Abstract
Diagnosis of Pasteurella pneumotropica in laboratory animals relies on isolation of the organism, biochemical characterization, and, more recently, DNA-based diagnostic methods. 16S rRNA and rpoB gene sequences were examined for development of a real-time PCR assay. Partial sequencing of rpoB (456 bp) and 16S rRNA (1368 bp) of Pasteurella pneumotropica isolates identified by microbiologic and biochemical assays indicated that either gene sequence can be used to distinguish P. pneumotropica from other members of the Pasteurellaceae family. However, alignment of rpoB sequences from the Pasteurella pneumotropica Heyl (15 sequences) and Jawetz (16 sequences) biotypes with other Pasteurellaceae sequences from GenBank indicated that although rpoB DNA sequencing could be used for diagnosis, development of diagnostic primers and probes would be difficult, because the sequence variability between Heyl and Jawetz biotypes is not clustered in any particular region of the rpoB sequence. In contrast, alignment of 16S rRNA sequences revealed a region with unique and stable nucleotide motifs sufficient to permit development of a specific fluorogenic real-time PCR assay to confirm P. pneumotropica isolated by culture and to differentiate Heyl and Jawetz biotypes.
Abbreviations: Ct, cycle threshold; Rn, relative fluorescence units; rpoB, RNA polymerase B subunit
The Pasteurellaceae family consists of several genera, including Pasteurella, Actinobacillus, and Haemophilus, commonly referred to as ‘the PAH group.’ Whereas most of the species of the family Pasteurellaceae cause subclinical infections in rodents, Pasteurella pneumotropica is considered to be an opportunistic pathogen that has been associated with respiratory disease and infection of the eye, genital tract, uterus, skin, mucous membranes, and intestinal tract.15 In our laboratory, mouse health monitoring most frequently detects eye lesions associated with P. pneumotropica.2
In 1963, P. pneumotropica was first reclassified into 2 distinct biotypes,9 which were later named as ‘Heyl’ and ‘Jawetz.’5 The third biotype, Henriksen, is not found in rodents and has been reclassified as P. dagmatis.13 Biotypes are most commonly identified by using microbiologic and biochemical methods. However, many discrepancies have been found in the diagnosis of P. pneumotropica by using commercial kits that are based on various biochemical characteristics, resulting in different interpretations by different laboratories. This problem has prompted the Federation of Laboratory Animal Science Associations (FELASA) to recommend reporting all Pasteurellaceae in health reports for laboratory rodents, leaving it to individual laboratories to make decisions regarding the use of the animals.17 Although there are different opinions regarding the need to remove all or selected Pasteurellaceae from animal facilities, there is general agreement on excluding P. pneumotropica. In a microbiologic screen of 25,973 mice, the prevalence of P. pneumotropica, P. multocida, and other Pasteurellaceae species was found to be 4.8%, 0%, and 0.4%, respectively, emphasizing the need to monitor for P. pneumotropica.19 Complication due to the ambiguity of taxonomy and diagnostic methods6 make it challenging for rodent vendors to ensure that their diagnostic assays are sensitive enough to detect P. pneumotropica while eliminating false-positive results due to other Pasteurellaceae. In our laboratory, we have seen several instances in which agents determined to be P. pneumotropica by commercially available biochemical tests are not confirmed by sequence analysis.3 Similar observations have been reported by others.18 Because commercial tests are optimized to speciate isolates of human origin, they offer only approximate speciation for rodent isolates.
Methods based on DNA sequence divergence potentially offer a more specific method for classification of P. pneumotropica. Various genes including 16S rRNA,1,18 atpD,1 infB,1 gyrB,8 and rpoB1,11 from various members of Pasteurellaceae family have been sequenced in an attempt to characterize Pasteurellaceae with more confidence than biochemical assays. However, even when relying on sequence data for diagnosis of P. pneumotropica, researchers should be careful in assay design and interpretation of results. Gel-based PCR assays, although sensitive, are prone to false positives due to nonspecific primer binding to related Pasteurellaceae;24 in comparison, real-time PCR assays add an additional level of specificity and sensitivity12 and can be used to determine copy number. 16S rRNA qualifies as the most comprehensive single gene database that can be used to classify bacteria phylogenetically. Although much of 16S rRNA is highly conserved among many bacterial families, portions of the gene are unique and can be used to speciate bacteria. Similarly, the Jawetz and Heyl biotypes have unique 16S rRNA sequence regions that can be used to differentiate them from all other bacterial 16S rRNA gene sequences (cultured and uncultured) in GenBank and from each other.1,7,11,18,23 The GenBank database contains 16S rRNA sequences from both Heyl CNP160 (AF012090) and Jawetz NCTC8141 (M75083) prototypes.
Although rpoB is used frequently for genotyping of bacteria, the usefulness of rpoB-based genotyping is limited due to the availability of far fewer sequences (approximately 19,000) in the GenBank compared with 16S rRNA (approximately 1,726,000 sequences). Because only the rpoB sequence for the prototype Jawetz strain (AY314034) but not that for Heyl is available currently in GenBank, this gene cannot yet be used to distinguish between the 2 biotypes. Additional rpoB sequence data for the 2 biotypes may provide target sequences for differentiating them from other Pasteurellaceae. Therefore, we decided to sequence segments of 16S rRNA and rpoB genes from various Pasteurella pneumotropica isolates obtained from laboratory mice and rats in an effort to design a real-time PCR assay for diagnosis of P. pneumotropica. Although PCR might be designed for direct detection of P. pneumotropica in samples collected from animals, the current study describes the characterization of isolated bacterial colonies.
Materials and Methods
Animal husbandry and samples collection.
