Abstract
Time-dependent drug–drug interactions (TDDIs) are drug combinations that result in a decreased drug effect due to coadministration of a second drug. Such interactions can be prevented by separately administering the drugs. This study attempted to reduce drug administration errors due to overridden TDDIs in a care provider order entry (CPOE) system. In four periods divided over two studies, logged TDDIs were investigated by reviewing the time intervals prescribed in the CPOE and recorded on the patient chart. The first study showed significant drug administration error reduction from 56.4 to 36.2% (p < 0.05), whereas the second study was not successful (46.7 and 45.2%; p > 0.05). Despite interventions, drug administration errors still occurred in more than one third of cases and prescribing errors in 79–87%. Probably the low alert specificity, the unclear alert information content, and the inability of the software to support safe and efficient TDDI alert handling all diminished correct prescribing, and consequently, insufficiently reduced drug administration errors.
Introduction
Care provider order entry (CPOE) systems frequently include integrated clinical decision support (CDS) components, with the goal of reducing errors and improving patient safety. 1–6 Drug safety alerts intended to prevent medication errors often are not read, or are misinterpreted or handled incorrectly. The result is diminished potential CDS effect on patient safety. 7
Time-dependent drug–drug interactions (TDDIs) are drug-drug interactions (DDIs) that typically decrease a drug's effect due to coadministration of a second drug that decreases absorption or affects metabolism of the first drug. Administering the two drugs separated by an appropriate time interval (generally 2–4 h) can prevent the TDDIs. For TDDIs, mechanisms that reduce absorption include complex formation (e.g., tetracyclines and divalent ions such as calcium or magnesium), increased pH (e.g., iron and antacids) or decreased enterohepatic circulation (e.g., mycophenolate mofetil and colestyramine). As drug administration is typically a nursing task, authors hypothesized that directing TDDI alerts to nurses might both reduce the burden of alerts seen by physicians and also decrease the number of TDDI-related administration errors. 7,8 Thus, the study objective was to reduce TDDI drug administration errors by educating nurses and physicians and by reducing the burden of TDDI alerts.
Questions posed by this study are:
1 How often do physicians prescribe TDDI drug combinations incorrectly, and how often do nurses administer them incorrectly?
2 What are the short and long term effects of educating physicians and nurses about TDDIs and related drug administration errors?
3 Can the burden of TDDI alerts be decreased by directing TDDI alerts to other people in the workflow, such as nurses (or pharmacy technicians)?
Methods
The Erasmus MC in Rotterdam, the Netherlands, is a 1,237 bed Academic Medical Center with three sites. It started using CPOE in December 2001, and since March 2005 all inpatient wards, intensive care units excluded, have used the CPOE system Medicatie/EVS (iSOFT, Leiden, the Netherlands). 7,9 As nurses are not legally allowed to prescribe drugs, physicians (and midwives) exclusively enter medication orders. During order entry sessions, physicians can select dosage regimens (e.g., thrice daily) that are translated to the corresponding drug administration times on the ward and these administration times can be adjusted when desired. Printed order labels are placed on paper charts, nurses write the intended drug administration times next to the prescribed times of the order labels, and sign to indicate that drug administration occurred.
Drug safety alerts and the corresponding alert texts in the CPOE system follow from the national Dutch drug database (“G-standard”) 10 and are presented intrusively (▶). When a TDDI alert occurs, physicians should either adjust drug administration times or place a remark in the order that indicates that the drugs should be administered separately, with at least the required time interval given in the alert text between them. Note that TDDI alerts arise irrespective of the drug administration times entered, which causes false positive alerts if time intervals are prescribed correctly.
