Abstract
This paper evaluates the modifications induced by ischemia and ischemia–reperfusion in mice after permanent or transient, respectively, ligation of the left coronary artery and establishes a correlation among the extent of ischemia, electrocardiograph features, and infarct size. The left coronary artery was ligated 1 mm distal from the tip of the left auricle. Histologic analysis revealed that 30-min ischemia (n = 9) led to infarction involving 9.7% ± 0.5% of the left ventricle, whereas 1-h ischemia (n = 9) resulted in transmural infarction of 16.1% ± 4.6% of the left ventricle. In contrast, 24-h ischemia (n = 8) and permanent ischemia (n = 8) induced similarly sized infarcts (33% ± 2% and 31.8% ± 0.7%, respectively), suggesting ineffective reperfusion after 24-h ischemia. Electrocardiography revealed that ligation of the left coronary artery led to ST height elevation (204 compared with 14 μV) and QTc prolongation (136 compared with 76 ms). Both parameters rapidly normalized on reperfusion, demonstrating that electrocardiography was important for validating correct ligation and reperfusion. In addition, electrocardiography predicted the severity of the myocardial damage induced by ischemia. Our results show that electrocardiographic changes present after 30-min ischemia were reversed on reperfusion; however, prolonged ischemia induced pathologic electrocardiographic patterns that remained even after reperfusion. The mouse model of myocardial ischemia–reperfusion can be improved by using electrocardiography to validate ligation and reperfusion during surgery and to predict the severity of infarction.
Abbreviation: AAR, area at risk; LCA, left coronary artery
Understanding the mechanisms involved in the progression of heart failure, including the formation of fibrous scar and cardiomyocyte loss, remains imperative for development of new therapies. For this reason, diverse experimental models, such as cultured cardiac cells, isolated hearts, and animal models, have been created to mimic human myocardial infarction and heart failure.7 The ready availability of laboratory mice and ability to generate genetic modifications in them has prompted the emergence of specific mouse surgical models.2 Of these, cardiac ischemia–reperfusion in mice is induced by transient ligation of the left coronary artery (LCA) and remains one of the most representative models of the ischemic cell death typical in humans.12 However, this model is associated with variability in infarct size of 20% to 90% of the entire left ventricle,8 thereby requiring a large number of mice for each study. Typically, variability between different laboratories was related to modifications to the procedure and different times of analysis, but high variability (infarct sizes varying from 20% to 30% of the left ventricle) has been reported even within the same experimental group.3,4
Standardization of key features during surgery could reduce variability markedly, thereby facilitating comparisons of results. To this end, one group demonstrated that the consistent ligation of the LAD immediately below the left auricular level ensured reproducible infarct size (27% ± 1.5% of the left ventricle after 3-wk ischemia).15 Another important source of variability arises from the configuration of the LCA and the difficulty in detecting it, which often lead to blind placement of the ligature.1 Here we describe a surgical procedure for LCA ligation in mice that takes advantage of electrocardiography to verify correct ligation and reperfusion and reduce variability in infarct size. The use of electrocardiography perhaps could replace subjective analysis of heart pallor, thereby eliminating incorrect evaluations. In addition, we used this model to evaluate the modifications induced by ischemia–reperfusion and established a correlation among extent of ischemia, pathologic changes in the electrocardiogram, and infarct size.
Materials and Methods
Animals.
The mice were housed and used in accordance with the Guide for the Care and Use of Laboratory Animals,5 and all experiments were approved by the Institutional Ethical Committee of Institute of Cellular Biology and Pathology ‘Nicolae Simionescu’(Bucharest, Romania). Male Rap mice (n = 40; age, 8 to 12 wk; weight, 25 to 30 g) were divided into 2 groups: 1) a myocardial infarction group (n = 34) that underwent 30 min (n = 9), 1 h (n = 9), or 24 h (n = 8) of ischemia followed by reperfusion until day 7 or 7 d of ischemia (permanent ischemia, n = 8) and 2) a sham-operated control group (n = 6). An additional group of 8 mice that underwent LCA ligation was used to determine the area at risk (AAR).
