Abstract
AIM: To assess the efficiency and toxicities of irinotecan (CPT-11)-involved regimens in patients with advanced gastric cancer.
METHODS: Randomized phases II and III clinical trials on chemotherapy for advanced gastric cancer were searched from MEDLINE, EMbase, Cochrane Controlled Trials Register, and EBSCO. Relevant abstracts were manually searched. A total of 657 patients were analyzed for their overall response rate (ORR), time to treatment failure (TTF), overall survival (OS) rate, and toxicities. Overall survival rate, reported as hazard ratio (HR) with 95% CI, was used as the primary outcome measure.
RESULTS: Four randomized controlled trials on chemotherapy for advanced gastric cancer were detected. The CPT-11-containing combination chemotherapy was not significantly advantageous over the non CPT-11-containing combination chemotherapy for OS rate (HR = 1.12, 95% CI: 0.92-1.36, P = 0.266) and ORR [risk ratio (RR) = 1.23, 95% CI: 0.71-2.14, P = 0.458]. However, the CPT-11-containing combination chemotherapy was significantly advantageous over the non CPT-11-containing combination chemotherapy for TTF (HR = 1.35, 95% CI: 1.12-1.64, P = 0.002). Grade 3/4 haematological toxicity (thrombocytopenia: RR = 0.20, 95% CI: 0.09-0.48; P < 0.001) and gastrointestinal toxicity (diarrhea: RR = 4.09, 95% CI: 2.42-6.93, P < 0.001) were lower in patients with advanced gastric cancer after CPT-11-containing combination chemotherapy than after non CPT-11 -containing combination chemotherapy.
CONCLUSION: CPT-11-containing combination chemotherapy is advantageous over non CPT-11 -containing combination chemotherapy for TTF with no significant toxicity. CPT-11-containing combination chemotherapy can be used in treatment of advanced gastric cancer.
Keywords: Meta-analysis, Advanced gastric cancer, Chemotherapy
INTRODUCTION
Although the incidence of gastric cancer has been sharply declined during the second half of the 20th century, it remains the second leading cause of cancer-related death in the world[1]. The morbidity and mortality rate of gastric cancer increase with age. The most effective treatment for gastric cancer is radical gastrectomy. A substantial number of patients, however, eventually die of recurrence after curative resection. Although systemic chemotherapy can improve the quality of life in patients with gastric cancer[2], the outcome of patients with unresectable gastric cancer is still extremely poor with a median survival time of 3-5 mo after the best supportive care[2-4].
Randomized clinical trial data demonstrate that the survival rate and quality of life are better in patients with advanced gastric cancer after chemotherapy than in those after the best supportive care[5]. Over the years, a number of single-agent chemotherapy trials have confirmed that gastric cancer is a relatively “chemosensitive” disease[6-9]. It is, therefore, necessary to investigate different combination chemotherapies, both in phase II and randomized phase III trial settings.
First line chemotherapy usually consists of different combination regimens with 5-fluorouracil (5-FU) and cisplatin, including FP (5-FU and cisplatin) and ECF (epirubicin, cisplatin, and 5-FU). It has been shown that the response rate and progression-free survival rate are better for patients with gastric cancer after FP therapy than after 5-FU or other combination therapies[10,11]. The additional survival advantage yielded by these combination therapies appears to be marginal. However, no standard regimen has yet been established. Thus, it is necessary to develop new agents and combination regimens to achieve greater survival benefits in advanced or recurrent unresectable gastric cancer. Since 2005, combination chemotherapy for advanced gastric cancer has been focused on the integration of other chemotherapy agents, including docetaxel, irinotecan, oxaliplatin, capecitabine, and S-1.
Irinotecan (CPT-11) is a water-soluble camptothecin derivative. CPT-11 and its active metabolite (SN-38) bind reversibly to the topoisomerase I-DNA complex and induce cancer cell death by preventing relegation of single-strand DNA breaks[12,13]. It has been shown that CPT-11 acts as a single agent in oesophago-gastric cancer. It was reported that the overall response rate of advanced gastric cancer patients to chemotherapy is 16%-20%[14,15]. It has been reported that CPT-11 in combination with leucovorin/5-FU (ILF)[16] or cisplatin (IP)[17] exhibits its antitumor activity against advanced gastric cancer.