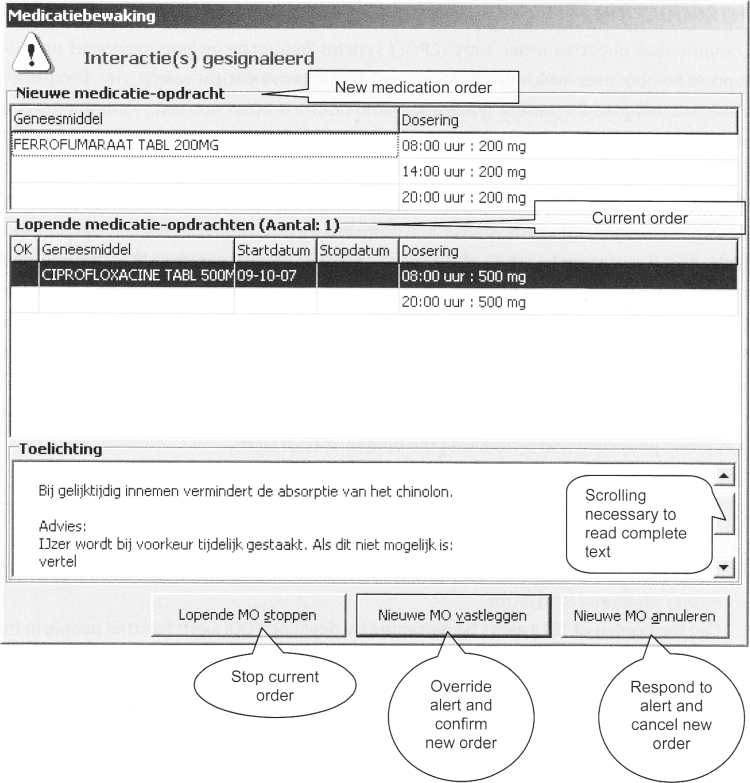
Figure 1.
Time-dependent drug–drug interaction alert quinolones and iron.
DDI alert presented to a physician ordering ferro fumarate when ciprofloxacin is already on the patient's medication list. Only part of the alert text (the boxed text below) is shown without scrolling down.
Complete (translated) alert text:
Taking these drugs concomitantly decreases quinolone absorption.
Recommendation:
Preferably stop iron temporarily. If this is not possible: tell the patient that the quinolone should be taken at least 2 hours before the iron.
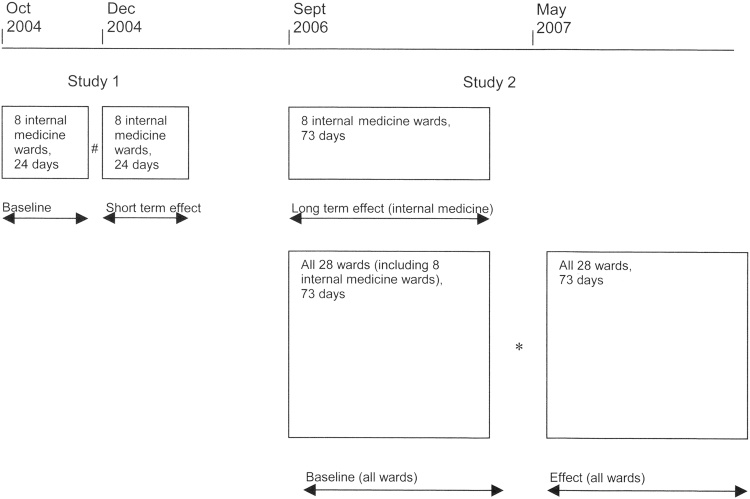
We analyzed all TDDI alerts logged in the Erasmus MC's 800-bed general hospital (Center Location) during four time periods divided over 2 studies. The study design is presented in ▶. In Study 1, after a 24 day preintervention period on eight internal medicine wards in October 2004, the study team provided feedback about drug administration errors to all nurses and physicians, and made available to them a table with TDDIs and their required dose separation timing intervals. During the 24 day postintervention period, the clinical pharmacologist communicated every incorrect time interval discovered on the patient chart personally to the attending nurse and physician; monitoring of TDDI handling continued.
Figure 2.
Study design.
#Intervention: education of physicians and nurses, a table with TDDIs and their required time intervals made available, followed by daily feedback by the clinical pharmacologist.
*Discussion of results of the baseline period with head nurse and medical co-ordinator, followed by feedback by pharmacy technicians.
The second study 73-day baseline period ran from Sept to Nov 2006 on all 28 wards of the general hospital, including the wards of the first study. The pharmacist then presented baseline results to the head nurse and the medical co-ordinator and suggested that nursing (or pharmacy) staff adjust administration times whenever TDDIs occurred. No TDDI table was made available to the wards that were new to the second study. In the 73 day effect period from May to July 2007, pharmacy technicians gave feedback to nurses about incorrect time intervals and asked the nurses to inform the prescribing physician. During this period the number of incorrectly handled TDDIs was again monitored.
A correct time interval was defined as a time interval that matched that given in the alert. For all TDDIs logged, study personnel checked the prescriptions for correct time interval prescription or for addition of an appropriate TDDI-related spacing comment. The study then compared nursing documentation of actual administration times written on the patient charts to the recommended TDDI-related spacing time interval. The TDDI was categorized as “unable to be evaluated” if the patient was discharged, if medication administration was under the patient's control, if the order for one or both TDDI-drugs had been stopped already, or if administration times were unclear. Statistical analysis was performed with the χ2 test on all TDDIs that could be evaluated.
