The migration of multilineage “passenger leukocytes” from transplanted organs and their ubiquitous survival in successfully treated human recipients for as long as 30 years (microchimerism) has been postulated to be an essential condition for organ allograft acceptance and the explanation for a number of previously enigmatic posttransplant events (1, 2). Following organ revascularization in either humans or rodents, donor leukocytes appear in large numbers in the recipient blood but usually diminish over a few days to a level undetectable with flow cytometry (2–5). We have construed the persistence of donor cells thereafter as prima facie evidence of the presence of hematopoietic stem and precursor cells (1,2,6).
When the blood compartment is serially sampled in long-surviving human organ recipients, the levels of donor cells fluctuate (7), presumably reflecting cyclic activity of these stem cells. Taniguchi et al. (8) showed that primitive CD45+ cells purified from adult mouse livers unfailingly reconstituted all hematolymphopoietic lineages in supralethally irradiated adult mouse recipients. We present here direct evidence that supralethally irradiated adult rats can be consistently rescued by syngeneic liver transplantation, and that heart transplantation has a less dramatic but significant therapeutic effect.
Inbred Lewis (RT11, LEW) rats weighing 200–250 g (8–10 weeks old) were purchased from Harlan Sprague Dawley (Indianapolis, IN) and kept in a laminar-flow specific-pathogen-free environment. Orthotopic liver and heterotopic (abdominal) heart transplantation were performed as described previously (5). After death of the donor by exsanguination, the grafts were flushed intravascularly with chilled lactated Ringer’s solution until the venous effluent was clear.
Bone marrow cells were harvested from the tibias and femurs, and processed in RPMI 1640 supplemented with 25 mM Hepes buffer, 2 mM L-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin (all from Gibco, Grand Island, NY) (5). Trypan blue exclusion testing uniformly showed >95% cell viability. The cell suspensions were administered into the recipient penile vein.
A reagent panel containing monoclonal antibodies against all of the principal leukocyte subsets was used to determine lineages of the control and reconstituted animals (panel available on request). Most experiments were performed with male donors and recipients. However, in 2 liver recipients, male → female transplantation was performed and the tissues and organs of the hematopoietically reconstituted recipient were studied with polymerase chain reaction and Southern hybridization, using rat Y-chromosome (sex determining region Y [SRY]) specific primers (9). This provided proof of hematopoietic sex change in the reconstituted animals as opposed to recovery of the cytoablated recipient stem cells.
Recipients were irradiated with 9.5 Gy, delivered from a cesium source. All cell or organ transplantations were done 4–8 hr after completion of irradiation. Survival was determined of animals receiving 4 different doses of syngeneic bone marrow, and of those given liver and heart grafts (Table 1). In addition, 6 irradiated animals of similar size were given 3 ml of fresh whole blood, which was an estimated 25% of their total blood volume. This amount of transfusion exceeded by an estimated thousand-fold or more any residual blood in the thoroughly flushed organs used for transplantation.
Table 1.
Survival of lethally irradiated (9.5 Gy) LEW rats after different types of syngeneic organ and cell transplantation
| Group | Organ/cells | n | Survival (days) | Survival rate | Median (days) | Pa |
|---|---|---|---|---|---|---|
| 1 | None | 6 | 10, 10, 10, 11, 12, 12 | 0/6 | 10.5 | — |
| 2 | Heart | 6 | 11, 14, 14, 16, 18, > 100 | 1/6 | 15.0 | <0.05 |
| 3 | Liver | 6 | 16, >100×5 | 5/6 | >100 | <0.005 |
| 4 | Bone marrow 0.5×106 | 6 | 10, 11, 12, 15, 16, 17 | 0/6 | 13.5 | NS |
| 5 | Bone marrow 1×106 | 6 | >100×6 | 6/6 | >100 | <0.005 |
| 6 | Bone marrow 5×106 | 6 | >100×6 | 6/6 | >100 | <0.005 |
| 7 | Bone marrow 10×106 | 6 | >100×6 | 6/6 | >100 | <0.005 |
| 8 | Whole blood 3 ml | 6 | 12, 13, 13, 13, 13, 13 | 0/6 | 13.0 | <0.01 |
Versus group 1 (Mann-Whitney U test). Group 2 vs. groups 4 and 8: NS; Group 3 vs. groups 5, 6, and 7: NS.
All untreated animals died within 12 days. Heart transplantation significantly prolonged survival (P<0.05). One of 6 cardiac recipients was permanently rescued and 4 of the 5 others lived 2–6 days beyond the longest surviving non-treated control. These results were at least as good as those obtained with a suboptimal dose of 0.5×106 bone marrow cells (P=0.109 vs. control) and with the infusion of 3 ml of whole blood (P=0.007 vs. control). Liver transplantation resulted in permanent survival of 5 of 6 irradiated recipients with full multilineage reconstitution, a rescue effect that was not significantly different from that following infusion of 1,5, or 10×106 bone marrow cells (Table 1).
