Abstract
AIM: To investigate the clinical significance of hepatic blood inflow occlusion without hemihepatic artery control (BIOwHAC) in the treatment of hepatocellular carcinoma (HCC).
METHODS: Fifty-nine patients with HCC were divided into 3 groups based on the technique used for achieving hepatic vascular occlusion: group 1, vascular occlusion was achieved by the Pringle maneuver (n = 20); group 2, by hemihepatic vascular occlusion (HVO) (n = 20); and group 3, by BIOwHAC (n = 19). We compared the procedures among the three groups in term of operation time, intraoperative bleeding, postoperative liver function, postoperative complications, and length of hospital stay.
RESULTS: There were no statistically significant differences (P > 0.05) in age, sex, pathological diagnosis, preoperative Child’s disease grade, hepatic function, and tumor size among the three groups. No intraoperative complications or deaths occurrred, and there were no significant intergroup differences (P > 0.05) in intraoperative bleeding, hepatic function change 3 and 7 d after operation, the incidence of complications, and length of hospital stay. BIOwHAC and Pringle maneuver required a significantly shorter operation time than HVO; the difference in the serum alanine aminotransferase or aspartate aminotransferase levels before and 1 d after operation was more significant in the BIOwHAC and HVO groups than in the Pringle maneuver group (P < 0.05).
CONCLUSION: BIOwHAC is convenient and safe; this technique causes slight hepatic ischemia-reperfusion injury similar to HVO.
Keywords: Hepatic blood inflow occlusion without hemihepatic artery control, Hepatocellular carcinoma, Intraoperative bleeding, Ischemia-reperfusion injury
INTRODUCTION
Intraoperative bleeding occurs most frequently during hepatic resection. Bleeding-associated blood transfusions increase the postoperative complications and mortality rate[1], especially in cases complicated with hepatocirrhosis[2]. Therefore, control of intraoperative bleeding is a prerequisite for reducing the number of cases requiring blood transfusions during liver resection. The common approaches to reduce blood transfusion include lowering the intraoperative central venous pressure[3-5], and using cut-ultrasound aspiration and microwave thermocoagulation in liver surgery[6,7], and hepatic inflow occlusion with or without outflow control. Hepatic inflow control (with or without outflow control) plays an important role in constructing a bloodless surgical field. However, hepatic ischemia-reperfusion injury (HIRI) occurs in all hepatic vascular occlusions to some extent. The normal liver receives 70%-75% of its blood supply from the portal vein and 40%-60% of its oxygen supply from arterial blood. Considering the differences between the distribution and oxygen content of the blood in the portal vein and that in the hepatic artery, we performed hepatic blood inflow occlusion without hemihepatic artery control (BIOwHAC)[8] to minimize HIRI by a modified surgical procedure. During BIOwHAC, the proper hepatic artery was surgically exposed, after which its left and right branches were separated. For the right and left hemihepatic occlusions, catheters were advanced and bypassed the portal vein, bile duct, and the respective branches of the hepatic artery before being tightened.
We designed a retrospective case-control study to compare BIOwHAC, Pringle maneuver and hemihepatic vascular occlusion (HVO) in terms of operation time, intraoperative bleeding, and postoperative liver function, and postoperative complications. We also assessed the merits and demerits of these three approaches.
MATERIALS AND METHODS
Inclusion criteria
Between March 2005 and January 2009, 162 patients were treated at our hospital for liver neoplasms. Of these, 59 patients who met with the following criteria were included in this trial: availability of complete data, presence of pathologically diagnosed hepatocellular carcinoma (HCC), cancers confined to half of the liver and being suitable for hepatic portal anatomical vascular occlusion, and resectable tumors. Patients who underwent pericardial devascularization, splenectomy, gastroenterostomy and biliointestinal anastomosis were excluded.
Vascular occlusion procedures
Of the 59 patients, 20 underwent Pringle maneuver, 20 underwent HVO, and 19 underwent BIOwHAC. The patients in the Pringle maneuver group underwent the Pringle’s occlusion by the conventional method at the hepatoduodenal ligament using urethral catheters. Patients in the HVO group underwent vascular occlusions via two approaches. (1) The catheters were introduced into the confluence of the left and right hepatic ducts along the common hepatic duct without dissecting the hepatoduodenal ligament; the tangential clamp was placed at the superior hepatic capsule or inferior transverse fissura ligamenti teretis and then the internal liver parenchyma and the external Glisson sheath were bluntly separated with care; if no resistance occurred at that time, the urethral catheter was exited at the portal vein bifurcation-caudate lobe junction towards the posterior hepatoduodenal ligament and then insufflated to achieve right hemihepatic occlusion; one end of the catheter was inserted through the lesser omentum foramen to the hepatogastric ligament and insufflated to achieve left hemihepatic occlusion; and (2) The affected side of the hepatic artery and the portal vein were separated to occlude them simultaneously or individually. Patients in the BIOwHAC group underwent BIOwHAC as follows: the proper hepatic artery was surgically exposed and the left and right branches of the proper hepatic artery were separated; for right hemihepatic occlusion, the catheter bypassed the portal vein, bile duct, and the right hepatic artery and was tightened; for left hemihepatic occlusion, the catheter bypassed the portal vein, bile duct, and the left hepatic artery and was tightened (Figure 1).
