Abstract
Fibromyalgia has been recognized as a central pain disorder with evidence of neuroanatomic and neurophysiologic alterations. Previous studies with techniques of noninvasive brain stimulation--transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS)--have shown that these methods are associated with a significant alleviation of fibromyalgia-associated pain and sleep dysfunction. Here we sought to determine whether a longer treatment protocol involving 10 sessions of 2 mA, 20 min tDCS of the left primary motor (M1) or dorsolateral prefrontal cortex (DLPFC) could offer additional, more long-lasting clinical benefits in the management of pain from fibromyalgia. Methods: Forty-one women with chronic, medically refractory fibromyalgia were randomized to receive 10 daily sessions of M1, DLPFC, or sham tDCS. Results: Our results show that M1 and DLPFC stimulation both display improvements in pain scores (VAS) and quality of life (FIQ) at the end of the treatment protocol, but only M1 stimulation resulted in long-lasting clinical benefits as assessed at 30 and 60 days after the end of treatment. Conclusions: This study demonstrates the importance of the duration of the treatment period, suggesting that 10 daily sessions of tDCS result in more long lasting outcomes than only five sessions. Furthermore, this study supports the findings of a similarly designed rTMS trial as both induce pain reductions that are equally long-lasting.
Keywords: Transcranial direct current stimulation, brain polarization, healthy subjects, fibromyalgia, pain
INTRODUCTION
Fibromyalgia is a chronic pain syndrome characterized by neuropathic tenderness in all four quadrants of the body in association with sleep alterations, mood dysfunction, musculoskeletal stiffness, and chronic fatigue (1). Recent evidence suggests that patients with fibromyalgia perceive pain differently from healthy individuals (2). Interestingly, although patients with fibromyalgia have similar detection thresholds for electrical, pressure, and thermal stimuli as compared to healthy controls, pain studies reveal that the pain threshold is significantly lower in fibromyalgia (3–5). In fact, converging evidence from neuroimaging (6) and electroencephalography (EEG) (7,8) suggest that fibromyalgia is a condition associated with brain dysfunction, and so fibromyalgia is now recognized as a central pain syndrome (2). Thus, therapeutic approaches should target the central nervous system.
Transcranial direct current stimulation (tDCS) has come to the forefront in the approach to novel treatments for fibromyalgia as this technique of noninvasive brain stimulation has been shown to significantly modulate the perception of sensory tactile, painful, and emotional stimuli (9–11). Furthermore, tDCS as well as other methods of invasive and noninvasive primary motor cortex (M1) stimulation -- including epidural motor cortex stimulation (MCS) and repetitive transcranial magnetic stimulation (rTMS)-- have all been shown to be effective in the alleviation of chronic pain (12). These methods of M1 stimulation are believed to mediate analgesic effects by modulating M1-thalamic inhibitory connections among other cortico-cortical and cortical-subcortical projections involved in pain processing pathways.
Indeed, previous studies by our group have shown that 5 daily sessions of M1 TDCS (2mA, 20 min) can induce significant improvements with respect to pain and sleep parameters in patients with fibromyalgia as compared to sham and dorsolateral prefrontal cortex (DLPFC) stimulation (13,14). Although a regimen of 5 daily sessions of M1 TDCS has already been shown to induce moderately long-lasting effects (up to 3 weeks after the end of stimulation) in our previous study with 32 patients, now we sought to determine the efficacy of a longer treatment protocol. Here we report the results obtained by performing 10 daily sessions of either sham stimulation or M1 or DLPFC tDCS (2 mA, 20 min) in a series of 41 patients with chronic, medically refractory fibromyalgia.
METHODS
We conducted a single-center, doubled-blinded, randomized, sham-controlled trial to determine the effect of ten daily sessions of tDCS on pain in women with fibromyalgia. This study conformed to the ethical standards of the Declaration of Helsinki and was approved by the local institutional ethics committee.
