Abstract
In the present study, we observed the effects of an α1-adrenoceptor agonist (phenylephrine), β-adrenoceptor agonist (isoprenaline), muscarinic cholinoceptor agonist (carbachol), and α1-adrenoceptor antagonist (doxazosin) on the bladder micturition function in anesthetized mice. Changes in bladder pressure in response to filling and blood pressure were recorded by using a data acquisition system. Phenylephrine (50 to 800 µg/kg) increased vesical micturition pressure in a dose-dependent manner but increased micturition basal pressure only at 800 µg/kg. Carbachol (3 to 7 µg/kg) increased the intercontraction interval and micturition time in a dose-dependent manner but increased micturition basal pressure only at 7 µg/kg. Isoprenaline (10 to 1000 µg/kg) increased micturition time and decreased vesical micturition pressure in a dose-dependent manner. Doxazosin (10 to 1000 µg/kg) did not affect bladder micturition function but dose-dependently inhibited phenylephrine-induced increases in vesical micturition pressure. Carbachol (7 µg/kg) and isoprenaline (1 mg/kg) caused a transient fall in blood pressure, whereas doxazosin (1 mg/kg) had a long-lasting hypotensive effect. The maximal decrease in systolic and mean blood pressure by carbachol did not differ from that by doxazosin and isoprenaline, respectively. Phenylephrine (800 µg/kg) transiently increased the blood pressure of anesthetized mice. These results indicate that activation of muscarinic cholinoceptors decreases voiding frequency and increases bladder capacity in anesthetized mice. Activation of α1-adrenoceptors mainly increases vesical micturition pressure, whereas activation of β-adrenoceptors decreases vesical micturition pressure and prolongs micturition time in anesthetized mice.
Aging is associated with declining function in nearly every physiologic system, and lower urinary tract symptoms are prevalent among the elderly. Clinical urodynamic studies have demonstrated that advancing age is associated with reduced bladder capacity, increased uninhibited detrusor contractions, decreased urinary flow rate, and increased postvoid residual urine volume.16,19,28,34 Voiding function is an integrated function requiring input from the parasympathetic system by means of the pelvic nerve, the sympathetic system by the hypogastric nerve, and the somatic system through innervation of the rhabdosphincter by the pudendal nerve. Mammalian bladder expresses much more M2 than M3 receptors. However, evidence from in vitro studies using many species including humans12 and an in vivo study using knockout mice33 indicates that physiologic bladder contraction largely is due to activation of M3 receptors. M2 receptors mediate bladder contraction in mice through an indirect mechanism.11 Contractile responses of the human prostate to nerve stimulation are mediated sympathetically, and the noradrenergic transmitters activate postjunctional α1A adrenoceptors.24 Available literature indicates that the mouse prostate has similar innervations to humans, and contractile responses to nerve stimulation in mice are noradrenergic and mediated by α1 adrenoceptors, suggesting the mouse prostate as a suitable model for functional studies of human prostate.14 The recent discovery of novel β-adrenergic receptor subtypes likely will provide new insight concerning the role of the sympathetic nervous system in bladder filling. In contrast to classic descriptions of β-receptor distribution, which state that only β2 receptors are present in smooth muscles, β1, β2, and β3 adrenergic receptors have been identified in bladder smooth muscle at both the pharmacologic and molecular levels.18,25,29,43 In the human detrusor, β-adrenergic receptors are much more numerous than α-adrenergic receptors, and the normal response to noradrenaline is relaxation.2
Cystometry is a measure of bladder pressure in response to filling, and anesthetized or conscious rats often are used to investigate the effects of various drugs on cystometry.20,41 In anesthetized mice, chemotherapy drugs affected urinary bladder micturition.30,31 A recent study found that a small dose of estrone had a positive effect on voiding in the mouse, suggesting that estrogens are needed for normal male voiding.35 Other researchers analyzed bladder function in mice lacking the vanilloid receptor TRPV1.4 In addition, urinary bladders obtained from P2X1 receptor-deficient mice were suggested as possible models for study of abnormal human bladder function.39 Although mice have attracted considerable attention as a useful animal model for investigating the bladder function in vivo, only limited data are available on the physiologic and pharmacologic profiles of bladder function in normal mice. Therefore, we assessed the effects of intravenous administration of an α1-adrenergic agonist, β-adrenergic agonist, muscarinic cholinergic agonist, and α1-adrenergic antagonist on bladder micturition function in anesthetized mice.
Materials and Methods
Mice.
Male adult albino KM strain mice (35 to 42 g; 8 to 9 wk of age; originally introduced from Swiss mice at the Hoffkine Institute, India, in 1944) were supplied by the Laboratory Animal Center of Hebei Medical University (China). Mice were housed 5 per cage, given standard laboratory chow and tap water ad libitum, and kept under a 12:12-h light:dark cycle in the animal care facility. All animals used in the present study received humane care in compliance with institutional animal care guidelines. The study was approved by the local institutional committee.
Chemicals.
Phenylephrine hydrochloride and isoprenaline hydrochloride were obtained from Sigma Chemical (St Louis, MO). Carbachol hydrochloride was obtained from ABCR (Karlsruhe, Germany). Doxazosin mesylate was provided by the New Drug Research and Development Centre of the North China Pharmaceutical Group (Shijiazhuang, China). All drugs were dissolved in distilled water.
