Abstract
Imatinib Mesylate, a Tyrosine Kinase inhibitor, is presently the drug of choice for Chronic myeloid leukemia (CML). During therapy, a few patients develop myelosuppression and present with cytopenias. To study the bone marrow morphology in imatinib treated CML patients presenting with persistent cytopenias. The cases were retrieved from the Hematopathology record files, Department of Pathology; the study period being January 2008–June 2009. Cases of CML on Imatinib presenting with grade 2 or more anemia, neutropenia and/or thrombocytopenias with bone marrow studies, were included in the study. The morphology of all cases was reviewed with cytogenetic studies. Follow-up details were obtained from the Medical Oncology records. During the study period, 683 Imatinib treated CML patients had bone marrow studies as part of their follow-up investigations. Of these, 60 patients (9%) had some form of persistent cytopenia. The patients ranged from 21 to 75 years of age with a median age of 38 years. The male:female ratio was 1:1. There were 46 patients with ≥grade 2 anemia, 25 patients with ≥grade 2 neutropenia and 37 patients with ≥grade 2 thrombocytopenia. Of these, 18 patients had bicytopenia and 13 cases had pancytopenia. The marrow evaluation revealed morphologic response in 30 patients, persistent marrow disease in five patients, marrow hypoplasia in six patients, extensive stromal changes including fibrosis in five patients, megaloblastic erythropoiesis in 11 patients and disease progression to accelerated or blast crisis in three patients. Various degrees of cytopenias may occur in few patients of CML on imatinib therapy. Regular hematologic follow-up is required so that the drug may be stopped or dose modified as per the individual’s needs.
Keywords: Imatinib, Cytopenias, Marrow morphology
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that originates in an abnormal pluripotent stem cell [1]. At diagnosis, 90–95% of cases of CML have the characteristic t (9; 22) translocation that results in the Ph chromosome. This translocation fuses sequences of the Bcr gene on chromosome 22 with regions of the Abl 1 gene from chromosome 9 resulting in the formation of Bcr–Abl chimeric protein with enhanced tyrosine activity. [2] The enhanced tyrosine kinase activity is associated with expansion of pluripotent stem cells, defective adhesion and decreased apoptosis of the hematopoietic cells. Imatinib mesylate (Gleevec, Glivec, formerly STI571) [3] is a tyrosine kinase inhibitor, which binds to the ATP binding site of the tyrosine kinases and maintains them in an inactive form. Imatinib is now the gold-standard for first-line pharmacotherapy of CML, in any phase.
Adverse side-effects of the drug include edema, nausea, vomiting, diarrhea, cramps and cutaneous reactions. Adverse hematologic side-effects include anemia, neutropenia, and thrombocytopenia.
The aim of this study was to evaluate the marrow morphology in diagnosed cases of CML on imatinib therapy, presenting with persistent cytopenias.
Materials and Methods
The cases were retrieved from the hematopathology record files of the Institute, the study period being 1.5 years. Diagnosed patients of CML (in any phase), who were on Imatinib mesylate and presenting with grade 2 or more anemia, neutropenia and/or thrombocytopenia with simultaneous bone marrow examinations, were included in the study. The degree of cytopenia was graded as per National Cancer Institute Common Toxicity criteria, version 1. Giemsa stained peripheral blood smears along with bone marrow aspirates were reviewed. In case of aparticulate aspirates, trephine imprints for cell counts and trephine sections were studied. The morphology of each case was reviewed along with cytogenetic studies. The cytogenetic evaluation was carried out on marrow aspirates or unstimulated peripheral blood samples using conventional G-banding techniques. For each case, 20 metaphases were analyzed and percentage of metaphases positive for Ph chromosome was counted. The patient management details were obtained from Medical Oncology department.
Results
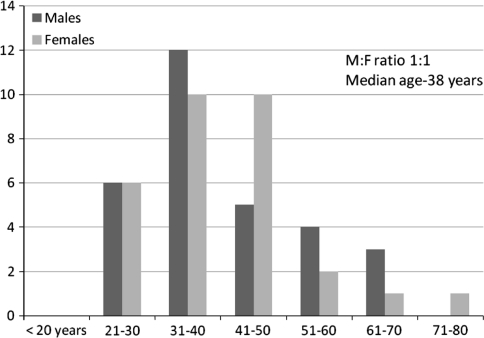
During the study period, 683 previously diagnosed patients of CML, on imatinib therapy had marrow evaluation as part of their follow-up studies. Of these, 60 patients (9.0%) had some form of persistent cytopenia, which was grade 2 or more. The patients ranged from 21 to 75 years of age with a median age of 38 years and a male:female ratio of 1:1. The patient characteristics are depicted in Fig. 1. The type and grade of cytopenia are shown in Table 1. Anemia was the commonest cytopenia followed by thrombocytopenia and neutropenia. Lower grades of anemia were frequently noted. There were 18 patients of bicytopenia and 13 patients of pancytopenia. A combination of anemia and thrombocytopenia was the most commonly noted bicytopenia.