Several phases II and III randomized trials are available on CPT-11-containing or non CPT-11-containing combination chemotherapy for advanced gastric cancer[18-21]. The meta-analysis in this study was to compare the two therapies by evaluating their clinical efficiency and toxicities.
MATERIALS AND METHODS
Literature search
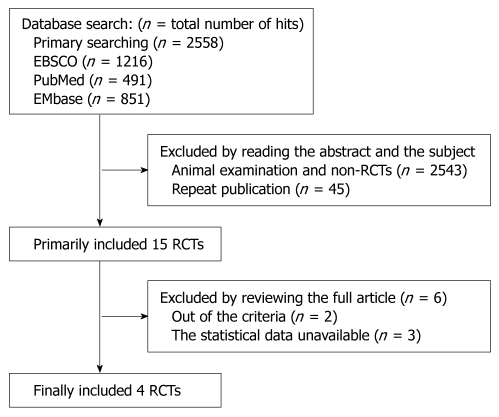
Trials were searched from MEDLINE, OLD MEDLINE, CancerLit, EMbase, and ISI Web of Science, incorporating Science Citation Index, Technology Proceedings, and Current Contents Databases as far back as they go. References of selected articles and previous systematic reviews were also searched for any other relevant trials (Figure 1). The search strategy included the following key words variably combined: metastatic, metastasis, gastric, cancer, CPT-11, randomized, trial.
Figure 1.
Article search and riddling progression. RCTs: Randomized controlled trials.
Two independent reviewers assessed the eligibility of the searched abstracts. If the eligibility of the abstract was unclear, the full article was retrieved for clarification. Any disagreements were solved by discussion. The selection criteria included study design (randomized or controlled trials), participants (patients with histologically confirmed advanced or recurrent adenocarcinoma, including diffuse type, intestinal type of the stomach or gastroesophageal junction). The exclusion criteria were nonrandomized trials, animal examination, single-arm phase II trial, or adequate statistical analysis with information missed. Care was taken to include only primary data or data that superseded earlier works. The deadline for trial inclusion was November 6, 2009.
Statistical methods
Overall survival rate was used as the primary outcome measure. Secondary outcome measures evaluated were overall response rate (ORR: number of partial and complete responses) and toxicities (published by the authors with the most frequently reported events analyzed). Hazard ratio (HR) and 95% CI as relevant effect measures were estimated directly or indirectly from the given data. Appropriate data, such as log-rank test P value, were extracted for the estimation of the log HR and its variance as previously described[22,23]. Summary statistical data were extracted from the published trials according to the standard methods for survival end points, with HR and CI as preferred sources for estimation, and log-rank P value/event count as a second choice[22]. Standard techniques for meta-analysis[24] were used to calculate the pooled estimates. All analyses were conducted using the Stata software version 8.2 (Stata Corp LP, College Station, TX). All tests were two sided. Fixed-(primarily) and random-effect model methodology was applied. All reported P values resulted from two-sided versions of the respective tests.
In consideration of possible heterogeneity across the studies, a statistical test for heterogeneity was performed as previously described[25]. P < 0.05 was considered statistically significant for the heterogeneity. A fixed effect approach was adopted unless there was evidence for significant unexplained heterogeneity, in which a random effect approach was used. In the absence of heterogeneity, the two methods provided identical results, because the fixed-effect model using the Mantel-Haenszel’s method assumes that studies are sampled from populations with the same effect size, making an adjustment to the study weights according to the in-study variance, whereas the random-effect model using the DerSimonian and Laird’s method assumes that studies are taken from populations with varying effect sizes, calculating the study weights both from in-study and between-study variances, considering the extent of variation or heterogeneity. Funnel plots and Egger’s linear regression test were used to show the potential publication bias in diagnosis of advanced gastric cancer[26].
RESULTS
The results of 4 randomized phases II and III trials, including 2 Europe randomized phase II trials[18,19], 1 Europe randomized phase III trial[20], and 1 Japanese study[21], that have been published or presented at major international meetings, were included in this analysis. These studies included 657 patients with metastatic gastric cancer, of whom 315 (48%) received the CPT-11-containing combination chemotherapy. Treatment schedule and quality of each trial were evaluated (Table 1).
Table 1.