Forty-one women (mean age of 54.8 ± 9.6 years, mean ± SD) with chronic, medically refractory fibromyalgia (diagnosed according to the ACR 1990 criteria) were recruited to participate in this study. Patients were selected from a specialized outpatient service. Subjects were regarded as suitable to participate in this study if they fulfilled the following criteria: 1) mean pain score of at least 4 on the visual analog scale (VAS) in the two weeks preceding the clinical trial, 2) sum of tender points score equal to or greater than 11, 3) no clinically significant or unstable medical, neuropsychiatric, or other chronic pain disorder (as assessed by the patient’s clinician); 4) not pregnant or lactating; 5) no history of substance abuse or dependence; 6) no use of central nervous system-effective medication in the past 1 month and 7) no history of brain surgery, tumor, or intracranial metal implantation. Patients were carefully evaluated by a licensed rheumatologist before entry into the trial. All study participants provided written, informed consent.
Experimental design
All subjects participated in a baseline observation period of two weeks duration during which baseline parameters were established. The patients were then randomized in a 1:1:1 ratio to receive 10 sessions of either sham tDCS, active tDCS of left M1, or active tDCS of left DLPFC. Randomization was performed using the order of entrance in the study and a previous randomization list generated by a computer using blocks of six (for each six patients, two were randomized to each group) in order to minimize the risk of unbalanced group sizes. The subjects then participated in follow-up assessment at 30 and 60 days after the final tDCS treatment session. Subjects remained blinded to treatment group throughout the study, and blinded raters carried out all assessments.
Transcranial direct current stimulation (tDCS)
tDCS is based on the application of low amplitude direct current to the scalp via two relatively large anode and cathode electrodes (15,16). Although there is substantial shunting at the scalp, a sufficient current penetrates the skull and enters the brain to modify transmembrane neuronal potentials (17,18). In this way, tDCS influence the excitability level of the underlying neurons, such that anodal stimulation generally increases cortical excitability, while cathodal stimulation decreases it (19–21).
In this study, patients received 10 daily sessions (Mon–Fri, 2 weeks) of either sham stimulation or anodal stimulation of left primary motor cortex (M1) or left dorsolateral prefrontal cortex (DLPFC). A pair of thick (.3 cm) rectangular surface sponge electrodes (5cm × 7cm; 35 cm2) were soaked in saline and applied to the scalp at the desired sites of stimulation. Rubber bandages were used to hold the electrodes in place for the duration of stimulation. For anodal stimulation of M1, the anode electrode was placed over C3 according to the 10–20 system for EEG electrode placement (referred in the text as “M1”). The reference cathode electrode was placed over the supraorbital area on the opposite side. Similarly, for anodal stimulation of DLPFC (referred in the text as “DLPFC”), the anode electrode was placed over F3, as confirmed reliable by neuronavigational techniques (22,23), and the cathode electrode was placed over the contralateral supraorbital area. For the active tDCS conditions, a constant current of 2 mA was applied for 20 min; tDCS delivered at a level of 2 mA has been shown to be safe for use in healthy volunteers (24) and patients with varying neurological disorders (25). For SHAM stimulation, the electrodes were placed in the same positions as for anodal M1 stimulation, but the stimulator was turned off after 30 s of stimulation as previously described as being a reliable method of blinding (26), indeed, extensive data from our laboratory suggests that tDCS in healthy subjects is comparable in sensation to sham stimulation regardless of the area stimulated. Finally it should be noted that although M1 is relatively close to DLPFC site given the electrode sizes, this design has shown to be adequate as we have compared cognitive performance (as indexed by working memory tasks) during stimulation of M1 and DLPFC and found differential results, only DLPFC stimulation resulted in a significant effect on working memory performance in healthy subjects (27) and patients with Parkinson’s disease (28).
The rationale for the choice of stimulation sites was the following: M1 stimulation via tDCS, rTMS, and epidural stimulation have all been associated with reduced pain in patients with chronic pain syndromes (29,30). DLPFC was chosen as an area of stimulation because the DLFPC is a critical component of the neural circuit involved in processing the cognitive and emotional aspects of pain (31), and because our previous study suggested that anodal stimulation of this area may be able to modulate the emotional processing of pain (11). We chose to stimulate the left M1 and DLPFC regions in keeping with previous studies (13,27,32), yet it should be noted that at least for the DLPFC, non-invasive brain stimulation may result in different modulatory and cognitive-emotional effects depending on the hemisphere used (33,34). Although we did not find significant results for DLPFC stimulation in our previous study (13), the reason might be the number of sessions – i.e., a longer duration of tDCS therapy might be necessary to induce significant effects with stimulation of DLPFC. Although we acknowledge that other studies prefer the use of noncephalic reference electrodes for tDCS to avoid confounding biases (33,36), we placed the reference electrode at the supraorbital cortex under all conditions of stimulation as in our previous tDCS studies with fibromyalgia (13,14).