Because frequent blood sampling and other manipulations jeopardized survival, hematologic studies were done in a separate cohort of irradiated animals, with subgroups that were untreated, infused with 1×106 or 1×107 unmodified bone marrow cells, or submitted to liver transplantation. The results, including weight gain, were compared with those in naive unaltered animals in the same environment (Figs. 1–3).
Figure 1.
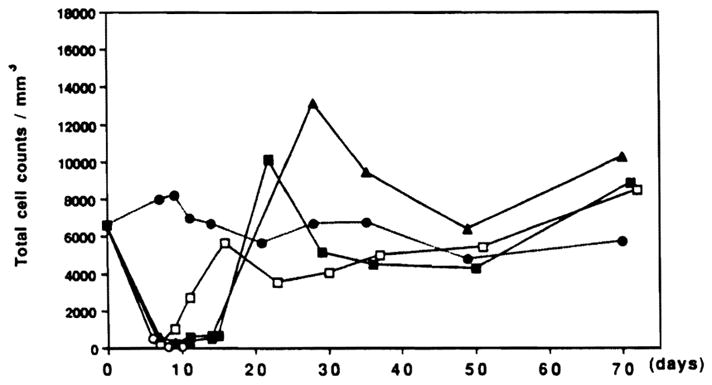
Total white cell counts (mm3) in normal and irradiated LEW rats (9.5 Gy) and rats given syngeneic transplants, after treatment with red blood cell lysing buffer. Cells were counted in a hemacytometer. ●, Normal Lewis (n=3); ○, radiation alone (n=3); ■, 1×106 bone marrow (n=6); □, 10×106 bone marrow (n=4); ▲, OLTX (n=3).
Figure 3.
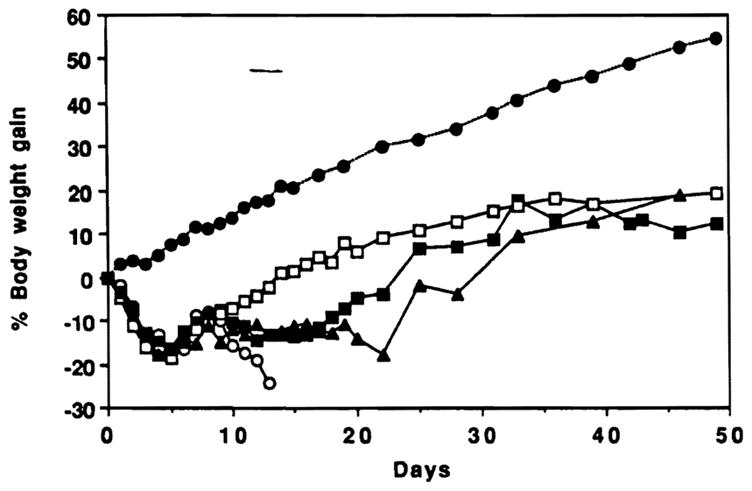
Body weight changes in animals of Figures 1 and 2. ●, Normal LEW (n=3); ○, radiation alone (n=3); ■ 1×106 bone marrow (n=6); □, 10×106 bone marrow (n=4); ▲ OLTx (n=3).
The rate and the extent of circulating leukocyte (Fig. 1) and hematocrit restoration (Fig. 2) were similar after liver transplantation and infusions of 1×106 bone marrow cells. Rats given a 10-fold higher dose of bone marrow cells (1×107) recovered more quickly (Figs. 1 and 2). The sequence of lineage recovery was similar in the liver and bone marrow recipients, and appeared to be dependent on the number of progenitor cells. The FACS profile of animals after various kinds of hematopoietic reconstitution is shown in Figure 4. The reconstituted cells in cross-sex liver transplantations (male → female) were predominantly donor (Fig. 5) and were, of course, least densely represented in the heart.
Figure 2.