Urinary bladder pressure.
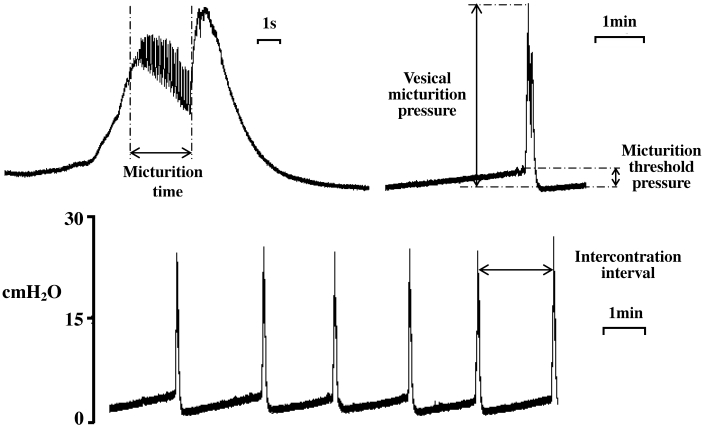
Each mouse was anesthetized with urethane (1.5 g/kg SC), and a catheter was inserted into the trachea to allow drainage of bronchial secretions and to facilitate breathing. A longitudinal midline incision (0.8 mm) was made in the skin just above the pubic symphysis, and the ureters were cut. The distal ends of the ureters were ligated, and a cotton ball was placed on the proximal ends to absorb urine. For bladder pressure measurements, an intravenous catheter (22 gauge, 1 in.) was inserted gently through the bladder apex into the lumen. The intravenous catheter was filled with physiologic saline and connected to a perfusion system consisting of a soft plastic bag, sphygmomanometer, and blood pressure monitoring kit (Becton Dickinson Infusion, Franklin Lakes, NJ). The cuff of sphygmomanometer was wrapped around the plastic bag containing to keep the pressure inside the bag at 170 mm Hg. A perfusion apparatus from an automated monitoring kit (Becton Dickinson, Research Triangle Park, NC) was connected to the bag by plastic tubing, and the perfusion rate was controlled at 0.043 mL/min. The bladder pressure created by perfusion of physiologic saline was recorded by using a 3-way stopcock connected to a pressure transducer. The perfused physiologic saline was kept at 37 °C. The following cystometric parameters (Figure 1) were investigated: vesical micturition pressure (maximal bladder pressure during micturition), micturition threshold pressure (bladder pressure immediately prior to micturition), micturition basal pressure (the lowest bladder pressure during filling), intercontraction interval (the interval(s) between each large amplitude spontaneous bladder contraction) and micturition time (high-frequency oscillations of bladder pressure associated with the urine flow). Vesical micturition pressure, micturition threshold pressure, micturition basal pressure, intercontraction interval, and micturition time were recorded by using a data acquisition system (PowerLab/8sp, ADInstruments, Sydney, Australia) interfaced through a personal computer running vendor-supplied software (PowerLab Chart version 5.0, ADInstruments). For drug administration, the tail vein was cannulated by using an intravenous catheter (26-gauge), which was connected with a short tube; the entire volume of the administration system was kept at 60 μL. Room temperature was kept at 25 to 30 °C during experiments. The mice were allowed to equilibrate for 70 min before drug administration.
Figure 1.
Representative traces of typical micturition cycle in a control mouse, showing the bladder pressure wave to filling in anesthetized mice.
Arterial blood pressure.
Male KM mice (additional to those used for other experiments) were anesthetized as mentioned previously. After tracheal intubation, an intravenous catheter (24-gauge) filled with physiologic saline containing 350 U/mL heparin was inserted into the left common carotid artery for blood pressure measurement. Systolic, diastolic, and mean arterial blood pressure were recorded by using a 3-way stopcock connected to a pressure transducer and the data acquisition system (PowerLab/8sp, ADInstruments). Mice were allowed to equilibrate for 30 min before drug administration.
Experimental protocols.
Effects of phenylephrine, carbachol, isoprenaline, and doxazosin on bladder micturition function in anesthetized mice.
Mice were allocated randomly into solvent (n = 12), phenylephrine (n = 15), carbachol (n = 15), isoprenaline (n = 15), and doxazosin (n = 12) groups. Phenylephrine was given at 50, 100, 200, 400, and 800 μg/kg; carbachol was given at 3, 4, 5, 6, and 7 μg/kg; and both isoprenaline and doxazosin were given at 10, 30, 100, 300, and 1000 μg/kg. Normal saline (solvent) was given at 0.2, 0.3, 0.2, 0.3, and 1.0 mL/kg. Five doses of each drug or solvent were injected into the tail vein at 30-min intervals. Three micturition cycles (duration, 6 to 12 min each) were recorded immediately before and after each administration, and their mean values (cystometric parameters) were calculated.
Effects of doxazosin on the phenylephrine-induced increase in vesical micturition pressure in anesthetized mice.