The animals (mice and rats) received for testing by our health monitoring department were housed for a maximum of 24 h; sufficient food, water, and bedding were available. Nasal aspirates from mice and rats were obtained after euthanasia by using CO2. Approximately 1 mL PBS in a pipette was flushed through the nasal cavity to collect a nasal aspirate sample. In some cases, exudates from eye lesions were collected by using sterilized inoculating loops. All the protocols were approved by Institutional Animal Care and Use Committee (Charles River, Wilmington, MA).
Culture and phenotypic characterization.
Nasal aspirates were plated on MacConkey agar plates and trypticase soy agar with 5% sheep blood for identification of P. pneumotropica. Plates were incubated for approximately 48 h at 35 °C and inspected for any colonies that exhibited colony morphology consistent with Pasteurella. These Pasteurella-like colonies were subcultured overnight at 35 °C on trypticase soy agar with 5% sheep blood for isolation. Biochemical identification was done by using both the API20NE strip (API–Biomérieux, Marcy l'Etoile, France) and GN card (API–Biomérieux) with a VITEK 2 Compact reader (API–Biomérieux) and reagents supplied by API–Biomérieux. After identification, the colonies were frozen in trypticase soy broth with 10% glycerol until further use. All media described were purchased from Becton Dickinson (Franklin Lakes, NJ). In addition, Proteus mirabilis and Pseudomonas aeruginosa isolates (American Type Culture Collection, Manassas, VA) were used as controls.
Biochemical analysis.
The biochemical properties of the isolates were determined on the basis of P. pneumotropica utilization of various carbon sources, including L+ arabinose, melibiose, raffinose, D+ xylose, trehalose, D– mannitol, D– salicin, esculin, and cellobiose, and measurement of a panel of enzyme activities including β-glucosidase, α-fucosidase, β-glucuronidase, phosphatase, urease, and lysine decarboxylase as previously described.14
DNA isolation.
DNA was extracted from isolated colonies by using a variation of the HotSHOT Alkaline lysis method.22 Stored glycerol stock (100 µL) was added to 1 mL solution no. 1 (25 mM NaOH, 0.2 mM EDTA) and incubated at 95 °C for 15 min; cell debris was removed by centrifuging at 16,000 × g for 1 min. A 100-µL aliquot of supernatant was neutralized with 100 μL solution no. 2 (40 mM Tris-HCL), and the DNA was used directly for PCR amplification.
DNA amplification and sequencing.
A region of the rpoB gene was amplified by using HotStar HiFidelity Taq polymerase (Qiagen, Hilden, Germany) and a previously published primer set (PasrpoB-L, GCA GTG AAA GAR TTC TTT GGT TC; rpoB-R, GTT GCA TGT TNG NAC CCA T); the expected PCR product size is 560 bp.11 Thermal cycling conditions were: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, primer annealing at 48 °C for 30 s, and extension at 72 °C for 45 s. The 16S rRNA gene (1368 bp) was amplified by using overlapping primers: 5′F46 (AAC ACA TGC AAG TCG AAC GGT A) and 5′R766 (TTT CGC ACA TGA GCG TCA GT) for the 5′ region and 3′F649 (CTA GAG TAC TTT AGG GAG GGG TAG AAT) and 3′R1537 (AAG GAG GTG ATC CAG CCG CA) for the 3′ region. Primer nomenclature is based on the nucleotide positions of GenBank reference sequence AF224296. Thermal cycling conditions used were: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, primer annealing at 55 °C for 30 s, and extension at 72 °C for 1 min. The PCR products were separated on 1% agarose gel and gel-eluted (Minelute Kit, Qiagen). Purified PCR products were sequenced (ABI 3130XL DNA Sequencer, Tufts University Core Facility, Boston, MA).
Sequence analysis.
Vector NTI software (Invitrogen, Carlsbad, CA) was used for sequence analysis, alignment, and construction of the phylogenetic tree by the Neighbor Joining method.20 Distance values (shown in parentheses) were calculated after the sequences were aligned. The sequence data of the 16S rRNA (M75083) and rpoB (AY314034) genes of Pasteurella pneumotropica Jawetz were used as prototypes for analysis.
Submission to GenBank.
All of the unique sequences with dissimilarity at one or more base positions that were obtained in this study at Research Animal Diagnostic Services, Charles River, for biotypes Heyl (CR1, CR5, CR10, CR16, CR18, CR24, CR26, CR 28, CR30, CR32) and Jawetz (CR3, CR13, CR17, CR19, CR51, CR53, CR54) and Pasteurellaceae species (CR4) were submitted to GenBank (Table 1).
Table 1.
List of strains and respective accessions numbers for 16S rRNA and rpoB sequences submitted to GenBank
| Organism | Strain | 16S rRNA | rpoB |
| P. pneumotropica Heyl | |||
| CR1 | GU809174 | GU809197 | |
| CR5 | GU809177 | GU809202 | |
| CR10 | GU809178 | GU809191 | |
| CR16 | GU809176 | GU809203 | |
| CR18 | GU809172 | GU809192 | |
| CR24 | GU809179 | GU809204 | |
| CR26 | GU809180 | GU809205 | |
| CR28 | GU809181 | GU809206 | |
| CR30 | GU809173 | GU809207 | |
| CR32 | GU809175 | GU809193 | |
| P. pneumotropica Jawetz | |||
| CR3 | GU809188 | GU809198 | |
| CR13 | GU809182 | GU809194 | |
| CR17 | GU809183 | GU809195 | |
| CR19 | GU809185 | GU809196 | |
| CR51 | GU809187 | GU809199 | |
| CR53 | GU809186 | GU809200 | |
| CR54 | GU809184 | GU809201 | |
| Pasteurellaceae | CR4 | GU809189 | GU809190 |
Sequences for all isolates with dissimilarity in either 16S rRNA or rpoB gene or both were submitted.