Figure 1.
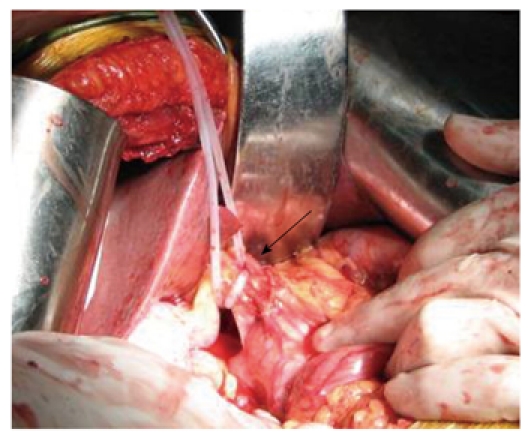
Application of hepatic blood inflow occlusion without hemihepatic artery control (arrow shows non-occlusive left hepatic artery).
Data collection
Complete background information, including age, sex, liver function, Child’s disease grade, level of serum markers of hepatitis, and α-fetoprotein levels was collected from all the patients.
Computed tomography (CT) and magnetic resonance imaging (MRI) images and operative record were carefully previewed and information regarding the size of HCC, hepatic vascular occlusion approach, occlusion time, type of liver resection, and operation time were precisely recorded.
The levels of albumin, total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were recorded 1, 3, and 7 d after operation to determine the deviations from preoperative values.
Patients were observed for postoperative complications such as bleeding from hepatic section, biliary fistula, subphrenic abscess, responsive pleural effusion, hepatic encephalopathy, pulmonary infection, hemorrhage from the digestive tract, and long-term (> 30 d) hepatic dysfunction.
Statistical analysis
Data were expressed as mean ± SD and variance analysis was performed; χ2 test was performed for countable data. P < 0.05 indicates significant difference.
RESULTS
Background information
Patients’ information on age, sex, preoperative Child’s disease grade, pathologic diagnosis, and preoperative hepatic function are listed in Table 1; and the information about tumor size and type of liver resection is shown in Table 2.
Table 1.
Patients’ background information1 (mean ± SD)
| Groups | Pringle maneuver (n = 20) | HVO (n = 20) | BIOwHAC (n = 19) |
| Age (yr) | 54.35 ± 10.03 | 54.20 ± 11.34 | 54.26 ± 7.05 |
| Gender (M:F) | 16:4 | 13:7 | 14:5 |
| Pathologic diagnosis | HCC | HCC | HCC |
| Complication hepatocirrhosis (cases) | 10 | 9 | 10 |
| Hepatitis virus positive (cases) | 18 | 18 | 17 |
| Increase in AFP (cases) | 12 | 11 | 10 |
| Child’s disease grade (cases, A:B) | 19:1 | 19:1 | 18:1 |
| Serum albumin (g/L) | 40.04 ± 3.42 | 40.87 ± 3.12 | 39.78 ± 2.71 |
| Serum bilirubin (μmol/L) | 12.86 ± 5.60 | 14.68 ± 3.37 | 13.08 ± 7.81 |
| Serum ALT (U/L) | 45.22 ± 32.99 | 45.04 ± 63.01 | 47.63 ± 40.48 |
| Serum AST (U/L) | 39.43 ± 17.20 | 40.72 ± 21.57 | 41.56 ± 41.01 |
1There is no significant intergroup difference (P > 0.05). BIOwHAC: Hepatic blood inflow occlusion without hemihepatic artery control; HVO: Hemihepatic vascular occlusion; AFP: α-fetoprotein; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
Table 2.