Mice were allocated randomly into solvent and doxazosin groups (n = 12 mice per group). Phenylephrine (220 μg/kg) was injected into the tail vein every 30 min for a total of 4 doses. Maximal increases in vesical micturition pressure induced by phenylephrine were recorded within 5 min immediately before and after each dose. The increase in vesical micturition pressure induced by the first injection of phenylephrine was taken as baseline. Doxazosin at 0.1, 0.3, and 1.0 mg/kg was given 5 min before the second, third, and fourth injections of phenylephrine (220 μg/kg), respectively. The effects of doxazosin on the increase in vesical micturition pressure induced by phenylephrine were observed. In the solvent group, normal saline was injected instead of doxazosin.
Effects of phenylephrine, carbachol, isoprenaline and doxazosin on blood pressure in anesthetized mice.
Carbachol (7.0 µg/kg), phenylephrine (800 µg/kg), and isoprenaline (1 mg/kg) were injected into the tail veins of 8 anesthetized mice. Because the 3 agents affected the carotid blood pressure only transiently and because the drug-induced change in blood pressure disappeared within 15 min (Figure 2), the 3 agents were given to each mouse at 30-min intervals. To eliminate the influence of potential drug interactions, carbachol, phenylephrine, and isoprenaline were given in random order. Because doxazosin at 1 mg/kg produced a long-lasting decrease in blood pressure, it was administered as a sole agent. Drug-induced maximal decrease or increase in blood pressure was recorded, and the blood pressure readings before drug administration were used as a control.
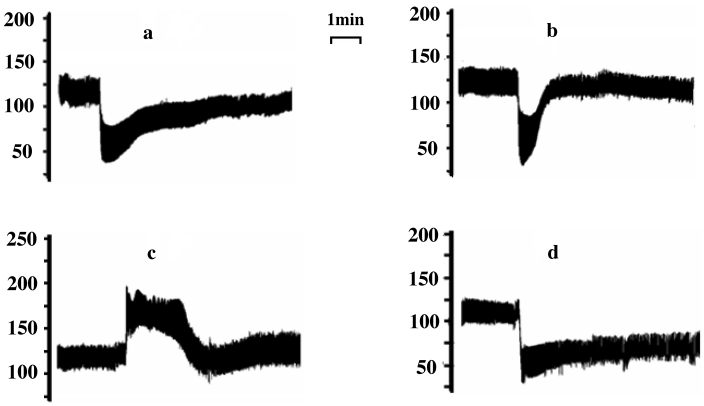
Figure 2.
Representative traces showing the effects of (A) isoprenaline (1 mg/kg), (B) carbachol (7 µg/kg), (C) phenylephrine (0.8 mg/kg), and (D) doxazosin (1 mg/kg) on blood pressure (mm Hg) in anesthetized mice.
Statistical analysis.
Data are expressed as mean ± SEM. One-way ANOVA followed by the Dunnett test was used to evaluate the concentration-dependent effects of drugs on bladder micturition function and the effects of doxazosin on the phenylephrine-induced increase in vesical micturition pressure. Paired t tests were used to evaluate the effects of drugs on blood pressure. Analyses were performed by using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA). P values less than 0.05 were considered statistically significant.
Results
Effects of phenylephrine, carbachol, isoprenaline, and doxazosin on bladder micturition function in anesthetized mice.
Intravenous administration of physiologic saline to anesthetized mice did not significantly change the cystometric parameters. Phenylephrine (50 to 800 µg/kg) administration increased (P < 0.05 to P < 0.01) vesical micturition pressure in a dose-dependent manner in comparison with before-drug values, whereas micturition basal pressure was increased (P < 0.05) by phenylephrine only at 800 µg/kg (Table 1). Carbachol (3 to 7 µg/kg) increased (P < 0.05 to P < 0.01) the intercontraction interval and micturition time in a dose-dependent manner but significantly (P < 0.01) increased micturition basal pressure only at 7 μg/kg and had a nonsignificant effect on vesical micturition pressure (Table 1). Isoprenaline (10 to 1000 µg/kg) increased (P < 0.05 to P < 0.01) micturition time and decreased (P < 0.05 to P < 0.01) vesical micturition pressure in a dose-dependent manner but did not significantly affect the intercontraction interval (Table 1). Doxazosin (10 to 1000 µg/kg) did not significantly affect the cystometric parameters in anesthetized mice (Table 1).
Table 1.