Results
During the four study periods a total of 1,031 TDDI alerts were logged, of which 749 (73%) could be evaluated. Sixty percent were due to the combination of any drug with calcium (of which half was due to the combination of bisphosphonates with calcium) and about 30% concerned antibiotics (quinolones and tetracyclines). In 17% of all TDDI alerts that could be evaluated, a TDDI alert was generated despite a correctly prescribed time interval (false positive alert).
One month after the first study intervention, the percentage of drug combinations administered incorrectly fell from 56 to 36% (p < 0.05) 11 and 2 years later this was still 39% (p < 0.05), without any study-generated feedback having been given meantime (▶). Figures for postintervention prescribing errors remained very high (79 and 87%). Even though nurses prevented many TDDI drug administration errors by adjusting incorrectly prescribed administration times, more than one third of TDDIs resulted in an actual administration error. The table indicating the required TDDI timing intervals still appeared to be present on many wards on the wall of the medication room.
Table 1.
Table 1 Number of TDDI Alerts and Handling Internal Medicine before and after Intervention of Verbal and Written Education Given in November 2004
| Pre-Intervention (24 d; Oct 2004) |
Short Term Effect (24 d; Dec 2004) |
p Value | Long Term Effect (73 d; Sep–Nov 2006) |
p Value | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |||
| TDDI alerts | 61 | 66 | 218 | |||||
| TDDI alerts/day | 2.5 | 2.8 | 3.0 | |||||
| TDDIs that could be evaluated | 55 | 90 | 58 | 88 | 193 | 89 | ||
| Prescribed incorrectly | 54 | 98 | 46 | 79 | < 0.02 | 167 | 87 | < 0.02 |
| Administered incorrectly | 31 | 56 | 21 | 36 | < 0.05 | 75 | 39 | < 0.05 |
TDDI = time-dependent drug–drug interactions.
In the baseline period of the second study, incorrect time intervals were communicated to the nurses. However, the percentages of incorrectly administered drug combinations per week, 44, 31, 39% and 40% respectively in the first four weeks did not show a learning effect. In this 4-week period, the percentage administration errors on wards given feedback earlier was significantly lower (24%) than on wards not included in the first study (54%; p < 0.05). This was not due to physicians prescribing time intervals correctly (15 versus 18%; p > 0.05), but to corrective action by nurses (58 versus 29%; p < 0.001).
Results of the baseline measurement of the second study and suggestions for TDDI handling by nurses were discussed on individual wards with each medical coordinator and head nurse. Directing TDDI alerts to nurses or pharmacy personnel was not deemed acceptable, and both groups agreed that physicians should prescribe correctly to prevent administration errors and that pharmacy technicians would give feedback on incorrectly prescribed and administered TDDI drug combinations in the effect period. The medical co-ordinators would inform their staff about the TDDI medication errors.
No significant reduction in drug administration errors (47–45%; p > 0.05) or prescribing errors (83–81%; p > 0.05) could be observed despite pharmacy technician feedback on incorrect time intervals (▶). Nurses said they would inform the physician about incorrect time intervals, but pharmacy requests for administration time adjustments more often resulted in correct time intervals on the patient chart (86%) than in the prescribed order (10%).
Table 2.
Table 2 Number of TDDI Alerts and Handling All Wards before and after Discussing TDDI Medication Errors with Head Nurse and Medical Coordinator
| Baseline (73 d; Sep–Nov 2006) |
Effect (73 d; May–Jul 2007) |
p Value | |||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | ||
| TDDI alerts | 454 | 450 | |||
| TDDI alerts/day | 6.2 | 6.2 | |||
| TDDIs that could be evaluated | 364 | 81 | 272 | 60 | |
| Prescribed incorrectly | 303 | 83 | 218 | 81 | > 0.05 NS |
| Administered incorrectly | 170 | 47 | 123 | 45 | > 0.05 NS |
TDDI = time-dependent drug-drug interactions.
Discussion
This study revealed several unexpected results. Firstly, the number of TDDI prescribing and administration errors was very high and could not easily be reduced to less than 10%.
Secondly, the initial intervention that included verbal education, written information and 24 days of intensive feedback had a long-lasting effect. In the baseline period of the second study, nurses of the internal medicine wards prevented many administration errors using the information leaflet with required time intervals that had been made available 2 years earlier. The information leaflet was often present in the medication room where nurses place order labels on paper charts and write down the drug administration times.