All isolates were obtained from mice, except for CR4, which came from a rat.
Real-time PCR assay.
Thermodynamic properties of the primers (Sigma-Aldrich, St Louis, MO) and the conventional probe with FAM detector and BHQ quencher (Sigma-Aldrich) were evaluated by using Net Primer Software. Real-time PCR assays were optimized for 300 nM of primers Forward (5′ CGG GTT GTA AAG TTC TTT CGG T 3′) and Reverse (5′ GGA GTT AGC CGG TGC TTC TTC) and 200 nM of probes specific for Jawetz (5′ AAT AAG GGT ATT AAC CTT ATC ACC TTC CTC ATC 3′) and Heyl (5′CAG CTT GGC TAT TAA CCA AAC TGC CT 3′). Calf thymus DNA was used as a negative control, and 100 copies of cloned 16S rRNA gene fragments of Heyl and Jawetz were used as respective positive controls.
The assay was run on an ABI 7300 thermocycler (Applied Biosystems, Carlsbad, CA), with 55 cycles of denaturation at 95 °C for 15 s and primer annealing and extension at 58 °C for 1 min. The real-time PCR results were analyzed by using vendor-supplied software (Applied Biosystems). Endpoint fluorescence values were obtained by using a plate-reading fluorometer (Fluoroskan Accent FL, ThermoLab Systems, Thermo Fisher Scientific, Waltham, MA).
Detection of P. pneumotropica Heyl DNA dilutions in various background DNA by real-time PCR assay.
Ten-fold serial dilutions of Heyl (isolate CR30) DNA were prepared, and 1 μL of each was added to 4 μL DNA from Proteus mirabilis (ATCC), Pseudomonas aeruginosa (ATCC), or ‘Pasteurellaceae–other CR4 isolate’ or water (as control). Pasteurella pneumotropica Heyl real-time PCR assay was performed in a reaction volume of 25 μL. A standard curve with known amounts of positive control was run in addition, and 1 μL of Heyl DNA was estimated to have 106 copies. The real-time PCR assay was run in triplicate wells.
Cloning in pCR4-TOPO vector plasmid.
PCR products were cloned into linearized pCR4-TOPO vector (Invitrogen) by using topoisomerase-mediated cloning, according to the manufacturer's instructions.
Results
Diagnosis of P. pneumotropica by using microbiologic and biochemical assays.
Bacteria initially were identified by isolation and growth on MacConkey and trypticase soy agar with 5% sheep blood and agar plates. Isolated colonies were biochemically identified as P. pneumotropica, after which 32 isolates from independent research facilities were selected for DNA isolation and sequence analysis. These isolates were obtained from client (pharmaceutical companies and academic institutions) samples collected between 2004 and 2005. The samples were from clients located in Massachusetts, Rhode Island, Connecticut, New Jersey, Pennsylvania, Georgia, California, Ottawa, and Calgary, but because of frequent exchange of genetically modified mice among research facilities, it is difficult to pinpoint the exact geographic origin of the isolates. The genetic background of the mice was not always known, but some nude mice were noted. All isolates, except CR4 (from rat), were isolated from mice.
Sequencing of 16S rRNA and rpoB genes.
By using overlapping primers, 1368 bp of 16S rRNA from various isolates was amplified and sequenced (Table 1). 16S rRNA gene sequence similarity was used to categorize them as Heyl and Jawetz on the basis of the respective gene sequences in GenBank (AF012090 for Heyl and M75083 for Jawetz). Sequence alignment using Vector NTI software showed that 15 isolates were nearly 100% identical to the P. pneumotropica Heyl biotype sequence DQ875933 and were characterized as Heyl. Another 16 isolates were 99% to 100% identical to P. pneumotropica Jawetz biotype sequence M75083 and therefore were characterized as Jawetz. Heyl and Jawetz biotypes are approximately 96% similar with respect to 16S rRNA gene sequences (Table 2).
Table 2.
Percentage identity (mean ± 1 SD) of the 16S rRNA (1368 bp) and rpoB (456 bp) genes in the isolates sequenced during the current study compared with Jawetz sequences from GenBank
BLAST analysis of the 16S rRNA sequence of CR4 isolate revealed no significant homology to P. pneumotropica; alignment showed only 96% homology to both Heyl (AF012090) and Jawetz (M75083) biotype. Therefore, this isolate was categorized as ‘Pasteurellaceae–other’. In addition, CR4 was the only rat isolate in this study, whereas all of the other isolates were obtained from mice. From here on, P. pneumotropica biotypes Jawetz and Heyl are referred to as Heyl and Jawetz, respectively, and sequences other than Heyl or Jawetz biotypes as Pasteurellaceae–other.
After characterization of isolates as Heyl or Jawetz according to 16S rRNA sequencing, 456 bp of rpoB from these isolates was PCR amplified and sequenced. The rpoB sequences of 12 of the 15 Heyl isolates were nearly 100% identical to each other. Because there is no rpoB sequence for the Heyl biotype in GenBank, we used CR5 as the reference sequence in our analysis. In addition, the rpoB sequence of reference strain CR5 was 99% to 100% similar to 2 recently submitted P. pneumotropica rpoB sequences in GenBank (AB479155.1 and AB479165.1). The rpoB gene sequence of the other 3 isolates (CR1, CR16, CR43) characterized as Heyl according to 16S rRNA sequence were 100% identical to each other and only 88% similar to that of our reference isolate CR5. Furthermore, the rpoB sequences of these 3 isolates were 100% identical to the Jawetz rpoB sequence from GenBank (AY314034).