Coronary artery ligation.
The procedure was carried out under aseptic conditions. The room and surgery table were sterilized under UV light at the beginning of the day and surgical instruments were sterilized in a dry sterilizer (Germinator 500, WPI, Berlin, Germany). Mice were anesthetized with chloral hydrate (400 mg/kg IP) and maintained in a dark box until the anesthetic achieved its effect. Surgical anesthesia was verified as a lack of response to toe pinch. The chest and neck regions of the mice were shaved and cleaned with 70% ethanol and the mice placed in a supine position under a stereomicroscope. During surgery, body temperature was maintained at 37 °C by using a temperature controller by means of a rectal probe (WPI). Mechanical ventilation was achieved by orotracheal cannulation of the trachea with a 20-gauge blunt needle attached to a mouse ventilator (28025 Ugo Basile, Comerio, Italy) by means of a plastic Y connector.4 Tidal volume and ventilation rate were adjusted according to the mouse's weight (for example, a ventilation rate of 133 breaths per minute and tidal volume of 0.2 mL for a 30-g mouse). Electrocardiography electrodes were connected to the limbs through small needles inserted subcutaneously; gel was used to increase the electrical contact between needles and electrodes.
For LCA ligation, the skin was incised, the layers of the chest muscles were separated, and a thoracotomy was made in the fourth intercostal space. The pericardium was removed, and the LCA was ligated 1 mm distal from the tip of the left auricle by using 7-0 polypropylene suture. A short 2-0 silk thread was inserted between the knot and LCA to protect the vessel from bleeding. The modifications induced by LCA ligation were monitored by electrocardiography. At the end of the procedure, the intercostal space was closed by joining together the 2 neighboring ribs and then the muscles and skin were sutured separately. Voluntary respiration was restored by gently removing the intubation needle. Mice were allowed to recover on cotton bedding and maintained under a heating lamp to prevent hypothermia. When completely recovered from anesthesia, mice were housed individually, with fresh water and food provided ad libitum under standard laboratory conditions. Mice were monitored postoperatively for behavior changes indicative of pain or distress. Most mice showed no signs suggestive of pain, such as reluctance to move, decreased appetite, vocalization, and self-mutilation. Two mice in the permanent ischemia group were reluctant to move 1 day after surgery and consequently were removed from analysis. Surgical lesions healed with no visible infection on the surface or within the body by the end of the experiment; therefore, there was no need for antibiotic treatment. Mice in the myocardial infarction group with 24-h ischemia underwent repeat surgery at the time of reperfusion.
Electrocardiography.
Three-lead electrocardiography was performed (PowerLab/4SP System and ML136 Animal Bio Amp module, ADInstruments, Spechbach, Germany). A recording time of 2 min was considered sufficient to provide a representative view of heart function (approximately 800 events). The data recorded were submitted to a 50-Hz notch filter with an automatic setting determined by the software (ECG module of Chart 5.5). Because in rodents the T wave is very close to QRS complex,19 the end of QRS complex of each signal was considered to be the point at which the T wave started and the ST segment was measured. Therefore, ST height represented the distance (in μV) from the baseline to the line where the T wave started.
Histologic analysis.
AAR was determined by retrograde perfusion of Evans blue dye (2%) into the abdominal aorta immediately after LCA ligation. The heart was excised immediately and sectioned in 4 transverse slices. AAR was calculated from 4 heart slices for each mouse and expressed as the mean percentage of the unstained area in the left ventricle. To evaluate histologic changes in heart muscle, sections from 8 different levels of the heart were stained with hematoxylin and eosin and Masson trichrome according to routine histologic protocols.