Effects of various drugs on bladder micturition function in anesthetized mice
| Vesical micturition pressure (cm H2O) | Micturition threshold pressure (cm H2O) | Micturition basal pressure (cm H2O) | Intercontraction interval (s) | Micturition time (s) | ||
| Physiologic saline (mL/kg) | ||||||
| 0.0 | 27.09 ± 2.00 | 4.06 ± 0.40 | 2.93 ± 0.37 | 193.25 ± 24.40 | 4.09 ± 0.88 | |
| 0.2 | 27.36 ± 1.99 | 4.00 ± 0.49 | 2.88 ± 0.39 | 180.71 ± 22.79 | 3.97 ± 0.67 | |
| 0.3 | 28.41 ± 2.24 | 4.20 ± 0.55 | 2.83 ± 0.42 | 183.51 ± 24.27 | 4.73 ± 0.94 | |
| 0.2 | 27.77 ± 9.32 | 3.84 ± 0.46 | 2.74 ± 0.43 | 174.91 ± 21.95 | 4.35 ± 0.61 | |
| 0.3 | 26.67 ± 9.54 | 3.48 ± 0.47 | 2.67 ± 0.43 | 171.31 ± 20.48 | 4.73 ± 0.74 | |
| 1.0 | 26.52 ± 8.70 | 3.50 ± 0.43 | 2.71 ± 0.46 | 167.64 ± 17.68 | 5.21 ± 0.85 | |
| Phenylephrine (µg/kg) | ||||||
| 0 | 23.04 ± 1.33 | 3.53 ± 0.37 | 2.53 ± 0.36 | 177.29 ± 29.10 | 3.56 ± 0.36 | |
| 50 | 25.04 ± 1.40 | 3.86 ± 0.41 | 2.49 ± 0.37 | 166.32 ± 23.85 | 3.84 ± 0.53 | |
| 100 | 26.40 ± 1.63 | 3.70 ± 0.36 | 2.61 ± 0.39 | 163.96 ± 21.55 | 4.01 ± 0.55 | |
| 200 | 31.00 ± 2.07a | 3.94 ± 0.48 | 2.60 ± 0.37 | 155.82 ± 15.01 | 4.73 ± 0.51 | |
| 400 | 34.57 ± 3.05b | 4.32 ± 0.58 | 2.98 ± 0.42 | 145.45 ± 9.91 | 5.77 ± 0.91 | |
| 800 | 37.20 ± 2.97b | 3.68 ± 0.57 | 4.32 ± 0.54a | 128.35 ± 17.65 | 5.41 ± 0.66 | |
| Carbachol (µg/kg) | ||||||
| 0.0 | 21.66 ± 1.02 | 3.77 ± 0.30 | 2.39 ± 0.30 | 160.38 ± 11.81 | 4.17 ± 0.48 | |
| 3.0 | 23.39 ± 1.34 | 4.20 ± 0.40 | 2.52 ± 0.33 | 173.57 ± 10.87 | 4.53 ± 0.56 | |
| 4.0 | 25.00 ± 1.75 | 4.39 ± 0.32 | 2.46 ± 0.34 | 185.83 ± 10.89 | 4.52 ± 0.35 | |
| 5.0 | 26.31 ± 2.08 | 4.22 ± 0.36 | 2.95 ± 0.41 | 186.74 ± 9.02 | 6.20 ± 0.64 | |
| 6.0 | 26.98 ± 1.73 | 5.16 ± 0.60 | 3.36 ± 0.43 | 205.69 ± 11.26a | 7.45 ± 0.85b | |
| 7.0 | 27.60 ± 1.77 | 4.84 ± 0.49 | 4.16 ± 0.51b | 214.42 ± 17.00b | 8.78 ± 0.95b | |
| Isoprenaline (µg/kg) | ||||||
| 0 | 27.24 ± 1.96 | 3.56 ± 0.30 | 2.16 ± 0.32 | 180.56 ± 12.99 | 2.88 ± 0.17 | |
| 10 | 27.09 ± 2.14 | 3.41 ± 0.39 | 1.96 ± 0.30 | 190.54 ± 15.91 | 3.05 ± 0.19 | |
| 30 | 25.19 ± 1.82 | 3.20 ± 0.35 | 1.81 ± 0.34 | 198.06 ± 22.77 | 3.29 ± 0.20 | |
| 100 | 23.61 ± 1.67 | 3.20 ± 0.29 | 1.63 ± 0.31 | 190.10 ± 20.53 | 3.63 ± 0.22 | |
| 300 | 21.42 ± 1.50 | 3.14 ± 0.28 | 1.65 ± 0.34 | 209.81 ± 21.69 | 4.31 ± 0.37a | |
| 1000 | 20.53 ± 1.49a | 3.55 ± 0.29 | 1.53 ± 0.28 | 239.15 ± 21.72 | 4.83 ± 0.58b | |
| Doxazosin (µg/kg) | ||||||
| 0 | 24.34 ± 1.74 | 2.67 ± 0.19 | 2.28 ± 0.42 | 156.57 ± 21.20 | 3.87 ± 0.36 | |
| 10 | 23.32 ± 1.79 | 2.84 ± 0.17 | 2.64 ± 0.49 | 151.40 ± 21.95 | 4.21 ± 0.32 | |
| 30 | 22.23 ± 1.48 | 2.37 ± 0.17 | 2.58 ± 0.31 | 150.68 ± 23.74 | 4.09 ± 0.28 | |
| 100 | 21.82 ± 1.62 | 2.39 ± 0.28 | 2.77 ± 0.53 | 152.62 ± 21.27 | 4.18 ± 0.30 | |
| 300 | 22.34 ± 1.68 | 2.97 ± 0.32 | 3.02 ± 0.53 | 173.14 ± 21.43 | 4.82 ± 0.40 | |
| 1000 | 24.12 ± 2.05 | 3.15 ± 0.41 | 2.85 ± 0.43 | 176.14 ± 23.82 | 5.29 ± 0.88 | |
Significantly different (P < 0.05, Dunnett test; n = 12 to 15) compared with value before drug administration.