The rpoB sequences for the 16 Jawetz isolates showed 99% to 100% identity to the rpoB gene sequence of P. pneumotropica Jawetz biotype NCTC 8141 in the GenBank; AY31403411 (Table 2) and also show 99% to 100% similarity to additional 28 sequences, 26 of these sequences are isolates from laboratory rodents that were recently submitted to GenBank.21 Comparison of the average percentage identity of 16S rRNA gene and rpoB gene sequences obtained with the respective reference sequences shows that rpoB is more variable than are 16S rRNA gene sequences.
Alignment of the obtained sequences and Pasteurellaceae–other sequences from GenBank showed that the rpoB-based phylogenetic tree is similar to that obtained by using 16S rRNA sequences. Therefore, sequences from either gene can be used to distinguish P. pneumotropica from other members of the Pasteurellaceae family (Figures 1 and 2).
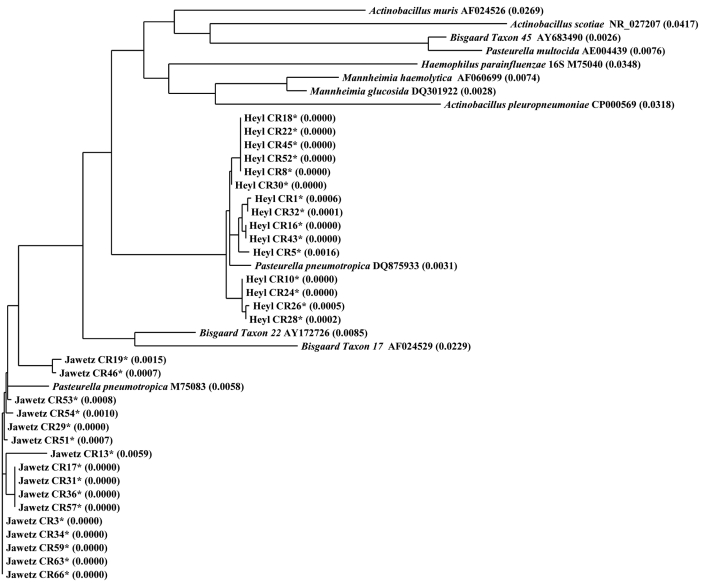
Figure 1.
Phylogenetic tree of the family Pasteurellaceae (Heyl, Jawetz, and Pasteurellaceae–other), based on partial 16S rRNA sequences. *, P. pneumotropica isolates sequenced during the current study. Representative sequences from GenBank were included for comparison. Sequences were aligned by using Vector NTI software. The distance values calculated by using the Neighbor Joining method are indicated in parenthesis. The alignment tree suggests that sequences from the Heyl and Jawetz biotypes cluster in 2 separate groups that are divergent from Pasteurellaceae–other.
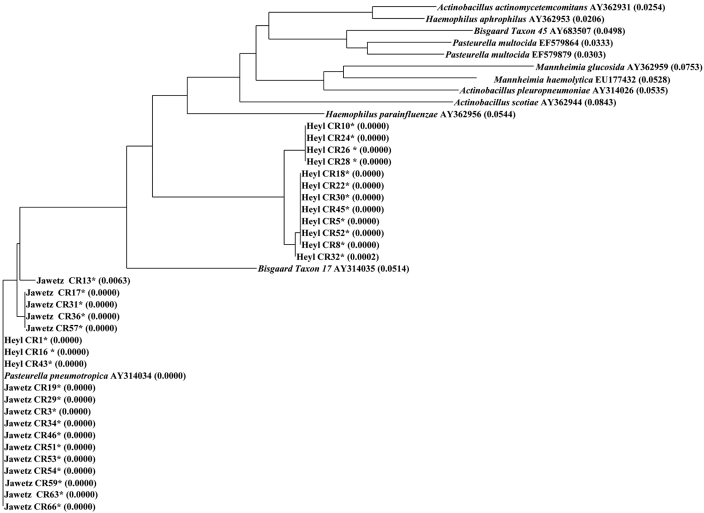
Figure 2.
Phylogenetic tree of the family Pasteurellaceae (Heyl, Jawetz, and Pasteurellaceae–other) based on partial rpoB gene sequences. *, P. pneumotropica isolates sequenced during the current study. Representative sequences from GenBank were included for comparison. Sequences were aligned by using Vector NTI software. The distance values calculated by using the Neighbor Joining method are indicated in parenthesis. The alignment tree suggests that P. pneumotropica clusters separately from Pasteurellaceae–other, although the distinction between Heyl and Jawetz biotypes was unclear.
Biochemical characterization.
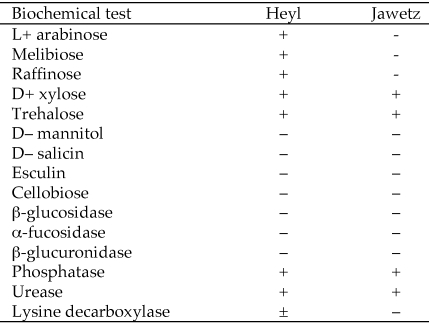
Detailed biochemical analysis 4 Heyl and 5 Jawetz isolates characterized by 16S rRNA gene sequencing was done as previously described 14 The results (Figure 3) meet the minimal criteria for these biotypes that were defined in a study of more than 2000 isolates,15 corroborating our classification based on sequence of the 16S rRNA gene.
Figure 3.
Biochemical characteristics of P. pneumotropica isolates. The scores are representative of 4 Heyl isolates (CR18, CR24, CR30, CR45*) and 5 Jawetz isolates (CR34, CR46, CR54, CR63,** and CR66**). The rpoB and 16S rRNA sequences of CR45 were the same as those of CR18, and those of CR63 and CR66 were the same as those of CR34.