Fig. 1.
Patient characteristics of CML patients on imatinib, presenting with cytopenia (n = 60)
Table 1.
Grades of cytopenia in the patients (n = 60) as per National Cancer Institute (NCI) common toxicity criteria version 1.0
| Grade | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|
| Anemia (gm/dl) | 8.0–10.0 | 6.5–8.0 | <6.5 | |
| 25 | 16 | 5 | 46 | |
| Neutropenia | ≥1000 < 1500 | ≥500 < 1000 | <500 | |
| (ANC-/cmm) | 12 | 9 | 3 | 24 |
| Thrombocytopenia | ≥50000 < 75000 | ≥25000 < 50000 | <25000 | |
| (platelet/cmm) | 14 | 10 | 10 | 34 |
| Bicytopenia | ||||
| Anemia + Thrombocytopenia | 9 | |||
| Anemia + Neutropenia | 7 | |||
| Neutropenia + Thrombocytopenia | 2 | |||
| Pancytopenia | 13 |
The morphologic changes seen in imatinib treated CML patients with persistent cytopenia were as shown in Table 2.
Table 2.
Marrow morphology in Imatinib treated CML patients presenting with cytopenia (n = 60)
| Bone marrow morphology | Number of Patients |
|---|---|
| Marrow morphologic response/Normal marrow | 30 |
| Persistence of disease | 5 |
| Marrow hypoplasia | 6 |
| Extensive stromal changes | 4 |
| Marrow fibrosis | 1 |
| Megaloblastoid erythropoiesis | 11 |
| Disease progression/transformation(blast/accelerated phase) | 3 |
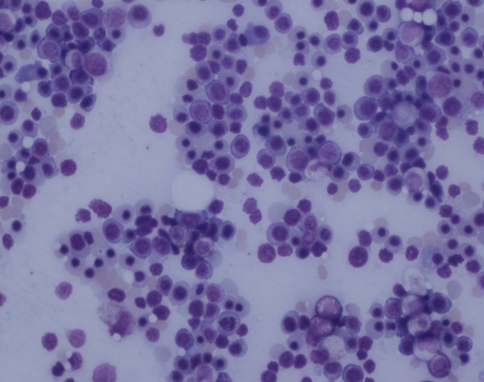
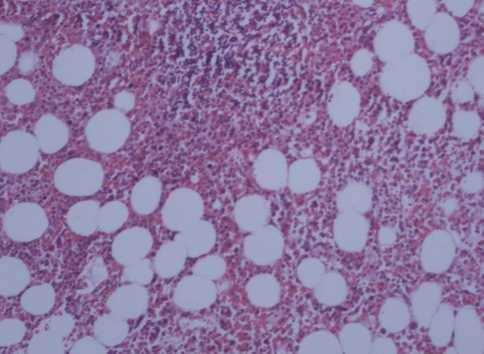
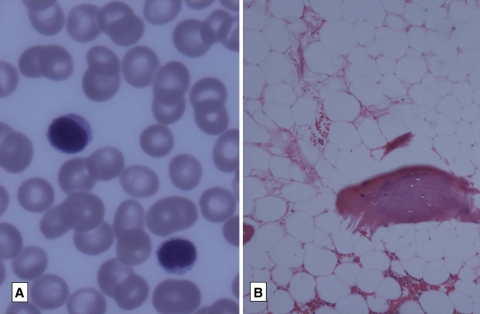
The bone marrow showed a morphologic response or near normal marrow in 30 patients (50%).A bone marrow is said to show morphologic response when there is reduction in marrow cellularity, reduction in myeloid to erythroid ratio accompanied by erythroid hyperplasia, reduction in numbers of blasts and megakaryocytes and reduction in fibrosis [2, 4–6] (Figs. 2, 3).
Fig. 2.
Erythroid hyperplasia following therapy (Giemsa X200)
Fig. 3.