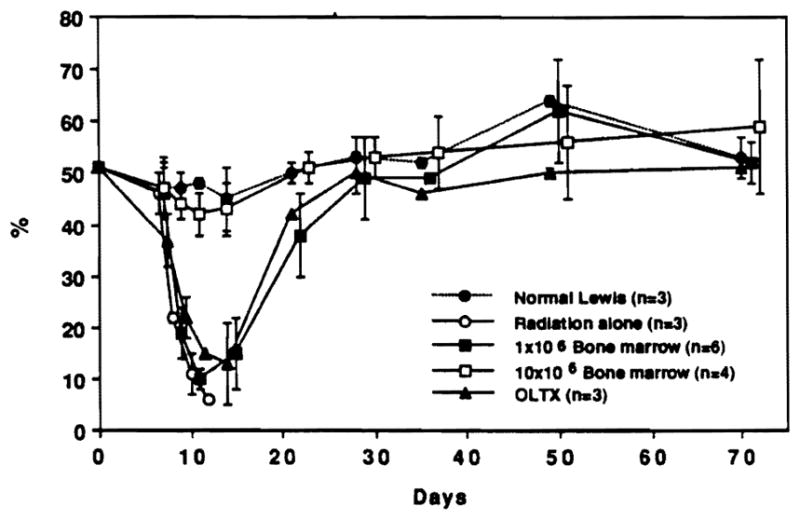
Hematocrit (tail vein blood, microcentrifuge method) in same samples as Figure 1.
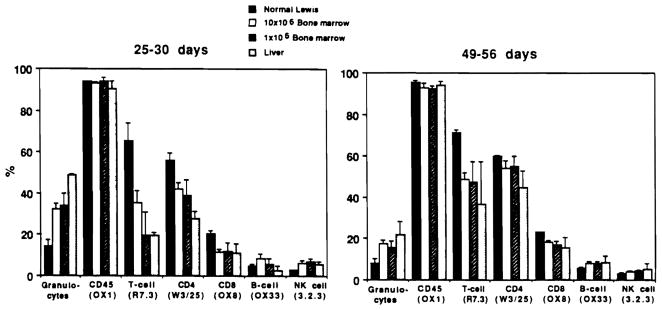
Figure 4.
Lineage of peripheral leukocytes shown in Figure 1. Granulocytes were determined on blood smear following α-naphthyl acetate esterase and hematoxylin staining. Other FACS analysis of percentage of subpopulations (mean ± SD) was by direct immunofluorescence with antibodies to surface markers, including OX1 (CD45), R7.3 (αβ TCR), W3/25 (CD4), OX8 (CD8), OX33 (B-cells), and NK3.2.3 (NK cells).
Figure 5.
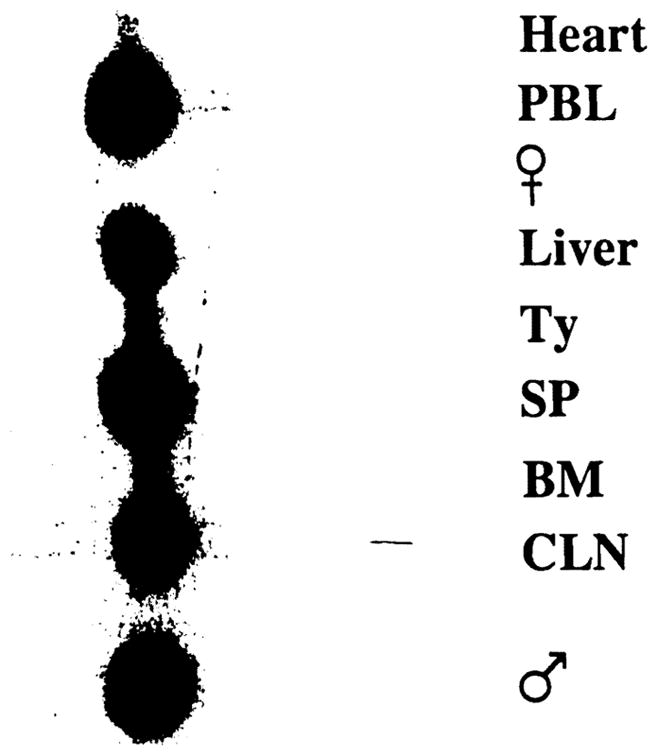
Polymerase chain reaction and Southern hybridization of organs and tissues 50 days after lethal irradiation and syngeneic liver transplantation. PBL, peripheral blood lymphocytes; Ty, thymus; SP, spleen; BM, bone marrow; CLN, cervical lymph nodes. Male and female (negative) controls are included.
The initial delay in postoperative weight gain of liver compared with bone marrow recipients (Fig, 3) was explained by the greater stress and metabolic depletion associated with the hepatic replacement compared with the less traumatic penile vein infusion of bone marrow cells. After 3 weeks, the liver recipients had catch-up weight gain, and they achieved parity with all other reconstituted groups by day 50 (Fig. 3).
