models (ie, perioperative beta blockers) demonstrate that even evidence from randomized trials is slow to affect clinical practice (23). Thus, the observed pattern of declining AST use in 2004–2005 likely represents a real effect of reimbursement change and not physician awareness of clinical evidence.
We chose to explore AST utilization trends in the extremes of indication—definitely indicated and non-indicated—so as to more clearly explore the effect of payment change on these two groups. Thus, we did not investigate AST utilization trends in intermediate groups such as locally advanced prostate cancer, for which indications for AST utilization in combination with radiotherapy or surgery have changed over our period of investigation (24–27). Furthermore, we emphasize that this investigation does not suggest that financial incentives led to the increase in AST seen in the 1990s. Our analysis only allows us to conclude that the reduction in reimbursement is associated with a decline in use in 2004–2005.
The national SEER registry with Medicare follow-up represents a relevant and robust method for examining trends in cancer care. Claims-based research has been shown to reliably collect treatment information when the claim is associated with a physician payment, especially high-reimbursing procedures such as surgery or chemotherapy (28–30). However, this study has several limitations. We followed a cohort of Medicare patients; thus, findings may not be valid in younger men. The change in the way SEER classified Gleason scores into WHO grade categories introduced some uncertainty into our trend analysis. Using our primary definition of low-risk disease, men in 2003–2005 had lower grade disease than men before 2003. This shift in the tumor grade of our low-risk cohort led to a false peak in the proportion and the odds of receiving primary AST in 2003. However, there was no change in the definition after 2003; thus, it did not affect measurement of our primary outcome (2004–2005 vs 2003). In addition, whereas methods were designed to minimize bias, residual confounding may exist. Until 2004, SEER did not report the PSA value, only whether it was elevated or not. Thus, our risk stratification did not include an important variable. Moreover, because our analysis covered a long period there is a risk that grade migration may have had an impact on prescription rates. The trend has been to classify the same tumor as higher grade in recent years (31), which could lead to less AST used in low-grade tumors; however, such migration would be expected to have a gradual impact on AST utilization rates, not the sudden impact that we observed.
Medicare changed the profitability of AST delivery in several ways in 2004–2005. Reimbursement for the drug itself was changed from paying 95% of the average wholesale price through 2003 to 80%–85% of the average wholesale price in 2004 to 106% of the average sales price in 2005 ($190 per 1-month depot in 2005) (32). These changes in pricing accounted for the decreased reimbursement we explored in this study. In 2004, Medicare also stopped paying for the nursing visit (CPT code 99211) associated with chemotherapy administration and in 2005 lowered the reimbursement for intramuscular or subcutaneous drug injection (CPT code 96400) by about $30. Because these latter reductions were relatively minor and would have introduced additional variability into our model, we did not include them in our analysis. However, future analyses may inquire about how the structure and timing of office visits for drug delivery changed as a result of these other payment changes.
Although the decline in non-indicated AST prescriptions was associated with a coincident decrease in AST payment, the payment change did not affect indicated therapy. Whereas only about 60% of men with metastatic disease receive indicated AST, this frequency did not suffer when reimbursement declined. The lack of a negative impact on indicated therapy may reflect the possibility that Medicare has identified a reimbursement level that is not far below the provider’s true cost of administration or it may represent an ethical constraint on withholding unprofitable beneficial therapy.
AST is one of the many classes of drugs covered under Medicare Part B, including other chemotherapeutics and asthma inhalers that were affected by recent payment changes; thus, our work has broad implications. Indeed, similar to our findings, Jacobson et al. showed that regional variations in payment for other chemotherapeutics in the 1990s did not affect the overall utilization rate of chemotherapy for metastatic breast, lung, or gastrointestinal tumors but did affect the selection of chemotherapeutic agents (33). Specifically, highly reimbursed physicians were more likely to prescribe more expensive agents. The interesting parallel to our study is that rates of indicated treatment (chemotherapy given or not) were not affected by reimbursement, but where discretion was allowed (ie, choice of which drug to use), reimbursement did affect practice. Future analyses should investigate the AST data with attention to whether the change in prescribing patterns in 2004–2005 was associated with particular physician characteristics. Such analyses could also exploit regional variations in physician reimbursement for AST that occurred because of variations in procedures used by National Drug Code carriers in setting the average wholesale price for AST within each code of the Healthcare Common Procedure Coding System before October 2002 (33). Similarly, one could examine how AST utilization is affected by regional variation in the adoption of the least costly alternative option for setting reimbursement, a policy that ended on April 19, 2010 (34).
Conclusions
A major reduction in physician reimbursement for AST was associated with a 39% decrease in the odds of receiving non-indicated AST but not indicated AST. These findings may help inform how payment changes will affect health-care utilization in other disease models.