Intraoperative status of patients (mean ± SD)
| Groups | Pringle maneuver (n = 20) | HVO (n = 20) | BIOwHAC (n = 19) |
| Right hemiliver (cases) | 2 | 3 | 1 |
| Left hemiliver (cases) | 1 | 2 | 1 |
| S2 + S3 (cases) | 3 | 5 | 3 |
| S6 + S7 (cases) | 2 | 2 | 2 |
| S5 + S6 (cases) | 2 | 2 | 1 |
| S5 + S8 (cases) | 1 | 2 | 1 |
| S6 (cases) | 2 | 2 | 3 |
| S7 (cases) | 3 | 1 | 2 |
| S5 (cases) | 2 | 1 | 2 |
| S4 (cases) | 2 | 0 | 2 |
| S8 (cases) | 0 | 0 | 2 |
| Tumor size (cm) | 6.33 ± 3.39 | 7.60 ± 4.03b | 6.11 ± 3.18bc |
| Operation time (min) | 158.50 ± 43.77a | 219.25 ± 58.09 | 151.84 ± 41.77a |
| Occlusion time (min) | 20.60 ± 4.91 | 25.70 ± 8.29b | 18.94 ± 5.13bc |
| Occlusion frequency (time) | 1 | 1 | 1 |
| Bleeding volumes (mL) | 700.00 ± 163.32 | 1017.5 ± 663.57b | 789.47 ± 683.04bc |
| Blood transfusion (cases) | 8 | 11 | 9 |
| Transfusion volumes (mL) | 775.00 ± 679.81 | 709.09 ± 317.66b | 766.66 ± 580.94bc |
P < 0.05 vs HVO;
P > 0.05 vs pringle maneuver;
P > 0.05 vs HVO. BIOwHAC: Hepatic blood inflow occlusion without hemihepatic artery control; HVO: Hemihepatic vascular occlusion.
Intraoperative information
In all the patients, the procedures were performed without intraoperative complications and deaths. Each group underwent vascular occlusion only once, and there were no statistically significant intergroup differences (P > 0.05) in occlusion time, type of liver resection, bleeding and blood transfusions (Table 2). BIOwHAC and Pringle maneuver required significantly shorter operation time than HVO (151.84 ± 41.77 min and 158.50 ± 43.77 min, respectively vs 219.25 ± 58.09 min, P < 0.05), but the time required for BIOwHAC was almost equivalent to that for Pringle maneuver (P > 0.05).
Postoperative hepatic function variation
Serum albumin level was reduced in all the patients 1 and 3 d after operation, but increased after 7 d, and no significant intergroup difference was observed (Figure 2A). The total serum bilirubin level increased 1 and 3 d after operation, but decreased after 7 d, with no significant intergroup differences (Figure 2B). In addition, there was an increase in serum ALT and AST levels 1 d after operation but a decrease after 7 d. Patients in the Pringle maneuver group had more significant variation in the serum ALT and AST levels (P < 0.05) before and 1 d after operation than the BIOwHAC and HVO groups, but there was no significant difference between BIOwHAC and HVO groups (P > 0.05). No significant intergroup differences were noted in the serum ALT and AST levels (P > 0.05) 3 and 7 d after operation (Figure 2C and D).
Figure 2.
Preoperative and postoperative albumin concentrations (A), total bilirubin levels (B), serum alanine aminotransferase levels (C) and aspartate aminotransferase levels (D) in the 3 approaches for achieving occlusions. HVO: Hemihepatic vascular occlusion; BIOwHAC: Hepatic blood inflow occlusion without hemihepatic artery control; ALB: Albumin; TBIL: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Postoperative complications and length of hospital stay
The most common postoperative complication in this study was pleural effusion with pulmonary infection, occurring in 4 cases (20%) of the Pringle maneuver group, 5 cases (25%) of the HVO group, and 4 cases (21.1%) of the BIOwHAC group. No significant intergroup differences were noted in length of hospital stay among the three groups (Table 3).
Table 3.