Reduction in cellularity with benign lymphoid aggregates (H&E X200)
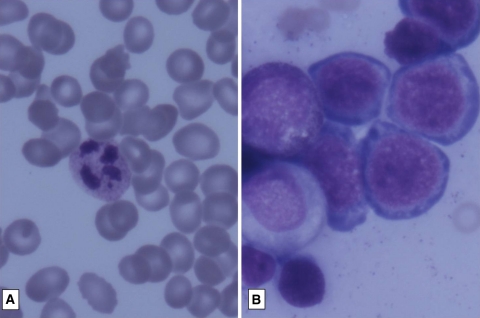
An unusual finding noted was of megaloblastic erythropoiesis in 11 patients. The details of these patients are given in Table 3 (Fig. 4).
Table 3.
Clinical details and follow-up of patients showing megaloblastosis in Imatinib treated patients with cytopenia (n = 11)
| S.No | Age/sex | Cytopenia | Past drug history | Months of Imatinib | M:E ratio | B12/folate levels | Response to B12/folate |
|---|---|---|---|---|---|---|---|
| 1 | M/35 | Pancytopenia | Nil, alcoholic | 8 m | 1:1.5 | ND | Response+ |
| 2 | F/41 | Bicytopenia | Nil | 9 m | 1:2 | ND | Response+ |
| 3 | F/38 | Bicytopenia | Nil | 13 m | 3:1 | ND | Not treated |
| 4 | M/23 | Bicytopenia | Hydrea | 19 m | 1:3 | ND | Not treated |
| 5 | M/67 | Bicytopenia | Nil | 11 m | 1:1.5 | ND | Not treated |
| 6 | M/40 | Pancytopenia | Hydrea, Interferon | 13 m | 1.5:1 | ND | Response+ |
| 7 | M/35 | Bicytopenia | Nil | 9 m | 1.5:1 | ND | Response + |
| 8 | M/36 | Pancytopenia | Nil | 6 m | 1:1 | ND | Not treated |
| 9 | F/36 | Bicytopenia | Hydrea | 7 m | 1:1 | B12↑,folate N | Treated→AP, expired |
| 10 | M/56 | Pancytopenia | Busulphan | 12 m | 1.5:1 | ND | Response+ |
| 11 | F/46 | Anemia | Hydrea | 11 m | 1.5:1 | ND | Not treated |
m months, M:E myeloid:erythroid ratio, B12 vitamin B12 levels, Response+ good response to therapeutic vitaminB12 and folate supplements, ND vitamin B12 and folate levels not done due to financial constraints,↑=increased levels, N normal levels, AP accelerated phase
Fig. 4.
a Peripheral smear showing macro-ovalocytes, hypersegmented neutrophils and thrombocytopenia (GiemsaX1000). b Marrow showing megaloblastoid erythropoiesis (GiemsaX1000)
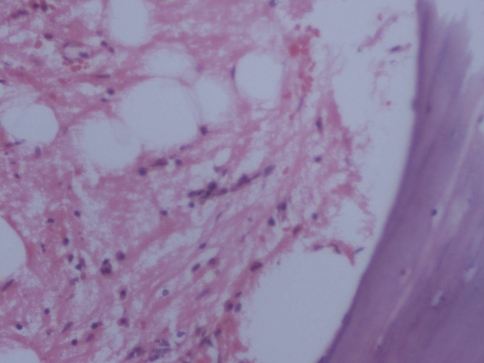
Other morphologic changes that were noted were persistent of disease (CML) in five patients, marrow hypoplasia in six patients, extensive stromal changes in four patients, marrow fibrosis in one patient and progression to accelerated phase or blast crisis in three patients (Figs. 5, 6).
Fig. 5.
a Peripheral smear showing pancytopenia (GiemsaX 200) b Trephine biopsy with severe hypoplasia (H&EX200)
Fig. 6.
Stromal changes following therapy (H&EX200)
The cytogenetic results of all the patients at the time of cytopenia are depicted in Table 4.
Table 4.
Cytogenetic results of Imatinib treated CML patients presenting with cytopenia (n = 60)
| Cytogenetic response (CR) | Percentage of metaphases positive | Number of patients |
|---|---|---|
| Complete CR | 0% +ve | 33 |
| Major CR | 1–35% +ve | 13 |
| Minor CR | 36–65% +ve | 10 |
| Minimal CR | 66–95% +ve | 2 |
| None | 96–100% +ve | 0 |
| No dividing cells | – | 2 |
Discussion
The marrow morphology was different in the patients presenting with various degrees of cytopenia. Untreated CML patients generally showed marrows with increased cellularity, increased M:E ratio, increased blasts and megakaryocytes with clustering and fibrosis [2, 4]. Post-imatinib therapy, the changes reported are reduction in cellularity, reduction in M:E ratio with relative erythroid hyperplasia, reduction in blast count, fibrosis and number of megakaryocytes [2, 4].Accompanying changes that have frequently been described are benign lymphoid aggregates (42%) and clusters of sea-blue histiocytes (47%) [5, 6].It is also reported that marrow morphologic response and cytogenetic response may lag behind the hematologic response [5].