Thirdly, formal adjustment of TDDI time intervals by nurses was not deemed acceptable, although these adjustments were common practice. The reason for this was not asked in the interviews, but the following assumptions can be made: (1) TDDIs were not well known because of the low frequency of 6.2 per d over 28 wards. (2) Because administration errors were often corrected after pharmacy requests, adverse events did not occur often. Therefore, these alerts perhaps were not perceived as serious enough to justify a formal responsibility shift from physicians towards nurses.
Fourthly, the initial intervention (Study 1) was effective and the second intervention (Study 2) was ineffective. The differences between the studies were fourfold:
1 In Study 2 no education was given by pharmacy personnel. The nature and handling of TDDIs were discussed only with the head nurse and the medical coordinator. The study did not ascertain whether the medical co-ordinators indeed informed their staff.
2 Written information was not made available to the new wards in the second study. Perhaps nurses required the leaflet with appropriate time intervals nearby to prevent drug administration errors.
3 Feedback was given by pharmacy technicians instead of the clinical pharmacologist and probably did not reach the physician. We assume that nurses often made corrections to inappropriate TDDI orders without communicating to physicians the nature of the original prescribing errors. Under such circumstances, physicians cannot be expected to learn.
4 More surgical wards (with less pharmacotherapy-minded caregivers and fewer TDDIs) were included.
Error Management
Incorrectly prescribed combinations (79–98%), due to erroneous TDDI alert overriding, were an important cause of administration errors. The authors hypothesize that alert fatigue, caused by error-producing conditions such as low specificity, unclear information given by the alert, and the software not allowing safe and efficient alert handling, may have played a role. 7 Therefore, the process of TDDI alert handling was studied in more detail.
The study's TDDI alerts were false positive in 17% of cases, generated even though the time intervals were prescribed correctly. At present, none of the Dutch CPOEs has functionality to prevent false positive TDDI alerts, perhaps because the Dutch drug database lacks time indications; it would be worthwhile to develop them to improve specificity.
Requirements for useful information from drug safety alert texts include brevity, nonambiguity, clearly indicated level of severity, and presentation of alternative courses of action. 7 The most relevant part of the alert text recommendation (quinolone has to be taken at least 2 h before iron) cannot be read without requiring the user to scroll down. The text is ambiguous, as it prompts the prescriber to “tell the patient” rather than being customized to the hospital setting, where nurses administer drugs. The seriousness of the effect of overriding the alert is not clearly indicated in the text. The alternative action of adjusting administration times is proposed only as a second option (after stopping iron temporarily). The first sentence should recommend adjusting administration times to the required time interval; it would be worthwhile to investigate whether this alert adjustment would result in fewer errors.
Handling of the TDDI alerts by the software appeared to be inefficient and error prone. In the CPOE system, three options are provided for handling the alert (▶): (1) stopping a current order, (2) overriding the alert and confirming a new order and, (3) canceling a new order, whereas the preferred option to adjust the new order is absent. The order has to be canceled and newly prescribed, or confirmed but adjusted afterward. The software is not helpful and may contribute to error generation. 7 Addition of a “adjust order” button is recommended for safe and efficient handling by the software, although future studies should investigate whether this indeed is prone to fewer errors.
After studying the whole process of prescribing and administering drugs, we postulate the following as an explanation for the unexpected study findings: low alert specificity, unavailability of clear information at the time of decision-making, inefficiency in responding to incorrect time intervals, and lack of clear responsibilities. The CPOE system generates many false positive TDDI alerts, which may provoke alert fatigue, important alerts being ignored along with unimportant ones. In TDDI alert recommendations, the relevant information is hidden and not tailored to the hospital setting, so the information needed is not effectively shown to the physician at the time of decision-making. Prescribing by physicians after TDDI alerts is inefficient and unsafe. Physicians will not learn about the TDDIs if relevant alert information is hidden and nurses do not give feedback. Nurses on internal medicine wards were able to use the information leaflet with required time intervals when deciding on appropriate drug administration times, whereas nurses on other wards were not. Nurses could efficiently adjust administration times by writing on the patient chart. As nurses generally administer (oral) drugs, residents may perceive the handling of these TDDIs as nurses' responsibility, although formalization of these roles was not deemed acceptable. In United States hospitals where nurses, medication administration record transcribers and/or pharmacists are responsible for drug administration times, the proposed workflow probably would be implemented easily. The culture of the health care setting may influence acceptance of CDS and the organizational entanglement of the CPOE requires change management that takes into account the social context. This may imply that the responsibility for drug administration times is left to the pharmacist and/or the nurses in one hospital and to the physician in another hospital. However, the responsibility of addressing prescription errors due to error-producing conditions in the CDS cannot be put on the shoulders of the nurses; these error-producing conditions should be counteracted. The unexpected long-term effect of the verbal and written education suggests that this intervention can be investigated as a short-term solution on the wards new to the second study, as long as the CDS contains the above-mentioned error-producing conditions.