Postoperative complications and length of hospital stay (mean ± SD)
| Groups | Pringle maneuver (n = 20) | HVO (n = 20) | BIOwHAC (n = 19) |
| Postoperative complications (cases) | 4 | 5 | 4 |
| Responsive pleural effusion (cases) | 3 | 2 | 3 |
| Pulmonary infection (cases) | 1 | 3 | 1 |
| Hepatic section bleeding (cases) | 0 | 0 | 0 |
| Bile leakage (cases) | 0 | 0 | 0 |
| Subphrenic abscess (cases) | 0 | 0 | 0 |
| Hepatic encephalopathy (cases) | 0 | 0 | 0 |
| Hemorrhage of digestive tract (cases) | 0 | 0 | 0 |
| Long-term liver dysfunction (cases) | 0 | 0 | 0 |
| Perioperative deaths (cases) | 0 | 0 | 0 |
| Length of hospital stay (d) | 21.75 ± 4.32 | 22.95 ± 5.30 | 21.47 ± 9.36 |
BIOwHAC: Hepatic blood inflow occlusion without hemihepatic artery control; HVO: Hemihepatic vascular occlusion
DISCUSSION
Owing to the recent improvement in the liver resection technique intraoperative hepatic vascular occlusion and perioperative management, surgical complications and mortality rate have been considerably reduced[8-10]. Pringle maneuver is the commonly used method for hepatic vascular occlusion, which enables effective control of bleeding. Multiple approaches for hepatic vascular occlusions have been developed; however the Pringle maneuver is more popular because it can be used in various types of liver resection[11,12]. Man et al[13] reported that the Pringle maneuver is superior to non-vascular occlusion, but can lead to obvious HIRI and is time consuming, especially in cases complicated with liver cirrhosis[14]. In 1987, Makuuchi performed HVOs through portal venous branch occlusion and hepatic arterial branch occlusion[15]. HVO helps conserve the contralateral hepatic vascular inflow and facilitates the hepatic operation and mildly affects postoperative liver function in favor of patients with liver cancer and hepatocirrhosis[15]. However, HVO must be performed by surgeons who are proficient in portal vein surgery so as to avoid incident damage to the interior conduit of the Glisson sheath, hemorrhage, and biliary fistula. If there are communicating branches between the non-occluded and the occluded hemilivers, hepatic bleeding may be quite severe[16].
Although no significant intergroup differences were observed in the changes of the serum AST and ALT levels 3 and 7 d after operation, patients in the HVO and BIOwHAC groups had significantly lower AST and ALT levels than those in the Pringle maneuver group 1 d after operation. This implies that HVO and BIOwHAC are superior to Pringle maneuver in minimizing the HIRI. In HVO, the vascular occlusion is continuous; but in the Pringle maneuver the circulation is maintained with occlusion for 15-20 min and reperfusion for 5 min, this interrupted occlusion gives rise to several problems[3]. In our study, the patients underwent only a single vascular occlusion, which is the reason why no significant intergroup differences were seen in intraoperative bleeding and in the number of cases requiring blood transfusions.
In humans, the confluence of the left and right hepatic ducts lies superiorly, the portal bifurcation is inferior to this bifurcation, and below is the proper hepatic arterial bifurcation. Therefore, it is convenient and safe to intraoperatively expose the proper hepatic artery and separate its left and right branches. Thus, surgeons can occlude the portal vein, bile duct, and occlusive-side artery branch in a single operation. BIOwHAC is convenient and safe as compared to Pringle maneuver. Our results showed that HVO required significantly longer operation time than BIOwHAC and Pringle maneuver (219.25 ± 58.09 min vs 151.84 ± 41.77 min and 158.50 ± 43.77 min) due to the net operation of vascular occlusion for 20-30 min. In addition, resection of the cholecyst is required in the right HVO, but not in BIOwHAC, which can satisfy the wish of the patients who want to conserve the cholecyst.
In the normal liver, 70%-75% of blood supply comes from the portal vein and 40%-60% of oxygen supply from arterial blood. Portal vein can not supply all parts of the liver with oxygen, and when the portal vein is completely occluded, the level of oxygen consumption in the liver remains the same as that before occlusion. This indicates that the hepatic artery alone is sufficient to meet the oxygen demand of the liver[17]. Our results showed that the patients in the BIOwHAC group had significantly lower serum AST and ALT levels than those in the Pringle maneuver group 1 d after operation, but no significant differences from that in the HVO group. This finding shows that hemihepatic artery conservation can provide enough oxygen to meet the demand of the intact hemiliver. In addition, no significant intergroup difference was noted in the postoperative serum albumin levels, which may be attributed to postoperative exogenous supplements.
Our results indicated no significant intergroup differences in the intraoperative bleeding and blood transfusion, liver function 3 and 7 d after operation, incidence of postoperative complications, and length of hospital stay. We recommend BIOwHAC because it is a convenient and safe technique similar to the Pringle maneuver and can protect the liver function injury as effectively as HVO.
In conclusion, among the various kinds of approaches for hepatic vascular occlusion available currently, the most suitable one should be selected on the basis of comprehensive preoperative examinations (CT or MRI imaging and liver function tests), intraoperative examinations (pathological examination and lesion location), examinations for invasion in the hepatic vein and inferior vena cava, cardiovascular status, as well as the experience and skill of surgeons and anesthesiologists.
COMMENTS
Background
Intraoperative bleeding occurs most commonly during hepatic resection. Hepatic inflow control plays an important role in constructing a bloodless surgical field.
Research frontiers
There are various techniques of hepatic vascular control, including Pringle maneuver, hemihepatic vascular occlusion (HVO), total hepatic vascular exclusion, liver hanging maneuver, etc.