BLAST search and analysis of percentage identity of 16S rRNA and rpoB sequences within Pasteurellaceae.
We used the BLAST program to search for all 16S rRNA and rpoB gene sequences from Pasteurellaceae family members that were submitted to GenBank. The analysis of percentage identity with respective reference sequences for 16S rRNA (M75083) and rpoB (AY314034) within Pasteurellaceae indicate that the variability within Pasteurella s.s. ranged from 78-100% for rpoB gene versus 89-99% for 16S rRNA gene. Similarly, rpoB gene sequences within Actinobacilus, Heamophilus, non-PAH and indeed total Pasteurellaceae appears to be comparatively more variable than 16S rRNA gene sequences (Table 3).
Table 3.
Percentage identity (as determined by BLAST analysis) of 16S rRNA and rpoB of reference strains of P. pneumotropica Jawetz with other sequences in GenBank
|
16S rRNA of Jawetz M75083 |
rpoB of Jawetz AY314034 |
|||
| Genus | % Identity | No. of sequences | % Identity | No. of sequences |
| Pasteurella s.s.a | 89 to 99 | 197 | 78 to 100 | 114 |
| Actinobacillus | 90 to 95 | 205 | 79 to 84 | 73 |
| Haemophilus | 86 to 94 | 858 | 79 to 88 | 152 |
| NonPAHb | 89 to 96 | 295 | 78 to 90 | 170 |
| Total Pasteurellaceae | 86 to 99 | 1555 | 78 to 100 | 509 |
Many species classified under Pasteurella in GenBank are genetically very divergent; and this diversity is reflected in the wide range of percentage identity.
All Pasteurellaceae in GenBank whose genera were not noted as either Pasteurella, Actinobacillus, or Haemophilus.
Real-time fluorogenic PCR assays for diagnosis of P. pneumotropica.
The 16S rRNA and rpoB sequences from the Heyl and Jawetz isolates obtained in our study and those available in GenBank were aligned. Alignment of the rpoB region showed that the distinguishing nucleotide bases in rpoB gene that identify P. pneumotropica (Figure 4) are not clustered together; therefore, it would be very challenging to develop primers and probes for PCR-based diagnosis based on rpoB. However, we identified unique island clusters in 16S rRNA that could distinguish P. pneumotropica (Figure 5).The region between nucleotides 437 and 470 (reference strain M75083) was identified as unique to both Heyl and Jawetz and therefore was predicted to distinguish the 2 biotypes from each other and from Pasteurellaceae–other. We designed 2 separate real-time PCR assays by targeting this region and using the respective real-time PCR assay probes for Heyl and Jawetz. We tested the Heyl and Jawetz isolates to establish the analytical sensitivity and specificity of the real-time PCR assays by calculating the endpoint relative fluorescence unit (Rn) and cycle threshold (Ct) values, respectively. The P. pneumotropica Heyl assay detected all isolates that were identified as Heyl by 16S rRNA sequencing (Ct = 13.8 ± 0.3, n = 15; Rn = 18.7 ± 0.9, n = 15 compared with Ct = 2.0 ± 0.1, n = 4 for calf thymus DNA [negative control]) but none of the Jawetz isolates (Rn = 2.0 ± 0.1, n = 16) or the CR4 (Pasteurellaceae–other) isolate (Rn = 2.0). Although there was a single-nucleotide mismatch at the third base position of the probe (adenosine instead of guanosine) for 4 Heyl isolates (CR10, CR24, CR26, and CR28), this mismatch did not affect the binding characteristics sufficiently to affect detection of these isolates as described previously for conventional probes.25 Neither amplification plot, cycle threshold, nor the endpoint fluorescence values of CR10, CR24, CR26, and CR28 were distinguishable from those of other Heyl isolates. Similarly, Jawetz isolates were detected specifically by the P. pneumotropica Jawetz assay (Ct = 13.9 ± 0.2, n = 16 and Rn = 19.2 ± 1.4, n = 16 compared with Rn = 7.4 ± 0.1, n = 4 for calf thymus DNA [negative control]). The 2 real-time PCR assays could detect as few as 10 copies of P. pneumotropica per well and did not crossreact with each other. In addition, the analytical specificity of the assays was confirmed against DNA of unrelated bacterial species, Proteus mirabilis and Pseudomonas aeruginosa. To test our hypothesis that the assays would not produce false-positive results from a contaminating dose of P. pneumotropica, we prepared 10-fold serial dilutions of genomic DNA preparations of the Heyl isolate CR30 and ran the Heyl real-time PCR assay in a background of approximately 106 copies of nonPasteurellaceae DNA (Pasteurellaceae–other isolate CR4). The assay prevented detection of Heyl CR30 at dilution of 10−2 or more (equivalent to 10,000 copies or fewer) in a background of CR4 (Figure 6). In contrast, the assay detected Heyl CR30 even at a dilution of 10−5 (10 copies) in a nonhomologous background (approximately 106 copies of Proteus mirabilis or Pseudomonas aeruginosa).
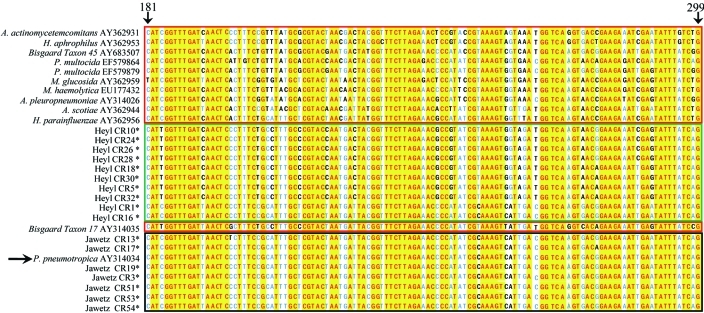
Figure 4.