Infarct size was determined by estimation of infarct midlines on Masson-stained sections obtained at 8 levels of the heart.16 Briefly, the left ventricular myocardial midline was drawn between the epicardial and endocardial surfaces and its length was measured as the midline circumference. The midline infarct length was taken as the midline of the length of infarct that included more than 50% of the wall thickness. Infarct size measurement of the heart was calculated by dividing the sum of midline infarct lengths by the sum of midline circumferences from all sections and multiplying by 100%. The mean infarct size for each group was obtained.
Statistics.
All data were expressed as mean ± 1 SD. Electrocardiography parameters were compared by using paired t tests (Excel Office 2007, Microsoft, Redmond, WA) with 2-tailed distribution. A P value of less than 0.05 was considered statistically significant.
Results
Electrocardiography to validate LCA ligation and reperfusion.
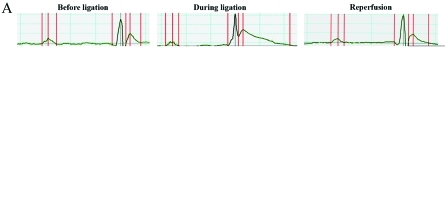
Electrocardiography of intubated mice (Figure 1 A) was used as a control for the modifications induced by ischemia and reperfusion. LCA ligation immediately induced a progressive increase in ST height and prolongation of the QTc segment (Figure 1 A). ST height increased by 14-fold (204 μV during ligation compared with 14 μV before ligation; Figure 1 B) and the QTc doubled (that is, 136 ms during ligation compared with 76 ms before ligation), due to increased T duration. These changes appeared within the first 5 min of ischemia, thereby confirming that LCA ligation was performed correctly. At 5 min after reperfusion, the ST height and QTc were within normal ranges (Figure 1 A), confirming appropriate reperfusion. Other ECG parameters (QRS interval, RR interval, and R amplitude) as well as heart rate did not differ significantly from the control values (Figure 1 B). Therefore, 2 traditional indicators of acute myocardial ischemia (ST height elevation and QTc prolongation) confirmed that electrocardiography was an appropriate tool to validate LCA ligation and reperfusion.
Figure 1.
Validation of LCA ligation and reperfusion by using electrocardiography. (A) Representative composite electrocardiographs from intubated mouse before LCA ligation, during ligation, and after releasing the ligation (reperfusion). Raw tracings are shown below each composite view. (B) Electrocardiographic parameters as calculated from the composite recordings in panel A.
Histologic changes induced by 30-min ischemia and ischemia–reperfusion.
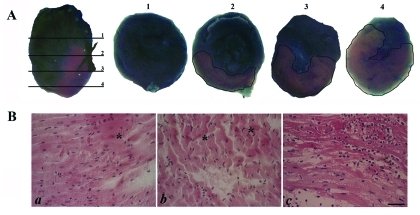
AAR induced by LCA ligation 1 mm distal from the tip of the left auricle was determined as the percentage of Evans blue-unstained tissue area in the total left ventricular region. AAR varied with the section level and increased as the level moved down (toward the apex; Figure 2 A), specifically from 25% at level 2 to 75% at level 4. A mean AAR value of 40% of the left ventricle was obtained by calculating the mean of the values obtained in the 4 heart slices from each of 8 mice.
Figure 2.
Histologic modifications induced by 30-min ischemia. (A) AAR induced by LCA ligation, as determined by Evans blue assay. Evans blue stain was visible throughout the heart, as well as in the sections numbered from 1 through 4. (B) Myocardial changes (a, b) immediately after 30-min ischemia (*, contraction band necrosis) and (c) after 24 h of reperfusion (*, stretching of myocardium). Bar, 50 μm.