Strengths and Limitations
To our knowledge, the topic of TDDIs has not been previously evaluated. All TDDI drug combinations irrespective of the prescribed time interval were available for review and it was feasible to study prescribing and administration errors, as well as the effect of two interventions.
Chart review was used to reveal incorrect administration times. Disguised observation, the preferred method for investigating drug administration errors, is very time-consuming and appeared to be too inefficient to study the relatively small number of about 6 TDDIs per day over 28 wards. A drug administration study performed with disguised observation in the ICU showed that 22% of drugs were administered more than 1 hour later or earlier than intended. 12 If we assume 78% of the incorrect time intervals to be indeed incorrect, this is still more than one third of all TDDI drug combinations.
In the effect period of the second study 60% of the TDDIs could be evaluated compared with more than 80% in the other study periods. This low percentage appeared to be due to the pharmacy technicians checking time intervals in the afternoon when many patients had been discharged already. Furthermore, administration times were not always written down clearly. In case of doubt, these TDDIs were categorized as TDDIs that could not be evaluated.
The study did not account for a possible repeated measure effect of nurses and physicians associated with different TDDI alerts within the study period.
This study did not include the clinical and financial effects of incorrectly administered drug combinations. Most of the TDDIs encountered are categorized in the G-standard as medium level seriousness with an increased risk of failure of therapy for a serious, nonlethal disease. 13 It is therefore likely that problems may arise due to drug administration at incorrect times. Several error-producing conditions appeared to be present in the software that should be eliminated to enable improvements on a patient level.
Acknowledgments
The authors thank Liselotte van der Meule-Soeting, Jan-Dietert Brugma and the pharmacy technicians for their help in checking time intervals on the patient charts.
References
- 1.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety. A systematic review. Arch Intern Med 2003;163:1409-1416. [DOI] [PubMed] [Google Scholar]
- 2.Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions. Benefits and barriers to using automated drug alerts. Med Care 2002;40:1161-1171. [DOI] [PubMed] [Google Scholar]
- 3.Walton R, Dovey S, Harvey E, Freemantle N. Computer support for determining drug dose: Systematic review and meta-analysis BMJ 1999;318:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events J Am Med Assoc 1998;280:1317-1320. [DOI] [PubMed] [Google Scholar]
- 5.Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance an patient outcomes J Am Med Assoc 1998;280:1339-1346. [DOI] [PubMed] [Google Scholar]
- 6.Rind DM, Safran C, Phillips RS, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients Arch Intern Med 1994;154:1511-1517. [PubMed] [Google Scholar]
- 7.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry J Am Med Inform Assoc 2006;13:138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krall MA, Sittig DF. Clinicians' assessment of outpatient electronic medical record alert and reminder usability and usefulness requirements Proc AMIA Symp 2002:400-404. [PMC free article] [PubMed]
- 9.Kalmeijer MD, Holtzer W, van Dongen R, Guchelaar H-J. Implementation of a computerized physician order entry system at the Academic Medical Center in Amsterdam Pharm World Sci 2003;25:88-93. [DOI] [PubMed] [Google Scholar]
- 10.van der Sijs H, Aarts J, van Gelder T, Berg M, Vulto A. Turning off frequently overridden drug alerts; limited opportunities for doing it safely J Am Med Inform Assoc 2008;15:439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Tweel AMA, van der Sijs IH, van Gelder T, Knoester PD, Vulto AG. Computerized medication alert signals: Does the MD no longer need the PharmD? Eur J Hosp Pharm 2006;12:30-32. [Google Scholar]
- 12.van den Bemt PM, Fijn R, Van der Voort PH, et al. Frequency and determinants of drug administration errors in the intensive care unit Crit Care Med 2002;30:846-850. [DOI] [PubMed] [Google Scholar]
- 13.van Roon EN, Flikweert S, le Le Comte M, et al. Clinical relevance of drug–drug interactions: A structured assessment procedure Drug Saf 2005;28:1131-1139. [DOI] [PubMed] [Google Scholar]