Trials comparing irinotecan-containing and nonirinotecan-containing combination chemotherapies, treatment schedule and quality of each trial
| Study | Regimen | Patients (n) | RR (%) |
Survival (mo) |
|
| Progression free | Overall | ||||
| Bouché et al[18] | FU | 45 | 13.0 | 3.2 | 6.8 |
| CF | 44 | 27.0 | 4.9 | 9.5 | |
| IF | 45 | 40.0 | 6.9 | 11.3 | |
| Moehler et al[19] | ILF | 56 | 42.9 | 4.5 | 10.8 |
| ELF | 58 | 24.1 | 2.3 | 8.3 | |
| Dank et al[20] | IF | 170 | 31.8 | 9.0 | |
| CF | 163 | 25.8 | 8.7 | ||
| Nakashima et al[21] | IP | 44 | 47.0 | 5.7 | 14.8 |
| SP | 32 | 80.0 | 7.8 | 15.6 | |
FU: Fluorouracil; CF: Cisplatin and FU; IF: Irinotecan and FU; RR: Response rate; ILF: Irinotecan, leucovorin and FU; ELF: Etoposide, leucovorin and FU; IP: Irinotecan and cisplatin; SP: S-1 and cisplatin.
Overall survival time
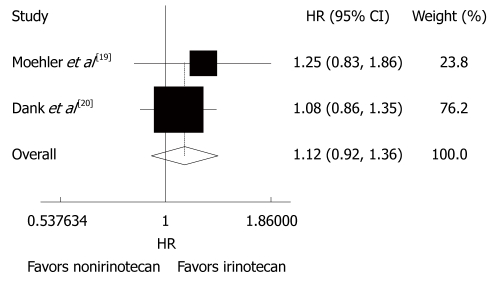
The overall survival rate was reported in the 4 trials[18-21], during which 315 patients received CPT-11-containing combination chemotherapy and 342 patients received non CPT-11-containing combination chemotherapy. However, only 2 trials reported the HR. The other trials showed that the overall survival time of patients with gastric cancer was 11.3-14.8 mo after CPT-11-containing combination chemotherapy and 6.8-15.6 mo after non CPT-1-containing combination chemotherapy. No striking inter-study heterogeneity was found (P = 0.535, I2 = 0.0%) in the 4 trials. Meta-analysis of the pooled data demonstrated that the overall risk of death was not different between the two chemotherapies (HR = 1.12, 95% CI: 0.92 -1.36, P = 0.266, Figure 2).
Figure 2.
Overall survival rate of patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies. HR: Hazard ratio.
Time to treatment failure
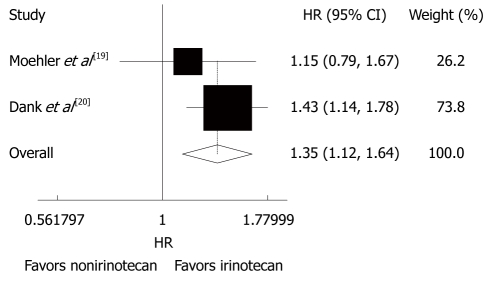
Two trials[19,20] analyzed the impact of time to treatment failure (TTF) with no striking inter-study heterogeneity (P = 0.327, I2 = 0.0%). The fixed-effect pooled estimation for TTF showed comparable results (HR = 1.35, 95% CI: 1.12-1.64, P = 0.002, Figure 3), suggesting that the outcome is significantly better in patients with advanced gastric cancer after CPT-11-containing combination chemotherapy than in those after non CPT-11-containing combination chemotherapy
Figure 3.
Time to treatment failure of patients with advanced gastric cancer irinotecan-containing and nonirinotecan-containing combination chemotherapies. HR: Hazard ratio.
Overall response rate
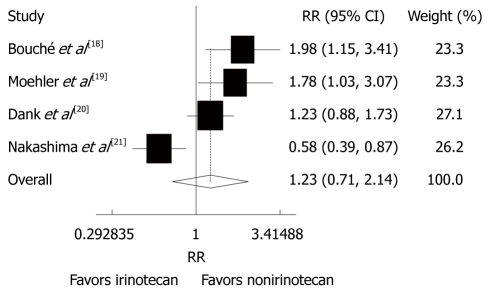
Risk ratio (RR) was reported in the 4 trials[18-21]. The overall response rate (ORR) of patients with advanced gastric cancer was 13%-80% to CPT-11-containing combination chemotherapy and 22%-47% to the non CPT-11-containing combination chemotherapy. A significant heterogeneity was observed (P = 0.000, I2 = 83.3%). The random-effect pooled estimate including 657 patients evaluated for ORR showed that the RR of CPT-11-containing combination chemotherapy was increased (RR = 1.23, 95% CI: 0.71-2.14, P = 0.458, Figure 4).