Clinical assessment
Pain was measured with Visual Analogue Scale for Pain (VAS-pain). Tender point scores were assessed to identify changes in pain quality or location. Quality-of-life and other domains of fibromyalgia were measured using the Fibromyalgia Impact Questionnaire (FIQ) (online at http://www.myalgia.com/FIQ/FIQ.htm) (37). Psychiatric symptoms were assessed with the Beck Depression Inventory (BDI), IDATE state-trait anxiety inventory for anxiety, and Geriatric Depression Scale. Cognition and safety were evaluated by the Mini-Mental State Examination. Finally, we monitored adverse events by asking patients, after each session of stimulation and during the follow-up period, whether they had experienced any adverse event and the relationship of these events to TDCS.
Statistical analysis
Analyses were done with STATA statistical software (version 9.1, Cary, NC, US). In order to compare the effects of stimulation on pain levels, we performed a mixed ANOVA model in which the dependent variable was the level of pain and the independent fixed variables were treatment (baseline, post-treatment, follow-up 1 and follow-up 2), group of stimulation (M1, DLPFC, and sham) and the interaction term group vs. treatment. In addition, we added the random variable subject ID in order to account for the within subjects variability. When appropriate, post-hoc comparisons were carried out using Bonferroni correction for multiple comparisons. We then performed similar models for the other variables. For the main outcome – pain as indexed by VAS – we calculated the mean of the first 3 days for the baseline evaluation and the mean of the last three days for the post-treatment assessment as to give a more reliable assessment as pain has an important variation. Finally, the time points are defined as: baseline, T1 (immediately after the 10 treatment sessions), T2 (30 days after treatment) and T3 (60 days after treatment).
Unless stated otherwise, all results are presented as means and standard deviation, and statistical significance refers to a p value < 0.05.
RESULTS
Fourteen patients were randomized to M1 and sham group and 13 patients to DLPFC group. There were no significant baseline differences in demographics, baseline clinical and pain characteristics (see table 1). There were no dropouts. Patients tolerated the tDCS treatment well. Adverse effects were minor and uncommon – such as skin redness and tingling - and distributed equally across groups of stimulation.
Table 1.
Demographic and baseline clinical characteristics.
| DLPFC | M1 | Sham | |||||
|---|---|---|---|---|---|---|---|
| mean | sd | mean | sd | mean | sd | p-value | |
| Age | ns | ||||||
| Duration of disease | ns | ||||||
| BMI | ns | ||||||
| Pain (VAS) | ns | ||||||
| FIQ | ns | ||||||
| BDI | ns | ||||||
| GDS | ns | ||||||
| IDATE | ns | ||||||
| MMSE | ns |
Demographic and baseline clinical characteristics: Age (years), duration of disease (years), BMI- body mass index (kg/m2), VAS-Pain Score (0= no pain, 10= worst pain), FIQ-Fibromyalgia Impact Questionnaire (0= no impact, 100= worst possible), BDI- Beck Depression Inventory (0= no depression, 29–63=severe depression), GDS- Geriatric Depression Scale (0= no depression, 10–19= mild depression, 20–30= severe depression), IDATE- Anxiety Metric: (0–30= low anxiety, 31–49= medium anxiety, 50–80= high degree of anxiety), MMSE- Mini-Mental Status Exam (scores of 23/30 and lower indicate relative cognitive impairment). ns indicates not significant (one-way ANOVA comparing the three groups).