The histologic changes induced immediately after 30-min ischemia and after 24 h of reperfusion were evaluated through hematoxylin and eosin staining (Figure 2 B). Compared with results from control hearts, which underwent surgical intervention for LCA ligation without tightening of the ligature (data not shown), 30-min ischemia induced contraction band necrosis and constricted cardiac cells (Figure 2 B). After 24 h of reperfusion, myocardial fiber necrosis was evident (Figure 2 B). The cytoplasm of some myocytes became more eosinophilic, and these cells lost their transversal striations and even nuclei. Furthermore, infiltration of inflammatory cells between cardiomyocytes was noted, indicating acute inflammation (Figure 2 B).
Correlation between duration of ischemia and infarct size.
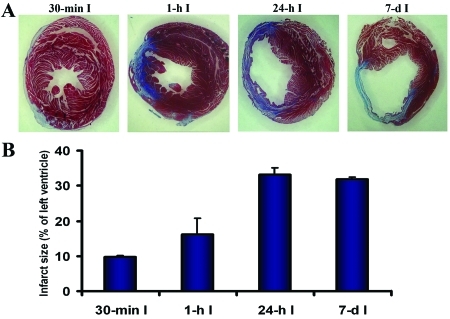
We questioned whether duration of ischemia and infarct size were correlated in the mouse model. Mice underwent ischemia for 30 min, 1 or 24 h, or 7 d followed by Masson trichrome staining on day 7 (Figure 3 A). Infarct severity progressed from a subepicardial infarct affecting 9.7% ± 0.5% of the left ventricle after 30-min ischemia to a transmural infarct affecting 16.1% ± 4.6% when ischemia lasted for 1 h (Figure 3 B). However, the infarct sizes produced by 24-h ischemia and 7-d ischemia were similar (33% ± 2% versus 31.8% ± 0.7%) and were nearly as large as AAR, indicating that reperfusion after 24-h ischemia was ineffective.
Figure 3.
Myocardial modifications induced by various periods of ischemia. (A) Masson staining showing a subepicardial infarct involving approximately 10% of the left ventricle that was induced by 30-min ischemia (I; far left) and transmural infarcts that increased in size with increasing ischemia duration. The sections were all obtained from the same level of the heart. (B) Quantification of infarct sizes induced by increasing periods of ischemia followed by reperfusion until day 7 and by 7-d ischemia.
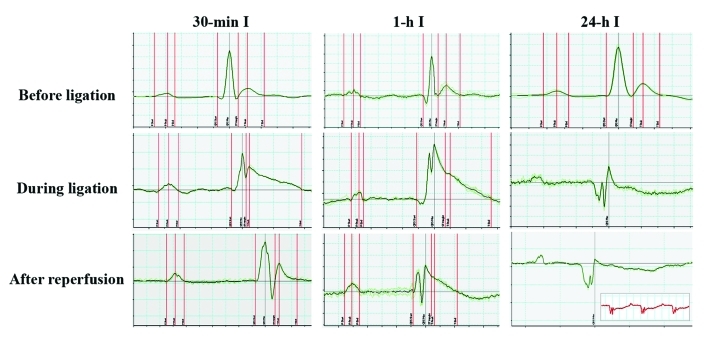
In addition, we used electrocardiography to evaluate the effect of increasing the duration of the ischemic period on the severity of myocardial modifications (Figure 4 A). Whereas 30-min ischemia resulted in reversible changes in the electrocardiogram (that is, abnormalities disappeared after reperfusion), 1-h ischemia induced more severe modifications, which were not completely abrogated after reperfusion. Thus, electrocardiographic patterns indicated various irreversible changes in the myocardium after 1-h ischemia. Accordingly, 24-h ischemia led to severe injury in the myocardium, which was associated with dramatic changes in the electrocardiogram (inverted T waves, pathologic Q waves, and microvoltage abnormalities). These changes did not reverse when the ligature was released and were similar to those noticed after 7-d ischemia (Figure 4, inset), indicating ineffective reperfusion after prolonged ischemia. In other words, ischemia for 24 h induced the same cardiac effects as 7-d (that is, chronic) ischemia. Taking together these findings, the electrocardiographic profile corroborates the histologic modifications and predicts the severity of the myocardial modifications induced by ischemia in the mouse model of LCA ligation and reperfusion.