These experiments suggest that the difference between the chimerism (and tolerance) following classical bone marrow transplantation and that produced by the donor (“passenger”) leukocytes of whole organs is purely semantic. While describing the multilineage character of the microchimerism in human and animal organ recipients (1, 2, 6, 10, 11), we have emphasized the invariable histopathologic prominence of dendritic cells because their presence was so much in conflict with the literature preceding 1992. The previous literature had associated these cells almost exclusively with rejection rather than graft acceptance. We have recently summarized elsewhere the impressive evidence acquired since then of a key tolerogenic role played by the dendritic cells, particularly when in their precursor stages (12). However, preoccupation with a single leukocyte lineage could obscure an understanding of the complex cell interactions that we are seeking to understand and manipulate. We believe it unlikely that transplantation tolerance can be fully explained, reliably induced, or efficiently sustained by any single lineage (5, 6, 13).
In the experiments reported herein, heart transplantation also had a significant effect on postirradiation survival, less dramatic than the liver but similar to that of a suboptimal dose of donor bone marrow or a large blood transfusion. The permanent hematopoietic reconstitution of one of the heart recipients and prolongation of survival of 4 of 5 others strengthen our earlier contention that tolerogenicity is an inherent capability common to all organized tissues and organs, with variations in outcome dictated by the quantity and lineage profile of bone marrow-derived leukocytes contained in the graft (1, 2, 5, 6, 13).
No record could be found in the literature suggesting that these simple and direct reconstitution experiments had been performed previously, presumably because of the assumption that organ transplantation involves a unidirectional host-versus-graft immune reaction (the one-way paradigm). Leszczynski et al. (14) showed that 1×106 recipient inflammatory cells extracted from rejecting rat kidney allografts contained enough host stem cells to fully reconstitute supralethally irradiated animals of the recipient strain. The experiments reported herein showed that the transplanted organ (especially the liver) brings enough donor stem cells to provide the basis of the sustained bidirectional immune reaction that we have postulated to be the basis of allograft acceptance and the usual form of transplantation tolerance (the two-way paradigm [5, 15]).
Acknowledgments
We thank Stanley H. Chia and Christopher S. Doughton for providing excellent technical assistance, and Dr. Lianfu Wang for help in conducting molecular assay.
ADDENDUM
Two historical contributions in addition to Reference 8 were brought to our attention after submission of this article, adding evidence that stem cells are present in the adult mouse liver. The first, by Hays, Hays, and Golde (1978), described multilineage reconstitution following supralethal irradiation with cultured syngeneic hepatic NPCs obtained from adult mouse livers, especially if the liver was regenerating (following partial hepatectomy). The second publication, by Decker, Lohmann-Matthes, and Baccarrine (1988), also emphasized that cultured syngeneic liver NPCs could substitute for bone marrow cells for rescue after irradiation.
- Hays EF, Hays DM, Golde DW. Hemopoietic stem cells in mouse liver. Exp Hematol. 1978;6:18. [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML, Baccarrini M. Liver-associated macrophage precursor cells proliferate under impairment of regular hemopoiesis. Eur J Immunol. 1988;18:697. doi: 10.1002/eji.1830180507. [DOI] [PubMed] [Google Scholar]
Footnotes
This study was supported by project grant DK 29961 from the National Institutes of Health, Bethesda. MD.
REFERENCES
- 1.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 3.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. 1991;337:818. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Demetris AJ, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and solid organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlitt HJ, Hundrieser J, Hisanaga M, et al. Patterns of donor-type microchimerism after heart transplantation. Lancet. 1994;343:1469. doi: 10.1016/s0140-6736(94)92584-4. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Evidence for the presence of hematopoietic stem cells in the adult liver. Transplant Proc. 1995;27:196. [PubMed] [Google Scholar]
- 9.Tashiro H, Fukuda Y, Kimura A, et al. Monitoring for rejection and engraftment following rat orthotopic liver transplantation by polymerase chain reaction. Transplantation. 1994;58:745. [PubMed] [Google Scholar]
- 10.Demetris AJ, Murase N, Fujisaki S, Fung JJ, Rao AS, Starzl TE. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 11.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson AW, Lu L, Murase N, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demetris AJ, Murase N, Rao AS, Starzl TE. The role of passenger leukocytes in rejection and “tolerance” after solid organ transplantation: a potential explanation of a paradox. In: Touraine JL, editor. Rejection and tolerance. Vol. 25. The Netherlands: Kluwer Academic Publisher; 1994. p. 325. [Google Scholar]
- 14.Leszczynski D, Halttunen J, Tiisala S, Ustinov J, Renkonen R, Hayry P. Properties of B cells and thy-1-antigen expressing cells infiltrating rat renal allografts. Hum Immunol. 1990;29:103. doi: 10.1016/0198-8859(90)90073-x. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ. Transplantation milestones: viewed with one- and two-way paradigms of tolerance. JAMA. 1995;273:876. doi: 10.1001/jama.273.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]