Figure 4.
Overall response rate of patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies. RR: Risk ratio.
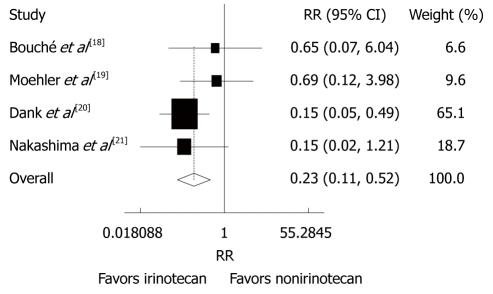
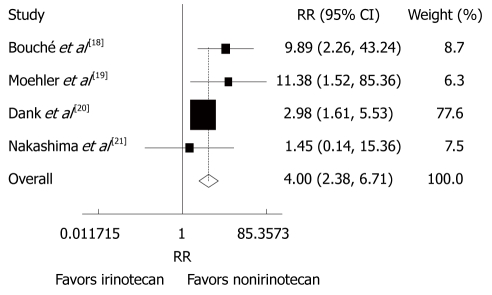
Toxicities
Reported toxicities were analyzed in all trials. The incidence of grade 3/4 thrombocytopenia (RR = 0.23, 95% CI: 0.11-0.52, P < 0.001) and diarrhea (RR = 4.00, 95% CI: 2.38-6.71, P < 0.001) was lower in patients after CPT-11-containing combination chemotherapy than in those after non CPT-11-containing combination chemotherapy (Figures 5 and 6). The incidence of other grade 3/4 haematological toxicities, such as neutropenia (RR = 0.60, 95% CI = 0.28-1.31, P = 0.201), febrile neutropenia (RR = 0.64, 95% CI: 0.37-1.10, P = 0.108) and leucopenia (RR = 0.85, 95% CI: 0.59-1.23, P = 0.388), as well as other grade 3/4 gastrointestinal toxicities, such as nausea (RR = 0.84, 95% CI: 0.46-1.54, P = 0.582), vomiting (RR = 0.83, 95% CI: 0.44 -1.56, P = 0.556), anorexia (RR = 0.66, 95% CI: 0.31-1.41, P = 0.278) was similar in patients with advanced gastric cancer after CPT-11-containing and non CPT-11-containing combination therapies. No evidence for heterogeneity was found except for neutropenia and alopecia.
Figure 5.
Grade 3/4 haematological toxicities (thrombocytopenia) in patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies. RR: Risk ratio.
Figure 6.
Grade 3/4 gastrointestinal toxicities (diarrhea) in patients with advanced gastric cancer after irinotecan-containing and nonirinotecan-containing combination chemotherapies. RR: Risk ratio.
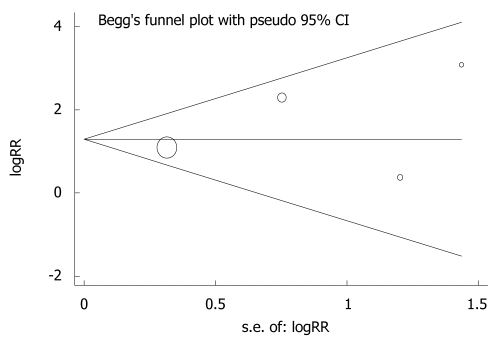
Publication bias
Evidence of publication bias was detected by plotting funnel plots of HR. Studies were plotted in order of decreasing variance of the log HR. Visual inspection of the funnel plots with respect to the 3 end points (OS rate, TTF and ORR) did not reveal any hint of publication bias (Figure 7). Funnel plots for all comparisons could not identify relevant publication bias, although the number of included studies was relatively small.
Figure 7.
Funnel plots and Egger’s linear regression test showing the potential publication bias in diagnosis of advanced gastric cancer[22]. RR: Risk ratio.
DISCUSSION
During the last decade, advances in treatment of patients with advanced gastric cancer have been achieved as a result of the integration of novel, effective agents into treatment algorithms. The availability of CPT-11 further enriches the options for combination therapy, because CPT-11 is effective and tolerable in patients with advanced gastric cancer.