Alignment of a region of rpoB from Heyl, Jawetz, and Pasteurellaceae–other. Sequences were aligned by using Vector NTI software; isolates with identical 16S rRNA and rpoB genes have been omitted from the alignment. Green box, Heyl isolates characterized during the current study; black box, Jawetz isolates (characterized during the current study and AY31404), red boxes, 16S rRNA gene sequences from Pasteurellaceae–other sequences; yellow, conserved residues; blue and black letters, mismatches. The sequences have been aligned to nucleotides 181 through 299 of reference strain AY314034. The reference strain and nucleotide position of the reference strain are indicated by arrows. Mismatches between rpoB sequences from Pasteurellaceae species and biotypes are evenly distributed, thereby complicating development of a P. pneumotropica-specific PCR-based diagnostic assay using this gene.
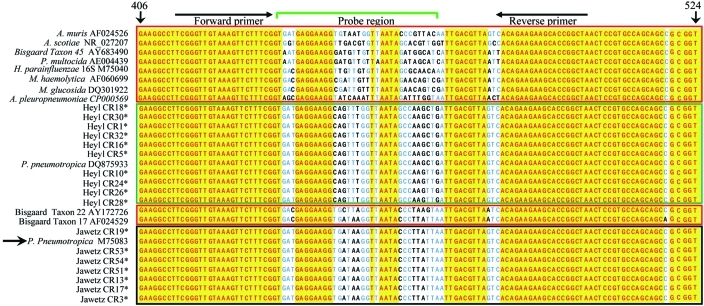
Figure 5.
Alignment of the PCR target region of 16S rRNA from Heyl, Jawetz, and Pasteurellaceae–other. Sequences were aligned by using Vector NTI software; isolates with identical 16S rRNA and rpoB genes have been omitted from the alignment. *, P. pneumotropica isolates sequenced during the current study; green box, Heyl isolates (those sequenced during the current study and DQ875933); black box, Jawetz isolates (those sequenced during the current study and M75083); red boxes, Pasteurellaceae–other sequences; yellow, conserved residues; blue and black letters, mismatches. The sequences have been aligned to nucleotides 406 through 524 of reference strain M75083. The reference strain and nucleotide positions of the reference strain are indicated by arrows. The positions of primers and probes used are indicated on top. The sequence data suggest that although 16S rRNA is highly conserved between various species and biotypes of Pasteurellaceae, small regions unique to P. Heyl and Jawetz biotypes are present and offer an opportunity to design a P. pneumotropica-specific PCR-based diagnostic assay.
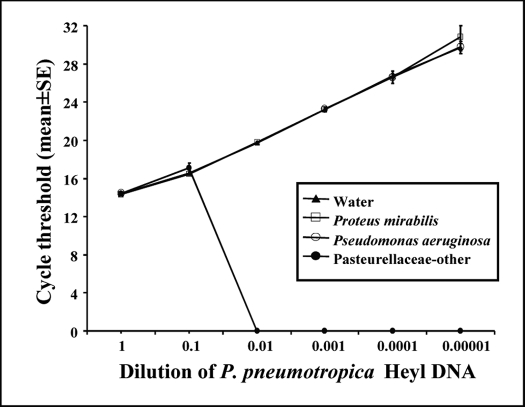
Figure 6.
Detection of decreasing amounts of P. pneumotropica Heyl DNA in backgrounds of Pasteurellaceae–other and nonPasteurellaceae DNA by using real-time PCR assays. The real-time PCR assay for Heyl was run in triplicate wells in presence of various background DNAs. At a dilution factor of 0.01, Heyl DNA (approximately 10,000 copies) could not be detected in a background of Pasteurellaceae–other (Ct = undetermined compared with 20 for the other 3 groups). However, Heyl DNA could be detected in water or a background of nonPasteurellaceae DNA (Proteus mirabilis and Pseudomonas aeruginosa), even at a dilution of 10−5 (approximately 10 copies; Ct = 30).
Discussion
DNA sequencing of various genes has been undertaken in an effort to improve taxonomic classification as well as detection of Pasteurellaceae.1,7,8,11,24 Here we have demonstrated the sensitivity and specificity of a 16S rRNA based real-time PCR assay to characterize P. pneumotropica from isolated bacterial colonies. Our study demonstrates multiple advantages of targeting the 16S rRNA gene over rpoB for PCR-based diagnosis of P. pneumotropica on a large scale, such as for routine health monitoring.
First, the repertoire of Pasteurellaceae-specific rpoB sequences in GenBank is far smaller than that for 16S rRNA: 746 rpoB sequences compared with 2851 sequences for 16S rRNA. Second, BLAST searches of regions of the 16S rRNA (M75083) and rpoB (AY314034) sequences of P. pneumotropica Jawetz biotype NCTC 8141 (Table 3) reveal 86% to 99% and 78% to 100% sequence identity, respectively, within Pasteurellaceae, illustrating that rpoB gene is less conserved than 16S rRNA gene among Pasteurellaceae family members. Nevertheless, on the basis of the rpoB gene sequences obtained in the current study, Heyl and Jawetz form unique clusters in the alignment tree (Figure 2). In agreement with previous reports,1,11 our data demonstrate that phylogenetic trees based on rpoB and 16S rRNA gene sequence correlate closely within the Pasteurellaceae family. Sequencing of either gene regions can distinguish P. pneumotropica from other members of Pasteurellaceae family. However, alignment of the rpoB gene sequences obtained from Heyl and Jawetz isolates identified herein and other Pasteurellaceae sequences from GenBank indicates that the sequence variability between the 2 biotypes is not clustered in any particular region. This distribution would make it challenging to develop distinguishing probes or primers for real-time or gel-based PCR without crossreactivity to other members of Pasteurellaceae family. Therefore, although rpoB gene sequencing can be used for diagnosis of P. pneumotropica, it presents a labor- and cost-intensive option. In contrast, the 16S rRNA sequence offers an opportunity to design gel-based or real-time PCR assays, which are more convenient than nucleotide sequencing for health status monitoring of laboratory rodents.