Pain assessment
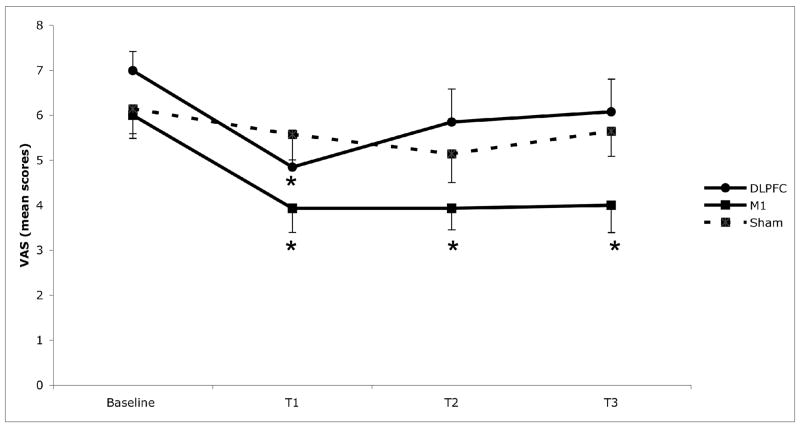
In order to assess pain (as indexed by VAS), we initially assessed the interaction term time vs. group of stimulation for the main model. This term was statistically significant (F(9,114)=3.05, p=0.0026); suggesting, therefore, that pain changed differently according to the group of stimulation. In fact, post-hoc comparisons showed that there were no significant changes in pain scores for the sham group (when comparing baseline vs. T1, T2, and T3). However, for M1 group, there was a significant difference between baseline vs. T1 (p=0.012), baseline vs. T2 (p=0.02) and baseline vs. T3 (p=0.03), indicating a significant pain reduction that lasted up to 2 months after the end of stimulation (see figure 1). Although there was also a significant effect for DLPFC group when comparing baseline vs. T1 (p=0.035), there was no difference between baseline vs. T2 (p=0.17) and baseline vs. T3 (p=0.27). Suggesting that although DLPFC induced a significant pain reduction, this effect was not long lasting. In fact, we then conducted additional models with each condition of stimulation separately to assess time effect and our results were confirmed: only the model for M1 group showed a significant time effect (F(3,52)=4.07, p=0.011). For the DLPFC and sham groups, there was no significant time effect (p>0.19 for both groups).
Figure 1.
Mean pain scores associated with the three conditions of stimulation: left M1 (primary motor cortex); left DLPFC (dorsolateral prefrontal cortex); and sham tDCS. Pain scores are reported on the Visual Analogue Scale for Pain; 0= no pain, 10= worst pain of life. * Indicates statistically significant (p<0.05) as compared with baseline. Each column represents mean score SEM (standard error of mean). T1: end of stimulation, T2: 30 day follow-up, T3: 60 day follow-up.
Analysis of tender point scores was not significant (p>.2 for all the analyses), showing that this outcome was not sensitive to assess the effects of tDCS treatment on pain reduction.
Quality of life
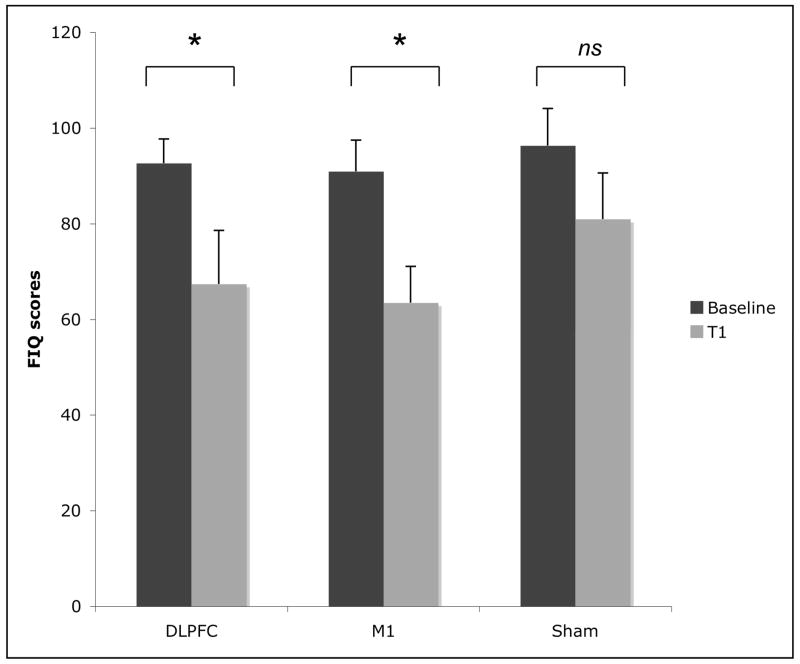
Changes in domains other than pain (e.g. function, fatigue, sleep disturbance, psychological distress) were assessed using the fibromyalgia impact questionnaire (FIQ). However FIQ was assessed at baseline and only immediately after treatment (T1). Repeated measures analysis for the FIQ showed a significant interaction term (time vs. group) effect (F(3,38) =6.54; p=0.001). Although the results showed that the three groups had a decrease in the FIQ scores over the course of the trial, only the decrease in the M1 group (28.3% (±37.1) reduction, p=0.0015) and DLPFC group (27.6% (±26.8) reduction, p=0.02) were significant. The small decrease in the sham group was not significant (13.8 (±39.4) reduction, p=0.15) (see figure 2).