Myelosuppression in patients receiving therapy may manifest as anemia, neutropenia and thrombocytopenia. Of the 60 patients presenting with any form of ≥2 cytopenia, 30 (50%) showed good marrow morphologic response or a histologically normal bone marrow.
Myelosuppression is significantly more common in CML patients on Imatinib than in patients of gastrointestinal stromal tumors (GISTs) [3]. Patients of CML in advanced phases are more prone for myelosuppression. It is unclear why some patients develop myelosuppression and some do not. Along with its potent inhibitory effect on Bcr/Abl, imatinib also inhibits c-kit, which is involved in early hematopoiesis. Hence myelosuppression may be the result of undesired suppression of normal progenitors [7]. Myelosuppression developing during therapy may have an adverse effect on overall response because (1) therapy being withheld for myelosuppression, decreases the exposure to imatinib (2) myelosuppression may be a manifestation of reduced normal stem pool which is unable to manifest as full normal hematopoiesis after suppression of the abnormal clone [7]. In addition to advanced disease, factors associated with a greater risk of myelosuppression include low hemoglobin, history of interferon induced cytopenias and previous busulphan therapy [3].
Myelosuppression has been identified as an independent adverse risk factor for achieving a cytogenetic response [7].
Of the 60 patients in the study, 6 showed marrow hypoplasia or replacement by fat. Marrow hypoplasia following imatinib therapy has infrequently been reported [7–9].Bone marrow hypoplasia is a potentially fatal adverse side-effect of imatinib and can present in any phase of CML. In one of the studies [9], there were five patients with severe hypoplasia (bone marrow cellularity between 5 and 10%) the Bcr/Abl was positive in all patients at the time of hypoplasia. In this study also, all six patients were positive for Ph chromosome at the time of diagnosis of hypoplasia. This reinforces the observation that myelosuppression is an independent adverse factor for achieving cytogenetic response.
One patient with cytopenia had secondary myelofibrosis and four patients had marrow with extensive stromal changes in the form of stromal edema (three patients) and gelatinous transformation (one patient). Cytopenias are noted when the marrow shows extensive stromal change [10]. Gelatinous transformation (serous atrophy) is a change where there is loss of fat cells and hematopoietic cells with replacement by acid mucopolysaccharide. It has been reported in severe malnutrition states, AIDS, renal failure and rarely in imatinib treated CML patients [11, 12].
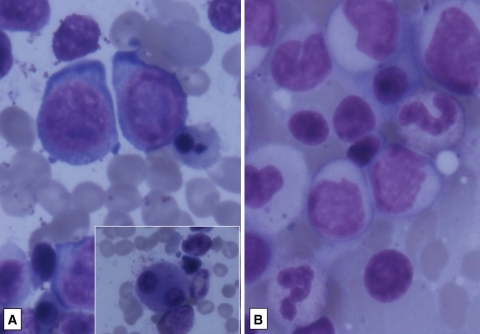
There were 11 patients with cytopenias, with the erythoid precursors in the marrow showing megaloblastoid change. The exact cause for this megaloblastosis is not known as most of the patients could not afford investigations to estimate vitamin B12 and folate levels. Of these, five responded to therapeutic doses of vitamin B12 and folate and hence nutritional deficiencies were thought to be the cause for megaloblastosis. Unlike hydroxyurea, imatinib is not known to cause folate deficiencies [5]. There was one patient who had high vitamin B12 (>2000 pg/ml) and normal folate levels. On follow-up, she went into accelerated phase and expired (Fig. 7). There is a report of myelodysplastic syndrome and acute leukemia developing in three patients of CML on imatinib therapy [13] where megaloblastoid change was described as part of the dyserythropoiesis. It is possible that the patient with high vitamin B12 levels with megaloblastosis and subsequent transformation may have had myelodysplastic syndrome following imatinib. Only a good follow-up, correlation with accurate vitamin B12, folate levels and cytogenetics could lead us to the cause of megaloblastosis in these patients.
Fig. 7.
a Dyspoietic erythroid precursors with bilobed megakaryocytes (Giemsa X1000), b same patient on follow up showing transformation to accelerated phase (GiemsaX400)
There were three patients presenting with cytopenias with marrows showing transformation into accelerated phase or blast crisis.