Figure 4.
Electrocardiograms at 7 d after 30-min, 1-h, and 24-h myocardial ischemia. Note that 24-h ischemia induced dramatic changes in the electrocardiogram which did not return to baseline after the ligature was released; these changes were similar to those observed after 7-d ischemia (inset).
Discussion
Since the first description of the mouse model of myocardial ischemia–reperfusion,13 various modifications have been made to improve the procedure. With few exceptions,4,14 these publications did not offer detailed descriptions, thereby complicating reproduction of the model. In addition, these studies typically used heart paleness to confirm ligation of the LCA,1,11,17 which practice contributed to the variability of the results.
Here we describe the surgical procedures for myocardial ischemia–reperfusion in adult mice. Our report stresses the importance of using electrocardiography during the procedure, as a rapid and inexpensive tool to validate LCA ligation and reperfusion. Three-lead electrocardiography is an easy monitoring system that adds minimal complexity to the procedure but offers great advantage by validating the procedure and thereby reducing variability in infarct size. Although a 12-lead system generally provides more detailed characterization of cardiac electrical activity, in the particular case of experimentally induced LCA ischemia, in which the precise location of the arterial occlusion is known, multilead ST segment analysis is unnecessary. In addition, 3-lead electrocardiography offers several advantages over 12-lead systems, including reduced signal noise in small animals, more rapid equipment set-up, and easier preparation of subjects.5
Increased ST height is a classic hallmark of acute myocardial ischemia10 that has been confirmed in several studies.3,14 Together with QTc prolongation, ST height elevation can be used to confirm ischemia associated with LCA ligation in mice, thus making the procedure more accurate. In addition, we here describe morphologic changes of myocardium that are induced by ischemia and ischemia–reperfusion and the correlations between the duration of ischemia and infarct size. Typically, 30-min ischemia led to a subepicardial infarct that affected less than 10% of the left ventricle. As the duration of ischemia increased, the size of the transmural infarcts increased, as confirmed by massive accumulation of collagen.
The mouse model of cardiac ischemia–reperfusion offers several advantages over other models, such as cryoinjury and chronic cardiac ischemia. The possibility of reperfusion makes this mouse model more clinically relevant (as compared with nonreperfused myocardial ischemia models), because reperfusion always occurs in vivo prior to therapeutic intervention. Although reperfusion is the routine therapeutic intervention to prevent progression of the infarct, additional cell death and scar formation are known to occur in the border zone, due to the process known as ‘reperfusion injury.’18 Although technically challenging, the ischemia–reperfusion model offers an opportunity to study the effect of reperfusion after ischemia, which is a known contributor to myocardial damage. In addition, this model enables researchers to control the ischemic period to better fit the aim of the study. Generally, variations in infarct severity resulted from proximal versus distal ligation of the coronary artery.1 For example, constriction of the coronary artery near its origin produced large myocardial infarcts with a short-term survival.9 In comparison, the area of the left ventricle that became infarcted varied when the occlusion was performed below the tip of the left auricle.4,11,13 With our model, different sizes of infarct can be obtained by varying the duration of ischemia, whereas the area at risk remains constant. To decrease the variations associated with the surgical procedure, we always ligated the LCA 1 mm distal from the tip of the left auricle. To further limit any potential variability, we used intraoperative electrocardiography. Without adding any major disadvantage in regard to the procedure, this tool offers the main advantage of validating LCA ligation and replaces the common procedure of blind identification of the coronary artery and confirmation through subjective assessment of heart pallor.
In conclusion, the clinically relevant experimental mouse model of myocardial ischemia–reperfusion can be improved by using electrocardiography during surgery to verify key components of the model, such as LCA ligation and reperfusion. This model can be adapted for use in diverse applications, from molecular signaling research to cellular transplantation studies, by varying the duration of the LCA ligation period.