Significantly different (P < 0.01, Dunnett test; n = 12 to 15) compared with value before drug administration.
Effects of doxazosin on phenylephrine-induced increase in the vesical micturition pressure in anesthetized mice.
In the solvent group, phenylephrine (220 µg/kg) was administered intravenously for a total of 4 doses and consistently increased vesical micturition pressure with each dose. Doxazosin (0.1 to 1.0 mg/kg) dose-dependently inhibited (P < 0.01) the phenylephrine-induced increase in vesical micturition pressure in anesthetized mice (Table 2).
Table 2.
Effects of doxazosin on phenylephrine (220 µg/kg)-induced increases in the vesical micturition pressure of anesthetized mice
| Doxazosin | Phenylephrine-induced increase in vesical micturition pressure (cm H2O) | |
| (mg/kg) | Solvent | Doxazosin |
| 0.0 | 9.00 ± 1.27 | 8.70 ± 1.27 |
| 0.1 | 9.89 ± 1.46 | 6.55 ± 0.93 |
| 0.3 | 8.09 ± 1.06 | 3.58 ± 0.79a |
| 1.0 | 7.34 ± 1.11 | 0.43 ± 0.16a |
Significantly different (P < 0.01, Dunnett test; n = 12) compared with value before treatment.
Effects of phenylephrine, carbachol, isoprenaline, and doxazosin on blood pressure in anesthetized mice.
Intravenous administration of carbachol (7 µg/kg), doxazosin (1 mg/kg), and isoprenaline (1 mg/kg) significantly (P < 0.01) decreased systolic, diastolic, and mean arterial blood pressure in anesthetized mice, respectively (Table 3). Whereas carbachol and isoprenaline caused only transient reductions in blood pressure, doxazosin had a long-lasting hypotensive effect (Figure 2). The carbachol-associated percentage maximal decrease in systolic and mean arterial blood pressure did not differ from the effects of doxazosin or isoprenaline (Table 3). Phenylephrine (800 μg/kg) significantly (P < 0.01) and transiently increased systolic, diastolic, and mean arterial blood pressure in anesthetized mice (Figure 2 and Table 3).
Table 3.
Effects of isoprenaline, carbachol, doxazosin, and phenylephrine on systolic, diastolic, and mean arterial blood pressure (mean ± 1 SD [% change]) in anesthetized mice
| Systolic (mm Hg) | Diastolic (mm Hg) | Mean arterial (mm Hg) | |
| Control | 130.33 ± 2.77 | 102.91 ± 4.83 | 112.05 ± 3.80 |
| Isoprenaline | 77.59 ± 2.21a | 40.76 ± 1.26a | 53.04 ± 1.34a |
| (1 mg/kg) | (–40.19 ± 2.47) | (–59.90 ± 1.94) | (–52.20 ± 2.23) |
| Control | 124.26 ± 3.88 | 98.85 ± 6.16 | 107.42 ± 5.36 |
| Carbachol | 83.31 ± 2.08a | 36.11 ± 3.03a | 51.85 ± 2.28a |
| (7 µg/kg) | (–32.59 ± 2.31) | (–63.41 ± 2.19) | (–51.39 ± 1.70) |
| Control | 118.54 ± 2.86 | 95.09 ± 4.26 | 102.91 ± 3.54 |
| Doxazosin | 78.59 ± 3.21a | 45.83 ± 4.96a | 56.75 ± 3.96a |
| (1 mg/kg) | (–33.69 ± 2.20) | (–52.38 ± 3.53) | (–45.02 ± 2.65) |
| Control | 122.24 ± 4.19 | 97.01 ± 6.18 | 105.42 ± 5.42 |
| Phenylephrine | 195.76 ± 5.70a | 141.84 ± 4.78a | 159.82 ± 4.72a |
| (800 µg/kg) | (61.39 ± 7.11) | (49.43 ± 8.09) | (53.80 ± 7.29) |
Significantly (P < 0.01, paired t test; n = 8) compared with control value.
Discussion
We have reported for the first time the effects of a selective α1-adrenoceptor agonist (phenylephrine), nonselective β-adrenoceptor agonist (isoprenaline), nonselective muscarinic cholinoceptor agonist (carbachol), and selective α1-adrenoceptor antagonist (doxazosin) on bladder micturition function in anesthetized mice. In light of the well-known hypotensive or hypertensive effects induced by the 4 agents, we monitored blood pressure to clarify the influence of any drug-induced change in blood pressure on its direct effect on bladder micturition function.