Innovations and breakthroughs
Based on the differences between the distribution and oxygen content of the blood in the portal vein and that in the hepatic artery, hepatic blood inflow occlusion without hemihepatic artery control (BIOwHAC) was performed in this study.
Applications
BIOwHAC is convenient and safe similar to Pringle maneuver; this technique causes as slight hepatic ischemia reperfusion injury as HVO.
Terminology
BIOwHAC: the proper hepatic artery was surgically exposed, after which its left and right branches were separated. For the right and left hemihepatic occlusions, catheters were advanced and bypassed the portal vein, bile duct, and the respective branches of the hepatic artery before being tightened.
Peer review
This is a retrospective analysis on a single center series of different methods of inflow hepatic occlusion during liver resection for liver cancer. The study is well written and the methodology is correct.
Footnotes
Supported by The Inner Mongolia Science Foundation, Grant No. 2009BS1103
Peer reviewer: Salvatore Gruttadauria, MD, Assistant Professor, Abdominal Transplant Surgery, ISMETT, Via E. Tricomi, 190127 Palermo, Italy
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
References
- 1.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869-870. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu CC, Kang SM, Ho WM, Tang JS, Yeh DC, Liu TJ, P'eng FK. Prediction and limitation of hepatic tumor resection without blood transfusion in cirrhotic patients. Arch Surg. 1998;133:1007–1010. doi: 10.1001/archsurg.133.9.1007. [DOI] [PubMed] [Google Scholar]
- 3.Wu CC, Yeh DC, Ho WM, Yu CL, Cheng SB, Liu TJ, P'eng FK. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients: a randomized comparison of hemihepatic and total hepatic occlusion techniques. Arch Surg. 2002;137:1369–1376. doi: 10.1001/archsurg.137.12.1369. [DOI] [PubMed] [Google Scholar]
- 4.Chen XP, Zhang ZW, Zhang BX, Chen YF, Huang ZY, Zhang WG, He SQ, Qiu FZ. Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis. Langenbecks Arch Surg. 2006;391:209–215. doi: 10.1007/s00423-006-0043-7. [DOI] [PubMed] [Google Scholar]
- 5.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Strasberg SM, Drebin JA, Linehan D. Use of a bipolar vessel-sealing device for parenchymal transection during liver surgery. J Gastrointest Surg. 2002;6:569–574. doi: 10.1016/s1091-255x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 7.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–563. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12:935–939. doi: 10.3748/wjg.v12.i6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan LN, Chen XL, Li ZH, Li B, Lu SC, Wen TF, Zeng Y, Yiao HH, Yang JY, Wang WT, et al. Perioperative management of primary liver cancer. World J Gastroenterol. 2007;13:1970–1974. doi: 10.3748/wjg.v13.i13.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang L, Tian F, Tao W, Cui J. Hepatocellular glycogen in alleviation of liver ischemia-reperfusion injury during partial hepatectomy. World J Surg. 2007;31:2039–2043. doi: 10.1007/s00268-007-9186-0. [DOI] [PubMed] [Google Scholar]
- 11.Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg. 1908;48:541–549. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–822, discussion 822-823. doi: 10.1097/01.sla.0000189121.35617.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man K, Lo CM, Liu CL, Zhang ZW, Lee TK, Ng IO, Fan ST, Wong J. Effects of the intermittent Pringle manoeuvre on hepatic gene expression and ultrastructure in a randomized clinical study. Br J Surg. 2003;90:183–189. doi: 10.1002/bjs.4027. [DOI] [PubMed] [Google Scholar]
- 14.Figueras J, Lopez-Ben S, Lladó L, Rafecas A, Torras J, Ramos E, Fabregat J, Jaurrieta E. Hilar dissection versus the "glissonean" approach and stapling of the pedicle for major hepatectomies: a prospective, randomized trial. Ann Surg. 2003;238:111–119. doi: 10.1097/01.SLA.0000074981.02000.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442–447. doi: 10.1001/archsurg.1996.01430160100022. [DOI] [PubMed] [Google Scholar]
- 16.Torzilli G, Makuuchi M, Inoue K, Takayama T, Sakamoto Y, Sugawara Y, Kubota K, Zucchi A. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg. 1999;134:984–992. doi: 10.1001/archsurg.134.9.984. [DOI] [PubMed] [Google Scholar]
- 17.Oda M, Yokomori H, Han JY. Regulatory mechanisms of hepatic microcirculatory hemodynamics: hepatic arterial system. Clin Hemorheol Microcirc. 2006;34:11–26. [PubMed] [Google Scholar]