Acknowledgments
We would like to thank Dr Emanuel Dragan, Ana Manole, and Nae Safta for their technical assistance. The work was supported by CNCSIS –UEFISCSU, PNII-IDEA Program, 250/2007
References
- 1.Ahn D, Cheng L, Moon C, Spurgeon H, Lakatta EG, Talan MI. 2004. Induction of myocardial infarcts of a predictable size and location by branch pattern probability-assisted coronary ligation in C57BL/6 mice. Am J Physiol Heart Circ Physiol 286:H1201–H1207 [DOI] [PubMed] [Google Scholar]
- 2.Conci E, Pachinger O, Metzler M. 2006. Mouse models for myocardial ischaemia/reperfusion. J Cardiol (Austria) 13:239–244 [Google Scholar]
- 3.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. 2006. Systematic evaluation of novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol 291:H2533–H2540 [DOI] [PubMed] [Google Scholar]
- 4.Fisher SG, Marber MS. 2002. An in vivo model of ischaemia–reperfusion injury and ischaemic preconditioning in the mouse heart. J Pharmacol Toxicol Methods 48:161–169 [DOI] [PubMed] [Google Scholar]
- 5.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 6.Kligfield P. 2001. Value and limitations of 12-lead ECG monitoring. Datex–Ohmeda Clin Window Web J 6:1–6 [Google Scholar]
- 7.Klocke R, Tian W, Kuhlmann MT, Nikol S. 2007. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 74:29–38 [DOI] [PubMed] [Google Scholar]
- 8.Leri A, Kajstura J, Anversa P. 2005. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev 85:1373–1416 [DOI] [PubMed] [Google Scholar]
- 9.Li B, Li Q, Wang X, Jana KP, Redaelli G, Kajstura J, Anversa P. 1997. Coronary constriction impairs cardiac function and induces myocardial damage and ventricular remodeling in mice. Am J Physiol 273:H2508–H2519 [DOI] [PubMed] [Google Scholar]
- 10.Li RA, Leppo M, Miki T, Seino S, Marbán E. 2000. Molecular basis of electrocardiographic ST-segment elevation. Circ Res 87:837–839 [DOI] [PubMed] [Google Scholar]
- 11.Lutgens E, Daemen MJ, de Muinck ED, Debets J, Leenders P, Smits JF. 1999. Chronic myocardial infarction in the mouse: cardiac structural and functional changes. Cardiovasc Res 41:586–593 [DOI] [PubMed] [Google Scholar]
- 12.Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Entman ML. 1999. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol 277:H660–H668 [DOI] [PubMed] [Google Scholar]
- 13.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. 1995. Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269:H2147–H2154 [DOI] [PubMed] [Google Scholar]
- 14.Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, Michael LH, Entman ML. 2000. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol 278:H1049–H1055 [DOI] [PubMed] [Google Scholar]
- 15.Salto-Tellez M, Lim SY, El Oakley RM, Tang TPL, ALmsherqi ZA, Lim SK. 2004. Myocardial infarction in the C57BL/6J mouse: a quantifiable and highly reproducible experimental model. Cardiovasc Pathol 13:91–97 [DOI] [PubMed] [Google Scholar]
- 16.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. 2007. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102:2104–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. 2004. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16:349–360 [DOI] [PubMed] [Google Scholar]
- 18.Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. 2006. Increased inflammatory response and neovascularization in reperfused versus nonreperfused murine myocardial infarction. Cardiovasc Pathol 15:83–90 [DOI] [PubMed] [Google Scholar]
- 19.Wehrens XH, Kirchhoff S, Doevendans PA. 2000. Mouse electrocardiography: an interval of 30 years. Cardiovasc Res 45:231–237 [DOI] [PubMed] [Google Scholar]