Activation of β adrenoceptors mediates relaxation of urinary bladder smooth muscle and increases bladder compliance. The β3-adrenoceptor subtype appears largely to be involved in this regulation, at least in human beings.27,42 One study reported that the β3-adrenoceptor subtype was the predominant receptor mediating isoprenaline-induced urinary bladder smooth muscle relaxation in monkeys.38 However, β1 and β3 adrenoceptors mediate urinary bladder smooth muscle relaxation in dogs, and β2 and β3 adrenoceptors are associated with this response in rats.43 In the present study, isoprenaline increased micturition time and decreased vesical micturition pressure in a dose-dependent manner and had a nonsignificant effect on the intercontraction interval in anesthetized mice. Some authors concurrently observed the bladder pressure and urine flow rate waves in anesthetized mice and showed that micturition time (the duration of intraluminal pressure high-frequency oscillations) was matched with urine flow wave.35 In the present study during bladder filling at a constant rate, a decrease in vesical micturition pressure induced by isoprenaline is consistent with relaxation of the bladder detrusor through β adrenoceptor activation. Moreover, the prominent increase in micturition time that was induced by isoprenaline suggests that β-adrenoceptor activation of the lower urinary tract in anesthetized mice might reduce detrusor contraction during the voiding response. Whether β-adrenoceptor subtypes mediate isoprenaline-induced changes in bladder micturition function needs to be investigated further.
Muscarinic receptors are distributed widely throughout the body and play a key physiologic role in the urinary bladder. Results from a study using isolated urinary bladder tissues from many species including humans and those from in vivo experiments indicate that bladder contraction under physiologic conditions is mediated largely through M3 receptors.1,17 Carbachol produces contractile responses in isolated urinary bladder strips from mouse.8 A study using M3 receptor knockout mice33 clearly demonstrated that M3 receptors are the predominant receptor mediating carbachol-induced bladder contraction. In the present study, intravenous administration of carbachol increased micturition time and the intercontraction interval in anesthetized mice. At the highest dose of carbachol, the prolonged micturition time was 2 times that before drug administration, and voiding frequency was decreased from 22.45 to 16.79 times hourly, indicating a carbachol-induced increase in bladder capacity. The muscarinic receptor antagonists oxybutynin and tolterodine are the most widely used to treat overactive bladder. However, in anesthetized or conscious rats, oxybutynin and tolterodine only decreased vesical micturition pressure, whereas the affect on bladder capacity was negligible.3 Therefore, the carbachol-treated mouse model may be helpful for studying cystometrographic increases in bladder capacity, which occur in humans after the administration of muscarinic receptor antagonists.
Benign prostatic hyperplasia is frequent in elderly men, and the resultant bladder outlet obstruction has 2 components: static (related to cellular mass) and dynamic (related to prostatic smooth muscle tone).26 α1 Adrenoceptors are expressed mainly in the smooth muscle of the trigone, bladder base, bladder neck region, and prostate, and they play a prominent role in the regulation of bladder outlet resistance.27 α1-Adrenoceptor agonists produce a strong contraction of the isolated trigonal muscle of human bladder,7 and the contractile responses of human prostate to phenylephrine are mediated by α1A adrenoceptors.26 A recent histochemical study14 suggested that the mouse prostate had a similar innervation to that of humans and other laboratory animals and might be a suitable model for functional studies of human prostate. In the present study, phenylephrine (50 to 800 µg/kg) administered intravenously increased vesical micturition pressure in a dose-dependent manner, and micturition basal pressure was increased by phenylephrine only at 800 µg/kg in anesthetized mice. These results indicate that phenylephrine stimulated α1 adrenoceptors in the prostate and bladder neck region, resulting in upregulation of smooth muscle tone and thereby increasing bladder outlet resistance.
α1-Adrenoceptor antagonists are the first-line of pharmacologic management for benign prostatic hyperplasia and lower urinary tract syndrome.13 These agents relax the smooth muscle of the bladder neck and prostate and decrease bladder outlet resistance by blocking α1 adrenoceptors. In anesthetized dogs, α1-adrenoceptor antagonists decreased urethral resistance.6,22,32,37 However, α1-adrenoceptor antagonists (tamsulosin, prazosin, and bunazosin) lacked significant effects on urinary bladder function in anesthetized rats.36 Similarly, we did not find any influence by doxazosin on the bladder micturition function in anesthetized mice. However, doxazosin was able to antagonize phenylephrine-induced increase in vesical micturition pressure in anesthetized mice suggesting a usefulness of this model in the investigation of α1-adrenoceptor antagonists.
The administration of isoprenaline, carbachol, phenylephrine, or doxazosin is known to change blood pressure. This change, in turn, may influence bladder micturition function in anesthetized mice. Results of the present study showed that although doxazosin, carbachol, and isoprenaline had obvious hypotensive effects in anesthetized mice, only carbachol and isoprenaline affected bladder micturition function, indicating that changes in bladder micturition function induced by carbachol and isoprenaline were not related to their hypotensive effects. Phenylephrine transiently increased systolic, diastolic, and mean arterial blood pressure in anesthetized mice, whereas carbachol transiently decreased blood pressure. However, because both phenylephrine and carbachol had similar effects on micturition basal pressure and vesical micturition pressure in anesthetized mice, the phenylephrine-associated effects on micturation physiology were not due to the drug's hypertensive effect. In addition, a CNS-related influence was excluded because isoprenaline,5 carbachol,40 and phenylephrine15 reportedly are unable to pass through the blood–brain barrier.