The present systematic review revealed the major findings in ongoing debatable questions. We wonder whether CPT-11-containing combination chemotherapy is better than non CPT-11-containing combination chemotherapy for advanced gastric cancer. One of the earlier systematic reviews on 3 randomized controlled trials (RCTs) of chemotherapy for advanced gastric cancer concluded that there is no convincing evidence that demonstrates a significant benefit in overall survival rate of patients after CPT-11-containing combination chemotherapy[27]. The resulting HR for the overall survival rate was 0.88% (95% CI: 0.73-1.06), which is in favor of CPT-11-containing combination chemotherapy. However, the study[23] did not assess other outcomes or toxicities. In our analysis, the 2 trials that assessed TTF showed that the overall summary estimate favored the CPT-11-containing combination chemotherapy with no significant inter-trial heterogeneity.
Most reported end points covered in the RCTs were searched and the most appropriate statistical methods for meta-analysis of time-to-event data extracted from published reports were used in our study. However, the quality of life was not stressed in patients with advanced gastric cancer due to the different methods used in reporting their quality of life. Although the data about response rate and adverse events were pooled to permit a clinically relevant analysis, these parameters varied. The response rate was reported according to the clinical parameters, WHO and RECIST criteria, whereas the CTC, WHO and ECOG scales were used in analysis of toxicity data.
In conclusion, there is insufficient evidence that the overall survival rate, overall response rate are better for patients with advanced gastric cancer after CPT-11-containing combination chemotherapy than after non CPT-11-containing combination chemotherapy. CPT-11-containing combination chemotherapy is advantageous over non CPT-11-containing combination chemotherapy for TTF and grade 3/4 thrombocytopenia.
Irrespective of the positive impact of presently available chemotherapy, the prognosis of patients with advanced gastric cancer remains poor, with a median survival time of 7-10 mo. Further RCTs are needed to assess which CPT-11 combination chemotherapy is least toxic.
COMMENTS
Background
The morbidity and mortality of gastric cancer increase with age. It has been shown that irinotecan (CPT-11) actis as a single agent in oesophago-gastric cancer. The aim of this meta-analysis was to assess the efficiency and toxicities of CPT-11 involved regimens in patients with advanced gastric cancer.
Research frontiers
Several phases II and III randomized trials have been reported comparing CPT-11-containing or non CPT-11-containing combination chemotherapy for advanced gastric cancer. The authors wonder whether CPT-11-containing combination chemotherapy is better than non CPT-11-containing combination chemotherapy for advanced gastric cancer.
Innovations and breakthroughs
This systematic review revealed the major findings in ongoing debatable questions. One of the earlier systematic reviews of 3 randomized controlled trials of chemotherapy for advanced gastric cancer concluded that there is no convincing evidence that demonstrates that the overall survival rate of patients with advanced gastric cancer is higher after CPT-11-containing combination chemotherapy than after non CPT-11-containing combination chemotherapy. However, this study did not assess other outcomes or toxicities. In the authors' analyses, 4 randomized phases II and III trials were included. A total of 657 patients were analyzed for their overall response rate, time to treatment failure (TTF), overall survival rate and toxicities. The two trials that assessed TTF showed that the overall summary estimate favored CPT-11-containing combination chemotherapy.
Applications
The findings in the study suggest that CPT-11 based combination chemotherapy is a candidate regimen for advanced gastric cancer. Further studies are needed to compare common chemotherapy with/without target therap.
Peer review
The authors in their meta-analysis showed that CPT-11-containing combination chemotherapy was advantageous over non CPT-11-containing combination chemotherapy for TTF, overall survival rate, overall response rate and toxicity. The availability of CPT-11 further enriches the options for combination therapy, because CPT-11 is effective and tolerable in patients with advanced gastric cancer. Further randomized control trials are needed to assess which chemotherapy provides favorable overall survival rate with less toxicity. Moreover, target therapy agents should be taken into consideration to see if they can achieve better clinical benefits in patients with advanced gastric cancer.