Figure 2.
Mean fibromyalgia impact scores associated with the three conditions of stimulation: left M1 (primary motor cortex); left DLPFC (dorsolateral prefrontal cortex); and sham tDCS. The Fibromyalgia Impact Questionnaire (FIQ) quantitates the overall impact of fibromyalgia over many dimensions (e.g. physical functioning, pain level, fatigue, sleep disturbance, psychological distress, etc.) and has been extensively validated; 0= no impact on quality of life, 100= worst impact possible. * Indicates statistically significant (p<0.05). ns – indicates not significant (p>0.05). Each column represents mean FIQ score SEM (standard error of mean). T1: end of stimulation, T2: 30 day follow-up, T3: 60 day follow-up.
Psychiatric assessment
For mood assessment, our results showed that there was no significant difference in BDI scores across the three groups of treatment (interaction term group vs. time - F(3, 38)=0.51; p=0.67), therefore revealing that treatment with tDCS was not associated with mood changes. In fact mean changes in depression scores was less than 10% for three groups of treatment. Similar results were obtained for IDATE state-trait anxiety inventory for anxiety, and Geriatric Depression Scale (F<1 for the interaction term for these two analyses).
Correlations
In an exploratory way, we performed correlation tests between pain improvement after M1 and DLPFC stimulation as indexed by VAS score changes (between baseline and after 10 days of treatment) with the following variables: age, duration of pain, sleep (VAS), body mass index (BMI), baseline scores of depression (BDI), pain (VAS) baseline, and fibromyalgia impact questionnaire (FIQ). The results showed no significant correlations.
DISCUSSION
Our results show that 10 daily sessions of 2 mA, 20 min tDCS of left M1 or DLPFC can both induce significant improvements with respect to pain and quality of life in patients with fibromyalgia. These findings are consistent with our previous study of tDCS in patients with fibromyalgia--where 5 daily sessions of M1 tDCS also induced a significant pain reduction. However, in contrast to our previous study where pain scores were noted to take an upward slope at two weeks after treatment completion, here we show that 10 sessions of M1 tDCS can be effective in maintaining the observed diminishment of pain scores for up to 60 days. This difference is in keeping with an rTMS study in patients with fibromyalgia, which shows that 10 daily sessions of 10Hz rTMS applied to the motor cortex can induce similarly long-lasting improvements in pain and measures of quality of life (38). Interestingly, the analgesic effects of the repeated sessions of rTMS were significant only after 5 days of stimulation.
These results are interesting as they underscore the impact of the number of treatment sessions in inducing and maintaining long-lasting clinical effects. Indeed, previous tDCS studies have come to a similar conclusion: Five daily sessions of tDCS in stroke patients yields greater improvements in motor function than a single session alone (39); and, rTMS studies corroborate this session-number dependent efficacy trend: more sessions results in longer-lasting or more significant effects (40–42). In addition, the importance lies not only in the total number of sessions administered, but also in their temporal proximity: 4 weekly sessions of tDCS in stroke patients does not result in changes that are significantly different from single session therapy (39). Therefore, these findings suggest that tDCS induces a cumulative effect that is maximized by consecutive sessions.
tDCS is believed to induce clinical effects through the modulation of synaptic connections (43). Nitsche et al. have shown that anodal stimulation of sufficient duration can enhance cortical excitability beyond the stimulation period (20,21). Further studies have revealed that these changes in post-tDCS cortical excitability are intimately dependent on Na+ channel and NMDA receptor activity (44,45) and that acetylcholine plays an important role in the consolidation of this neuroplasticity (46). These results suggest that long term potentiation of new adaptive synaptic connection is what underlies the improvements in working memory (27), motor function (39), and pain modulation (47) that have been attributed to tDCS. Because LTP underlies the mechanism behind the long-lasting effects of repeated sessions of tDCS, it is therefore not surprising that the changes in cortical excitability and synaptic connections induced by tDCS are prone to extinction and that they can be reinforced with longer and/or additional treatment sessions. Indeed, the difference between our results with 10 sessions of tDCS vs. 5 daily sessions may be attributed to greater synaptic strengthening.