Urethane has been used widely as a long-term anesthetic for small experimental animals,9,21 because it has less of a CNS-mediated effect on cardiovascular functions than do other anesthetics.23 In urethane-anesthetized animals, peripheral stimuli are still able to activate the CNS and produce reflexive changes in autonomic functions.10 However, the pontine urine storage center and pontine micturition center are controlled by the cerebral cortex as well. An important consideration in interpretation of our data is that the results were obtained from anesthetized mice.
In conclusion, activation of muscarinic cholinoceptors in anesthetized mice decreases voiding frequency and increases bladder capacity. In addition, activation of α1 adrenoceptors mainly increases vesical micturition pressure, whereas activation of β adrenoceptors decreases vesical micturition pressure and prolongs micturition time in anesthetized mice.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (no. 30940088) and Hebei Natural Science Foundation (C2009001070).
References
- 1.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, De Groat WC, Fryer AD. 2006. Muscarinic receptors: their distribution and function in body systems and the implications for treating overactive bladder. Br J Pharmacol 148:565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KE. 1993. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev 45:253–308 [PubMed] [Google Scholar]
- 3.Angelico P, Velasco C, Guarneri L, Sironi G, Leonardi A, Testa R. 2005. Urodynamic effects of oxybutynin and tolterodine in conscious and anesthetized rats under different cystometrographic conditions. BMC Pharmacol 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder LA, Nakamura Y, Kiss S, Neaten ML, Barrick S, Kanai AJ, Wang E, Ruiz G. 2002. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5:856–860 [DOI] [PubMed] [Google Scholar]
- 5.Borges N, Sarmento A, Azevedo I. 1999. Dynamics of experimental vasogenic brain oedema in the rat: changes induced by adrenergic drugs. J Auton Pharmacol 19:209–217 [DOI] [PubMed] [Google Scholar]
- 6.Breslin D, Fields DW, Chou TC, Marion DN, Kanem K, Vaughan ED, Felsen N. 1993. Medical management of benign prostatic hyperplasia: a canine model comparing the in vivo efficacy of α1-adrenergic antagonists in the prostate. J Urol 149:395–399 [DOI] [PubMed] [Google Scholar]
- 7.Caine M, Raz S, Zeigler M. 1975. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule, and bladder neck. Br J Urol 47:193–202 [DOI] [PubMed] [Google Scholar]
- 8.Choppin A. 2002. Muscarinic receptors in isolated urinary bladder smooth muscle from different mouse strains. Br J Pharmacol 137:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte B, Maggi CA, Parlani M, Lopez G, Manzini S, Giachetti A. 1991. Simultaneous recording of vesical and urethral pressure in urethane-anesthetized rats: effect of neuromuscular blocking agents on the activity of the external urethral sphincter. J Pharmacol Methods 26:161–171 [DOI] [PubMed] [Google Scholar]
- 10.Cross BA, Silver IA. 1963. Unit activity in the hypothalamus and the sympathetic response to hypoxia and hypercapnia. Exp Neurol 7:375–393 [DOI] [PubMed] [Google Scholar]
- 11.Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, Matsui M. 2005. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J Pharmacol Exp Ther 313:368–378 [DOI] [PubMed] [Google Scholar]
- 12.Fetscher C, Fleichman M, Schmidt M, Krege S, Michel MC. 2002. M3 muscarinic receptors mediate contraction of human urinary bladder. Br J Pharmacol 136:641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine SR, Ginsberg P. 2008. α-Adrenergic receptor antagonists in older patients with benign prostatic hyperplasia: issues and potential complications. J Am Osteopath Assoc 108:333–337 [PubMed] [Google Scholar]
- 14.Gray KT, Ventura S. 2005. Evaluation of the mouse prostate as a suitable model for the study of human prostate function. J Pharmacol Toxicol Methods 51:41–50 [DOI] [PubMed] [Google Scholar]
- 15.Guo TZ, Tinklenberg J, Oliker R, Maze M. 1991. Central α1-adrenoceptor stimulation functionally antagonizes the hypnotic response to dexmedetomidine, an α2-adrenoceptor agonist. Anesthesiology 75:252–256 [DOI] [PubMed] [Google Scholar]
- 16.Hampel C, Artibani W, Espuna Pons M, Haab F, Jackcon S, Romero J. 2004. Understanding the burden of stress urinary incontinence in Europe: a qualitative review of the literature. Eur Urol 46:15–27 [DOI] [PubMed] [Google Scholar]
- 17.Hegde SS. 2006. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol 147:S80–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igawa Y, Yamazaki Z, Takeda H, Hayakawa K, Akahane M, Ajisawa Y, Yoneyama T, Nishizawa O, Andersson KE. 1999. Functional and molecular biological evidence for a possible β3 adrenoceptor in the human detrusor muscle. Br J Pharmacol 126:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin DE, Milsom I, Hunskaar S, Reillly K, Kopp Z, Herschom S. 2006. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in 5 countries: results of the EPIC study. Eur Urol 50:1306–1315 [DOI] [PubMed] [Google Scholar]