Footnotes
Supported by Affiliated Nanjing First Hospital, Nanjing Medical University and Medical school of Southeast University
Peer reviewer: Susumu Ohwada, Associate Professor, Department of Surgery, Gunma University Graduate School of Medicine, 3-39-15 Shoma-Machi, Maebashi 371-8511, Japan
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1–9. doi: 10.1016/s0895-4356(02)00534-6. [DOI] [PubMed] [Google Scholar]
- 2.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Glimelius B, Hoffman K, Haglund U, Nyrén O, Sjödén PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol. 1994;5:189–190. doi: 10.1093/oxfordjournals.annonc.a058778. [DOI] [PubMed] [Google Scholar]
- 4.Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 6.González Barón M, Espinosa E, Feliu J, Ordóñez A, Zamora P, de Castro J, García Girón C, García Alfonso P, Garrido P, Belón J, et al. The UFT/leucovorin/etoposide regimen for the treatment of advanced gastric cancer. Oncopaz Cooperative Group. Oncology (Williston Park) 1997;11:109–112. [PubMed] [Google Scholar]
- 7.Köhne CH, Wils JA, Wilke HJ. Developments in the treatment of gastric cancer in Europe. Oncology (Williston Park) 2000;14:22–25. [PubMed] [Google Scholar]
- 8.Caponigro F, Facchini G, Nasti G, Iaffaioli RV. Gastric cancer. Treatment of advanced disease and new drugs. Front Biosci. 2005;10:3122–3126. doi: 10.2741/1768. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Lee KW, Kim YH, Lee KH, Oh do Y, Kim J, Yang SH, Im SA, Choi SH, Bang YJ. Individualized tumor response testing for prediction of response to Paclitaxel and Cisplatin chemotherapy in patients with advanced gastric cancer. J Korean Med Sci. 2010;25:684–690. doi: 10.3346/jkms.2010.25.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993;71:3813–3818. doi: 10.1002/1097-0142(19930615)71:12<3813::aid-cncr2820711205>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205) J Clin Oncol. 2003;21:54–59. doi: 10.1200/JCO.2003.04.130. [DOI] [PubMed] [Google Scholar]
- 12.Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- 13.Tanizawa A, Fujimori A, Fujimori Y, Pommier Y. Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst. 1994;86:836–842. doi: 10.1093/jnci/86.11.836. [DOI] [PubMed] [Google Scholar]
- 14.Futatsuki K, Wakui A, Nakao I, Sakata Y, Kambe M, Shimada Y, Yoshino M, Taguchi T, Ogawa N. [Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. CPT-11 Gastrointestinal Cancer Study Group] Gan To Kagaku Ryoho. 1994;21:1033–1038. [PubMed] [Google Scholar]
- 15.Chun JH, Kim HK, Lee JS, Choi JY, Lee HG, Yoon SM, Choi IJ, Ryu KW, Kim YW, Bae JM. Weekly irinotecan in patients with metastatic gastric cancer failing cisplatin-based chemotherapy. Jpn J Clin Oncol. 2004;34:8–13. doi: 10.1093/jjco/hyh006. [DOI] [PubMed] [Google Scholar]
- 16.Bouché O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, Arsène D, Paitel JF, Guérin-Meyer V, Mitry E, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol. 2004;22:4319–4128. doi: 10.1200/JCO.2004.01.140. [DOI] [PubMed] [Google Scholar]
- 17.Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, Charnsangavej C. CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer. 2002;94:641–646. doi: 10.1002/cncr.10279. [DOI] [PubMed] [Google Scholar]
- 18.Bouché O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, Arsène D, Paitel JF, Guérin-Meyer V, Mitry E, et al. Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study--FFCD 9803. J Clin Oncol. 2004;22:4319–4328. doi: 10.1200/JCO.2004.01.140. [DOI] [PubMed] [Google Scholar]
- 19.Moehler M, Eimermacher A, Siebler J, Hohler T, Wein A, Menges M, Flieger D, Junginger T, Geer T, Gracien E, et al. Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer. 2005;92:2122–2128. doi: 10.1038/sj.bjc.6602649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima K, Hironaka S, Boku N, Onozawa Y, Fukutomi A, Yamazaki K, Yasui H, Taku K, Kojima T, Machida N. Irinotecan plus cisplatin therapy and S-1 plus cisplatin therapy for advanced or recurrent gastric cancer in a single institution. Jpn J Clin Oncol. 2008;38:810–815. doi: 10.1093/jjco/hyn109. [DOI] [PubMed] [Google Scholar]
- 22.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 24.Wilson IA. Don C. Wiley. Nat Struct Biol. 2002;9:164–166. doi: 10.1038/nsb0302-164. [DOI] [PubMed] [Google Scholar]
- 25.Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil. 2006;87:875. doi: 10.1016/j.apmr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Thakkinstian A, McElduff P, D'Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24:1291–1306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
- 27.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]