In addition to demonstrating the efficacy of M1 tDCS in relieving pain in fibromyalgia, our study here also demonstrates a potential role for DLPFC stimulation. Whereas our previous study failed to show a significant effect for DLPFC stimulation, here 10 sessions of DLFPC stimulation significantly diminished pain scores compared to sham stimulation. Thus, it is possible that DLPFC is also useful but it is inevitably less effective than M1 for the treatment of fibromyalgia-associated pain. Mechanistically, M1 stimulation produces an analgesic effect by modulating the sensory aspects of pain, while DLPFC stimulation mediates its effects by modulating affective-emotional networks regulating the unpleasantness associated with pain (11). Pain in fibromyalgia has been associated with abnormal information processing characterized by a lack of inhibitory control over somatosensory processing (7,8); thus, it appears appropriate that M1 tDCS would have a more primary analgesic effect in this population.
The treatment protocols resulted in no change to tender points or measures of depression or anxiety as compared to sham stimulation; these parameters may be secondary to central pain in fibromyalgia. In addition we found no significant correlations between baseline characteristics and response to treatment, yet this might be due to low power to perform these correlations.
Here we show that 10 daily sessions of M1 and DLPFC tDCS both generate clinical improvements in patients with fibromyalgia, but that only M1 stimulation results in long-lasting clinical benefits as assessed at 30 and 60 days after the end of treatment. This study demonstrates the importance of the duration of the treatment period, suggesting that protocols with 10 daily sessions of tDCS would result in more long lasting outcomes than protocols with only 5 daily sessions. Furthermore, this study supports the findings of a similarly designed rTMS trial (38), although it should be noted that the magnitude of the effect of 10 sessions of tDCS appears to be greater than the respective rTMS trial— nevertheless, this needs to be compared in a head to head comparison trial of tDCS vs. rTMS using the same study population. Noninvasive forms of brain stimulation hold great promise, yet further studies are indicated to determine the role of maintenance therapy in treatment planning.
References
- 1.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Schweinhardt P, Sauro KM, Bushnell MC. Fibromyalgia: A disorder of the brain? Neuroscientist. 2008;14(5):415–21. doi: 10.1177/1073858407312521. [DOI] [PubMed] [Google Scholar]
- 3.Dadabhoy D, Clauw DJ. Therapy Insight: fibromyalgia. A different type of pain needing a different type of treatment. Nat Clin Pract Rheumatol. 2006;2(7):364–72. doi: 10.1038/ncprheum0221. [DOI] [PubMed] [Google Scholar]
- 4.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13(3):189–96. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 6.Cook DB, Stegner AJ, McLoughlin MJ. Imaging pain of fibromyalgia. Curr Pain Headache Rep. 2007;11(3):190–200. doi: 10.1007/s11916-007-0190-8. [DOI] [PubMed] [Google Scholar]
- 7.Montoya P, Sitges C, Garcia-Herrera M, Rodriguez-Cotes A, Izquierdo R, Truyols M, et al. Reduced brain habituation to somatosensory stimulation in patients with fibromyalgia. Arthritis Rheum. 2006;54(6):1995–2003. doi: 10.1002/art.21910. [DOI] [PubMed] [Google Scholar]
- 8.Diers M, Koeppe C, Yilmaz P, Thieme K, Markela-Lerenc J, Schiltenwolf M, et al. Pain ratings and somatosensory evoked responses to repetitive intramuscular and intracutaneous stimulation in fibromyalgia syndrome. J Clin Neurophysiol. 2008;25(3):153–60. doi: 10.1097/WNP.0b013e31817759c5. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga K, Nitsche MA, Tsuji S, Rothwell JC. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2004;115(2):456–60. doi: 10.1016/s1388-2457(03)00362-6. [DOI] [PubMed] [Google Scholar]
- 10.Rogalewski A, Breitenstein C, Nitsche MA, Paulus W, Knecht S. Transcranial direct current stimulation disrupts tactile perception. Eur J Neurosci. 2004;20(1):313–6. doi: 10.1111/j.0953-816X.2004.03450.x. [DOI] [PubMed] [Google Scholar]
- 11.Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. 2008;15(10):1124–30. doi: 10.1111/j.1468-1331.2008.02270.x. [DOI] [PubMed] [Google Scholar]