To conclude, various degrees of cytopenia may occur in few patients of CML on imatinib. The cause of the cytopenia is varied and the marrow morphology could be different in each case. Regular hematologic follow-up with blood counts and if necessary, bone marrow studies are required for patient management; so that the drug may be withheld or dose modified, as per individual needs.
References
- 1.Vardiman JW, Melo JV, Baccarani M, Thiele J, et al. Chronic myeloid leukemia, BCR-ABL1 positive. In: Swerdlow SH, Elias C, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 32–37. [Google Scholar]
- 2.Hasserjian RP, Boecklin F, Parker S, Chase A, Dhar S, Zaiac M, et al. STI571 (imatinib mesylate) reduces bone marrow cellularity and normalizes morphologic features irrespective of cytogenetic response. Am J Clin Pathol. 2002;117:360–367. doi: 10.1309/NR81-VCU0-CKW1-4HT9. [DOI] [PubMed] [Google Scholar]
- 3.Guilhot F. Indications for imatinib mesylate therapy and clinical management. Oncologist. 2004;9:271–281. doi: 10.1634/theoncologist.9-3-271. [DOI] [PubMed] [Google Scholar]
- 4.Frater JL, Tallman MS, Variakojis D, Druker BJ, Resta D, Riley MB, et al. Chronic myeloid leukemia following therapy with imatinib mesylate (Gleevec). Bone marrow histopathology and correlation with genetic status. Am J Clin Pathol. 2003;119:833–841. doi: 10.1309/A4RGP4LF12GGH8MW. [DOI] [PubMed] [Google Scholar]
- 5.Braziel RM, Launder TM, Druker BJ, Olson SB, Magenis RE, Mauro MJ, Sawyers CL, et al. Hematopathologic and cytogenetic findings in imatinib mesylate-treated chronic myelogenous leukemia patients: 14 months’ experience. Blood. 2002;100:435–441. doi: 10.1182/blood.V100.2.435. [DOI] [PubMed] [Google Scholar]
- 6.Joshi S, Sunita P, Deshmukh C, Gujral S, Amre P, Nair CN. Bone marrow morphological changes in patients of chronic myeloid leukemia treated with imatinib mesylate. Ind J Cancer. 2008;45:45–49. doi: 10.4103/0019-509X.41769. [DOI] [PubMed] [Google Scholar]
- 7.Sneed TB, Kantarjian HM, Talpaz M, O’Brien S, Rios MB, Bekele BN, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100:116–121. doi: 10.1002/cncr.11863. [DOI] [PubMed] [Google Scholar]
- 8.Lokeshwar N, Kumar L, Kumari M. Severe bone marrow aplasia following imatinib mesylate in a patient with chronic myelogenous leukemia. Leuk Lymphoma. 2005;46:781–784. doi: 10.1080/10428190500046778. [DOI] [PubMed] [Google Scholar]
- 9.Srinivas U, Pillai LS, Kumar R, Pati HP, Saxena R. Bone marrow aplasia–a rare complication of imatinib therapy in CML patients. Am J Hematol. 2007;82:314–316. doi: 10.1002/ajh.20776. [DOI] [PubMed] [Google Scholar]
- 10.Khan KA, Junaid A, Siddiqui NS, Mukhtar K. Siddiqui S Imatinib-related bone marrow aplasia after complete cytogenetic response in chronic myeloid leukemia. J Coll Physicians Surg Pak. 2008;18:176–178. [PubMed] [Google Scholar]
- 11.Bain BJ, Clark CM, Lampert IA, Wilkins BS, editors. Bone marrow pathology. Oxford, UK: Blackwell Science; 2001. pp. 130–131. [Google Scholar]
- 12.Ram R, Gafter-Gvili A, Okon E, Pazgal I, Shpilberg O, Raanani P. Gelatinous transformation of bone marrow in chronic myeloid leukemia during treatment with imatinib mesylate: a disease or a drug effect? Acta Haematol. 2008;119:104–107. doi: 10.1159/000121825. [DOI] [PubMed] [Google Scholar]
- 13.Kovitz C, Kantarjian H, Garcia-Manero G, Abruzzo LV, Cortes J. Myelodysplastic syndromes and acute leukemia developing after imatinib mesylate therapy for chronic myeloid leukemia. Blood. 2006;108:2811–2813. doi: 10.1182/blood-2006-04-017400. [DOI] [PubMed] [Google Scholar]