Funding
National Institutes of Health (5K12-RR023247-04, a Multidisciplinary Scholar Development Program to Advance Clinical Research to S.P.E.).
Footnotes
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. This study used the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services , Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database
References
- 1.Horsely S. “Obama: Overtreatment drives health care costs.”. All Things Considered on National Public Radio. July 21, 2009. http://www.npr.org/templates/story/story.php?storyId=106859968. [Google Scholar]
- 2.Brownlee S. Overtreated: Why Too Much Medicine is Making us Sicker and Poorer. 1st U.S. ed. New York, NY: Bloomsbury: Distributed to the trade by Holtzbrinck Publishers; 2007. [Google Scholar]
- 3.Gawande A. The cost conundrum. The New Yorker 2009. June 1, 2009 [Google Scholar]
- 4.Arrow K. Uncertainty and the welfare economics of medical care. Am Econ Rec. 1963;53:941–973. [Google Scholar]
- 5.Wennberg JE, Cooper MM. Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 6.Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 2001;166(2):508–515. doi: 10.1016/s0022-5347(05)65972-1. [DOI] [PubMed] [Google Scholar]
- 7.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 8.Keating NL, O’Malley AJ, McNaughton-Collins M, Oh WK, Smith MR. Use of androgen deprivation therapy for metastatic prostate cancer in older men. BJU Int. 2008;101(9):1077–1083. doi: 10.1111/j.1464-410X.2007.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24(24):3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 11.Oefelein MG. Health related quality of life using serum testosterone as the trigger to re-dose long acting depot luteinizing hormone-releasing hormone agonists in patients with prostate cancer. J Urol. 2003;169(1):251–255. doi: 10.1016/S0022-5347(05)64079-7. [DOI] [PubMed] [Google Scholar]
- 12.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 13.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 14.Practice Guidelines Committee of the American Urological Association. Guideline for the management of clinically localized prostate cancer: 2007 update. Americal Urological Association. 27–28. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/proscan07/content.pdf. [Google Scholar]
- 15.NCCN. National Comprehensive Cancer Network Practice Guidelines in Oncology: Prostate Cancer. Fort Washington, PA: National Comprehensive Cancer Network; Version 2.2009; 2004. [Google Scholar]
- 16.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103(8):1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 17.McKoy JM, Lyons EA, Obadina E, et al. Caveat medicus: consequences of federal investigations of marketing activities of pharmaceutical suppliers of prostate cancer drugs. J Clin Oncol. 2005;23(34):8894–8905. doi: 10.1200/JCO.2005.01.4605. [DOI] [PubMed] [Google Scholar]
- 18.Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer. 2008;112(10):2195–2201. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Hu JC, Hevelone ND, Ferreira MD, et al. Patterns of care for radical prostatectomy in the United States from 2003 to 2005. J Urol. 2008;180(5):1969–1974. doi: 10.1016/j.juro.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 22.Conti PD, Atallah AN, Arruda H, Soares BG, El Dib RP, Wilt TJ. Intermittent versus continuous androgen suppression for prostatic cancer. Cochrane Database Syst Rev. 2007;(4):CD005009. doi: 10.1002/14651858.CD005009.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui AK, Ahmed S, Delbeau H, Conner D, Mattana J. Lack of physician concordance with guidelines on the perioperative use of beta-blockers. Arch Intern Med. 2004;164(6):664–667. doi: 10.1001/archinte.164.6.664. [DOI] [PubMed] [Google Scholar]
- 24.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167(1):112–116. [PubMed] [Google Scholar]
- 25.Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood DP, Jr, Puras-Baez A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. J Urol. 1995;154(2 pt 1):424–428. [PubMed] [Google Scholar]
- 26.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 27.Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997;15(3):1013–1021. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 28.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 29.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV-43–48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 30.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(8 suppl):IV-49–54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kondylis FI, Moriarty RP, Bostwick D, Schellhammer PF. Prostate cancer grade assignment: the effect of chronological, interpretive and translation bias. J Urol. 2003;170(4 pt 1):1189–1193. doi: 10.1097/01.ju.0000085675.96097.76. [DOI] [PubMed] [Google Scholar]
- 32.Painter MR. Reimbursement issues with hormonal therapies for prostate cancer. Rev Urol. 2005;7(suppl 5):S44–S47. [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson M, O’Malley AJ, Earle CC, Pakes J, Gaccione P, Newhouse JP. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25(2):437–443. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- 34.Services DoHaH. Medicare Reimbursement for Lupron. Washington, DC: Office of the Inspector General, Department of Health and Human Services; http://oig.hhs.gov/oei/reports/oei-03-03-00250.pdf Pub. No. OEI-03-03-00250. [Google Scholar]