- 20.Ishizuka O, Mattiasson A, Andersson KE. 1995. Urodynamic effects of intravesical resiniferatoxin and capsaicin in conscious rats with and without outflow obstruction. J Urol 154:611–616 [DOI] [PubMed] [Google Scholar]
- 21.Katafuchi T, Koizumi K. 1990. Fastigial inputs to paraventricular neurosecretory neurones studied by extra- and intracellular recordings in rats. J Physiol 421:535–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny BA, Read AM, Naylor AM, Greengrass PM, Carter AJ, Wyllie MG. 1994. Effect of α1-adrenoceptor antagonists on prostatic pressure and blood pressure in the anesthetized dog. Urology 44:52–57 [DOI] [PubMed] [Google Scholar]
- 23.Lalley PM. 1980. Inhibition of depressor cardiovascular reflexes by a derivative of γ-aminobutyric acid (GABA) and by general anesthetics with suspected GABA-mimetic effects. J Pharmacol Exp Ther 215:418–425 [PubMed] [Google Scholar]
- 24.Lepor H, Tang R, Shapiro E. 1993. The α-adrenoceptor subtype mediating tension of prostatic smooth muscle. Prostate 22:301–307 [DOI] [PubMed] [Google Scholar]
- 25.Longhurst PA, Levendusky M. 1999. Pharmacological characterization of β adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br J Pharmacol 127:1744–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall I, Burt RP, Chapple CR. 1995. Noradrenaline contractions of human prostate mediated by α1A-(α1c)-adrenoceptor subtype. Br J Pharmacol 115:781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel MC, Vrydag W. 2006. α1, α2, and β adrenoceptors in the urinary bladder, urethra, and prostate. Br J Pharmacol 147:S88–S119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milsom I, Abrams P, Cardozo L, Roberts RG, Thürff JW, Wein AJ. 2001. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. Br J Urol Int 87:760–766 [DOI] [PubMed] [Google Scholar]
- 29.Oshita M, Hiraoka Y, Watanabe Y. 1997. Characterization of β adrenoceptors in urinary bladder: comparison between rat and rabbit. Br J Pharmacol 122:1720–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Post JG, te Poele JAM, Oussoren YG, Sterwart FA. 1995a. Radiation tolerance of normal mouse bladders after intravesical chemotherapy. Radiother Oncol 34:30–38 [DOI] [PubMed] [Google Scholar]
- 31.Post JG, te Poele JAM, Oussoren YG, Sterwart FA. 1995b. The influence of intravesical photodynamic therapy on subsequent bladder irradiation tolerance. Radiother Oncol 37:124–130 [DOI] [PubMed] [Google Scholar]
- 32.Shibasaki M, Sudoh K, Inagaki O, Uchida W, Honda K. 1992. Effect of the optimal isomers of YM12617 on increased intraurethral pressure induced by phenylephrine in anesthetized dogs. J Auton Pharmacol 12:263–268 [DOI] [PubMed] [Google Scholar]
- 33.Stengel PW, Yamada M, Wess J, Cohen ML. 2002. M3-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol 282:R1443–R1449 [DOI] [PubMed] [Google Scholar]
- 34.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R. 2003. Prevalence and burden of overactive bladder in the United States. World J Urol 20:327–336 [DOI] [PubMed] [Google Scholar]
- 35.Streng TK, Talo A, Andersson KE, Santti R. 2005. A dose-dependent dual effect of oestrogen on voiding in the male mouse?. Br J Urol Int 96:1126–1130 [DOI] [PubMed] [Google Scholar]
- 36.Sudoh K, Inagaki O, Takenaka T. 1997. Effect of α1-adrenoceptor antagonists on urinary bladder function in urethane-anesthetized rats. Gen Pharmacol 28:521–524 [DOI] [PubMed] [Google Scholar]
- 37.Sudoh K, Tanaka H, Inagaki O, Asano M, Takenaka T. 1996. Effect of tamsulosin, a novel α1-adrenoceptor antagonist, on urethral pressure profile in anesthetized dogs. J Auton Pharmacol 16:147–154 [PubMed] [Google Scholar]
- 38.Takeda H, Yamazaki Y, Akahane M, Akahane S, Miyata H, Igawa Y, Nishizawa O. 2002. Characterization of β-adrenoceptor subtype in bladder smooth muscle in cynomolgus monkey. Jpn J Pharmacol 88:108–113 [DOI] [PubMed] [Google Scholar]
- 39.Vial C, Evans RJ. 2000. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol 131:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Chen WH, Zhu DM, She JQ, Ruan DY. 2007. Effects of carbachol on lead-induced impairment of the long-term potentiation–depotentiation in rat dentate gyrus in vivo. Food Chem Toxicol 45:412–418 [DOI] [PubMed] [Google Scholar]
- 41.Watanabe T, Perkash I, Constantinou CE. 1997. Modulation of detrusor contraction strength and micturition characteristics by intrathecal baclofen in anesthetized rats. J Urol 157:2361–2365 [PubMed] [Google Scholar]
- 42.Yamaguchi O, Chapple CR. 2007. β3-Adrenoceptors in urinary bladder. Neurourol Urodyn 26:752–756 [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki Y, Takeda H, Akahane M, Igawa Y, Nishizawa O, Ajisawa Y. 1998. Species differences in the distribution of β-adrenoceptor subtypes in bladder smooth muscle. Br J Pharmacol 124:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]