- 12.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70(24):2329–37. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 13.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 14.Roizenblatt S, Fregni F, Gimenez R, Wetzel T, Rigonatti SP, Tufik S, et al. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pract. 2007;7(4):297–306. doi: 10.1111/j.1533-2500.2007.00152.x. [DOI] [PubMed] [Google Scholar]
- 15.Sparing R, Mottaghy FM. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction. Methods. 2008;44(4):329–37. doi: 10.1016/j.ymeth.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114(4):589–95. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 17.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117(7):1623–9. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 21.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553(1):293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi S, Cappa SF, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, et al. Prefrontal [correction of Prefontal] cortex in long-term memory: an “interference” approach using magnetic stimulation. Nat Neurosci. 2001;4(9):948–52. doi: 10.1038/nn0901-948. [DOI] [PubMed] [Google Scholar]
- 23.Herwig U, Lampe Y, Juengling FD, Wunderlich A, Walter H, Spitzer M, et al. Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res. 2003;37(4):267–75. doi: 10.1016/s0022-3956(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 24.Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–5. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- 25.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72(4–6):208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 28.Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249(1):31–8. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 29.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–93. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 30.Lefaucheur JP. Use of repetitive transcranial magnetic stimulation in pain relief. Expert Rev Neurother. 2008;8(5):799–808. doi: 10.1586/14737175.8.5.799. [DOI] [PubMed] [Google Scholar]
- 31.Duquette M, Roy M, Lepore F, Peretz I, Rainville P. Cerebral mechanisms involved in the interaction between pain and emotion. Rev Neurol (Paris) 2007;163(2):169–79. doi: 10.1016/s0035-3787(07)90388-4. [French] [DOI] [PubMed] [Google Scholar]
- 32.Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11(2):249–54. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci. 2007;27(23):6212–8. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avery DH, Holtzheimer PE, 3rd, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. Transcranial magnetic stimulation reduces pain in patients with major depression: a sham-controlled study. J Nerv Ment Dis. 2007;195(5):378–81. doi: 10.1097/NMD.0b013e31802f58d1. [DOI] [PubMed] [Google Scholar]
- 35.Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci. 2007;26(1):242–9. doi: 10.1111/j.1460-9568.2007.05633.x. [DOI] [PubMed] [Google Scholar]
- 36.Priori A, Mameli F, Cogiamanian F, Marceglia S, Tiriticco M, Mrakic-Sposta S, et al. Lie-specific involvement of dorsolateral prefrontal cortex in deception. Cereb Cortex. 2008;18(2):451–5. doi: 10.1093/cercor/bhm088. [DOI] [PubMed] [Google Scholar]
- 37.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire (FIQ): development and validation. J Rheumatol. 1991;18:728–33. [PubMed] [Google Scholar]
- 38.Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130(10):2661–70. doi: 10.1093/brain/awm189. [DOI] [PubMed] [Google Scholar]
- 39.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–9. [PubMed] [Google Scholar]
- 40.Rumi DO, Gattaz WF, Rigonatti SP, Rosa MA, Fregni F, Rosa MO, et al. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol Psychiatry. 2005;57(2):162–6. doi: 10.1016/j.biopsych.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Avery DH, Holtzheimer PE, 3rd, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187–94. doi: 10.1016/j.biopsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76(6):833–8. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568(1):291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacology. 2004;29(8):1573–8. doi: 10.1038/sj.npp.1300517. [DOI] [PubMed] [Google Scholar]
- 45.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125(10):2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 46.Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neurosci. 2007;27(52):14442–7. doi: 10.1523/JNEUROSCI.4104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol. 2007;6:188–91. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]