Third, the rpoB sequences of 3 Heyl (CR1, CR16, CR43) isolates were in fact homologous to the Jawetz sequence, indicating that sequencing of the targeted rpoB gene region cannot be used to differentiate the biotypes. These 3 isolates largely contribute to the observed increased variability in the rpoB sequence (Table 2). To eliminate the possibility of technical error, we repeated rpoB sequencing for these 3 samples and confirmed the results. Interestingly, 16S rRNA sequences of these 3 isolates show multiple single-nucleotide variations at the same locations. The 16S rRNA-based real-time PCR assays described here have an additional capability of distinguishing Heyl and Jawetz biotypes from each other as well as distinguishing them from Pasteurellaceae–other. This feature is especially valuable for pathogenesis studies. In another study performed in our laboratory4 118 Pasteurella pneumotropica isolates identified by API 20 NE assay and further characterized by PCR yielded 66 Heyl, 44 Jawetz, and 8 Pasteurellaceae–other. Interestingly, all the 6 isolates that were associated with lesions (eye, muzzle, urinogenital) were Heyl. In the current study, 1 Heyl (CR8) and 2 Jawetz isolates (CR31, CR66) were associated with eye lesions. Further screening of a larger population is required to establish whether the Heyl biotype is indeed more pathogenic than Jawetz.
Although a random amplified polymorphic DNA method targeting 16S rRNA gene has been described for differentiating Heyl and Jawetz,10 diagnosis based on the gel-based assays remains vulnerable to false positives due to contamination by non P. pneumotropica isolates, and investigators should be alert to the conditions applied for diagnosis before deciding to discontinue using a colony in further research applications.
We occasionally have detected inconsistencies between results obtained from the commercially available biochemical profile cards and 16S rRNA-based real-time PCR assay, such as those described in the Results section for isolate CR4. This rat isolate was identified as P. pneumotropica after isolation on a blood agar plate, followed by characterization by using the API 20 NE biochemical card; however the 16S rRNA sequence obtained did not match that for P. pneumotropica. According to the BLAST results, the closest match (98% homology) was with Bisgaard Taxon 22 (AY172726), which (as expected) did not give a positive signal in either the Heyl or Jawetz real-time PCR assay. The 16S rRNA sequence of CR4 is most closely related to a group of organisms that have in common a negative reaction for trehalose and that belong to the Jawetz-like-group but that are not related to the Jawetz type strain according to various molecular criteria (for example, 16S rRNA gene sequences, DNA: DNA hybridization). This group of organisms consists of V-factor-dependent and -independent strains and is found primarily in rats but occasionally in mice.16 Pasteurellaceae in rats and mice may show variation only in 4 reactions of the API 20 NE panel; therefore, inconsistency when using biochemical methods for diagnosis is not surprising. Therefore, investigators should obtain sequence data on 16S rRNA or rpoB gene after microbiologic and biochemical characterization and not base a decision regarding subsequent research use of a colony, solely on the results of biochemical characterization.
The described assays were designed for PCR amplification from bacteria after growth and isolation on selective media, but, low-copy environmental contaminants are unavoidable in a microbiology laboratory. Most research laboratories currently consider elimination of only P. pneumotropica but not other Pasteurellaceae. We aimed to design a real-time PCR assay that would be specific for Heyl and Jawetz but at the same time avoid detection of minute environmental contamination of P. pneumotropica. To this end, the forward and reverse primers flanking the Heyl- and Jawetz-specific probes targeted regions that are highly conserved among Pasteurellaceae. Such an approach for real-time PCR approach in lieu of a 2-primer gel-based assay for characterization of isolated colonies surmounts the problem of false-positive results. As we described (Figure 6), PCR products due to a contaminating P. pneumotropica isolate with similar primer binding sites would be masked by the vastly more numerous PCR products arising from the principal Pasteurellaceae–other colony. In this situation, a gel-based assay with differentiating sequences in the primers might yield a visible band of the expected PCR product size, making it impossible to differentiate a true P. pneumotropica isolate from an environmental contaminant. Regardless of the diagnostic testing method, results should always be confirmed by the same or other method of detection. The real-time PCR assays described in this paper should not be used for direct detection of P. pneumotropica in samples collected from rodents, given that the presence of larger numbers of other Pasteurellaceae may lead to competitive inhibition and mask detection.
Complete sequencing of rpoB from numerous isolates from various members of Pasteurellaceae family may be useful for its taxonomic characterization. The real-time PCR assay method described here is only useful for characterization of isolated bacterial colonies. However, additional unique motifs in 16 rRNA could be targeted for specific detection of P. pneumotropica from samples collected directly from animals, thereby eliminating the need for bacteriology and offering a more sensitive and faster detection method.
In conclusion, our results demonstrate that the 16S rRNA but not rpoB gene of the Pasteurellaceae family is a useful target for development of real-time PCR-based diagnostic assays that can provide conclusive differentiation without the need for DNA sequencing.
Acknowledgments
We are grateful to Panagiota Momtsios for technical support, Bob Zaccardi for assistance with preparation of figures, and Drs Charles B Clifford and Kathleen R Pritchett-Corning for providing unpublished data.
References
- 1.Christensen H, Kuhnert P, Olsen JE, Bisgaard M. 2004. Comparative phylogenies of the housekeeping genes atpD, infB, and rpoB and the 16S rRNA RNA gene within the Pasteurellaceae. Int J Syst Evol Microbiol 54:1601–1609 [DOI] [PubMed] [Google Scholar]
- 2.Fister RD.2010. Unpublished results.
- 3.Fister RD, Henderson KS.2010. Unpublished results.
- 4.Fister RD, Pritchett KR, Clifford CB. 2005. Characterization of Pasteurella spp. in populations of genetically modified mice: prevalence, lesions and biotype. ASM Conference on Pasteurellaceae, 23–26 Oct 2005. Kohala Coast, HW [Google Scholar]
- 5.Frederiksen W. 1973. Pasteurella taxonomy and nomenclature, p 170–176. : Winblad S. Contributions to microbiology and immunology, vol 2. Basel (Switzerland): Karger [Google Scholar]
- 6.Hayashimoto N, Aiba T, Itoh K, Kato M, Kawamoto E, Kiyokawa S, Morichika Y, Muraguchi T, Narita T, Okajima Y, Takakura A, Itoh T. 2005. Identification procedure for Pasteurella pneumotropica in microbiologic monitoring of laboratory animals. Exp Anim 54:123–129 [DOI] [PubMed] [Google Scholar]
- 7.Hayashimoto N, Takakura A, Itoh T. 2005. Genetic diversity of 16S rRNA rDNA sequence and phylogenic tree analysis in Pasteurella pneumotropica strains isolated from laboratory animals. Curr Microbiol 51:239–243 [DOI] [PubMed] [Google Scholar]
- 8.Hayashimoto N, Ueno M, Takakura A, Itoh T. 2006. Phylogenetic analysis of isolates of Pasteurella pneumotropica from laboratory animals based on the gyrB gene sequence. Exp Anim 55:487–490 [DOI] [PubMed] [Google Scholar]
- 9.Heyl JG. 1963. A study of Pasteurella strains from animal sources. Antonie van Leeuwenhoek 29:79–83 [DOI] [PubMed] [Google Scholar]
- 10.Kodjo A, Villard L, Veillet F, Escande F, Borges E, Maurin F, Bonnod J, Richard Y. 1999. Identification by 16S rDNA fragment amplification and detection of genetic diversity by random amplified polymorphic DNA analysis of Pasteurella pneumotropica isolated from laboratory rodents. Lab Anim Sci 49:49–53 [PubMed] [Google Scholar]
- 11.Korczak B, Christensen H, Emler S, Frey J, Kuhnert P. 2004. Phylogeny of the family Pasteurellaceae based on rpoB sequences. Int J Syst Evol Microbiol 54:1393–1399 [DOI] [PubMed] [Google Scholar]
- 12.Leutenegger CM. 2001. The real-time TaqMan PCR and applications in veterinary medicine. Veterinary Sciences Tomorrow [electronic resource] 1:1–15 [Google Scholar]
- 13.Mutters R, Ihm P, Pohl S, Frederiksen W, Mannheim W. 1985. Reclassification of the genus Pasteurella Trevisan 1887 on the basis of deoxyribonucleic acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa. Int J Syst Bacteriol 35:309–322 [Google Scholar]
- 14.Nicklas W. 1989. Haemophilus infection in a colony of laboratory rats. J Clin Microbiol 27:1636–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicklas W. 2007. Pasteurellaceae, p 469–505. : Fox JG, Barthold SW, Newcomer CE, Quimby F, Smith A. The mouse in biomedical research, 2nd ed. New York (NY): Academic Press [Google Scholar]
- 16.Nicklas W.2010. Personal communication.
- 17.Nicklas W, Baneux P, Deeny RAA, Fumanelli M, Illgen-Wilcke B. 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42 [DOI] [PubMed] [Google Scholar]
- 18.Nozu R, Goto K, Ohashi H, Takakura A, Itoh T. 1999. Evaluation of PCR as a means of identification of Pasteurella pneumotropica. Exp Anim 48:51–54 [DOI] [PubMed] [Google Scholar]
- 19.Pritchett-Corning KR, Cosentino JM, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 20.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425 [DOI] [PubMed] [Google Scholar]
- 21.Sasaki H, Kawamoto E, Tanaka Y, Sawada T, Kunita S, Yagami K. 2009. Comparative analysis of Pasteurella pneumotropica isolates from laboratory mice and rats. Antonie van Leeuwenhoek 95:311–317 [DOI] [PubMed] [Google Scholar]
- 22.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and Tris (HotSHOT). Biotechniques 29:52, 54. [DOI] [PubMed] [Google Scholar]
- 23.Wang RF, Campbell W, Cao WW, Summage C, Steele RS, Cerniglia CE. 1996. Detection of Pasteurella pneumotropica in laboratory mice and rats by PCR. Lab Anim Sci 46:81–85 [PubMed] [Google Scholar]
- 24.Weigler BJ, Wiltron LA, Hancock SI, Thigpen JE, Goelz MF, Forsythe DB. 1998. Further evaluation of a diagnostic polymerase chain reaction assay for Pasteurella pneumotropica. Lab Anim Sci 48:193–196 [PubMed] [Google Scholar]
- 25.Yao Y, Nellåker C, Karlsson H. 2006. Evaluation of minor groove binding probe and TaqMan probe PCR assays: influence of mismatches and template complexity on quantification. Mol Cell Probes 20:311–316 [DOI] [PubMed] [Google Scholar]