Abstract
Objective
This study evaluated the effect of a Computerized Physician Order Entry system with basic Clinical Decision Support (CPOE/CDSS) on the incidence of medication errors (MEs) and preventable adverse drug events (pADEs).
Design
Interrupted time-series design.
Measurements
The primary outcome measurements comprised the percentage of medication orders with one or more MEs and the percentage of patients with one or more pADEs.
Results
Pre-implementation, the mean percentage of medication orders containing at least one ME was 55%, whereas this became 17% post-implementation. The introduction of CPOE/CDSS has led to a significant immediate absolute reduction of 40.3% (95% CI: −45.13%; −35.48%) in medication orders with one or more errors.
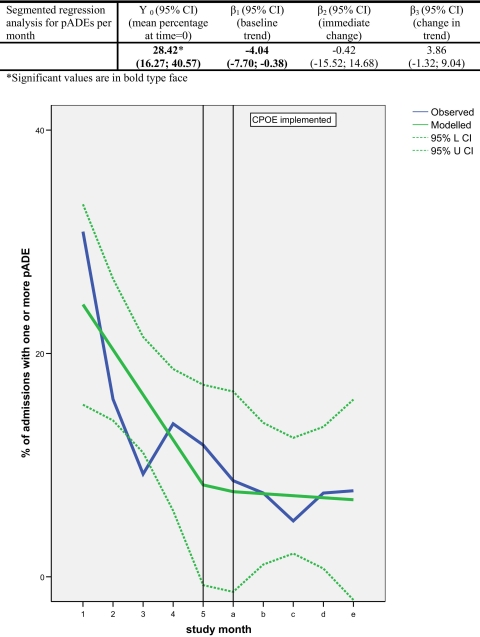
Pre-implementation, the mean percentage of admitted patients experiencing at least one pADE was 15.5%, as opposed to 7.3% post-implementation. However, this decrease could not be attributed to the introduction of CPOE/CDSS: taking into consideration the interrupted time-series design, the immediate change was not significant (−0.42%, 95% CI: −15.52%; 14.68%) because of the observed underlying negative trend during the pre-CPOE period of −4.04% [95% CI: −7.70%; −0.38%] per month.
Conclusions
This study has shown that CPOE/CDSS reduces the incidence of medication errors. However, a direct effect on actual patient harm (pADEs) was not demonstrated.
Introduction
Since the publication of the Institute of Medicine (IOM) report, “To Err is Human”, many strategies for making health care safer have been created and implemented. 1 One of these strategies is electronic prescribing through the use of a Care Provider Order Entry (CPOE) system. Before the first introduction of this system in the United States in the 1970s, expectations about CPOE systems reducing medication errors and patient harm were high. Legibility and completeness of prescriptions would be ensured 2 and Clinical Decision Support Systems (CDSS) incorporated in the CPOE systems would be able to assist physicians by triggering alerts in case of drug–drug interactions and inappropriate dosing. These were reasons to suppose that CPOE/CDSS systems would be effective in reducing medication errors and adverse drug events, and thereby improving medication safety.
Meanwhile, a number of studies (predominantly from the United States) showed that CPOE/CDSS systems were indeed successful strategies for reducing medication errors, and there was some indication of patient harm being reduced. 3–9 Other studies showed negative effects in the sense that new medication errors were being introduced through CPOE/CDSS 10 or that mortality increased after implementation of CPOE/CDSS in a Children's Hospital. 11 However, most of these CPOE/CDSS studies used a pre/post analysis to evaluate the effect. This is not a robust study design, because it does not take into account other factors during the introduction and eventual use of CPOE/CDSS that might explain the change in outcome. An interrupted time-series (ITS) design with segmented linear regression analysis is more robust, because it evaluates the longitudinal effect of CPOE/CDSS and controls for trends in the outcome. 12
Moreover, studies that looked into the effect of electronic prescribing were predominantly performed in the United States, because it was here that CPOE/CDSS was first introduced into clinical practice. The findings from these studies may not apply to the European hospital setting due to differences in computer systems and work processes between the two continents.
Therefore, this study has used an ITS design with segmented linear regression analysis in order to evaluate the effect that CPOE/CDSS has had on the incidence of medication errors and to relate this to patient harm in two Dutch hospitals.
Methods
Setting and Study Population
This study was performed in two medical wards of the 1300-bed University Medical Center Groningen (a general internal medicine and a gastroenterology/rheumatology ward) and in two medical wards (a geriatric and a general internal medicine ward) of the 600-bed teaching hospital “TweeSteden” in Tilburg and Waalwijk, the Netherlands. All patients admitted to these wards for more than 24 h were included. A waiver of the Medical Ethical Committee was obtained for this study, as the study fell within the boundaries of quality of care improvement. Patients received information about the study and they could decline to participate.
Design
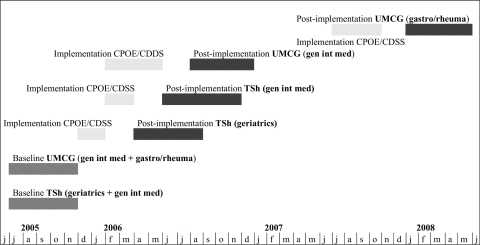
The study was set up as an interrupted time series that is characterized by a series of measurements over time interrupted by an intervention. In this study the intervention was the implementation of a Computerized Physician Order Entry system in combination with a basic Clinical Decision Support System (CPOE/CDSS). Data collection took place during a 5-month pre-implementation period (during which the hand-written medication order system continued to be used) and during a 5-month post-implementation period (when the CPOE/CDSS system continued to be used). The post-implementation data collection period started 8 weeks after finishing the implementation process in order to make sure that initial problems were solved. Because CPOE/CDSS was not simultaneously implemented in all study wards, the starting date for the post-implementation period was different for each ward.
In both hospitals, pre-implementation data were collected from Jul through Nov 2005 (▶). In the TweeSteden Hospital, the post-implementation data collection on the geriatric ward was from Apr through Aug 2006, and on the general internal medicine ward from mid-Jun through mid-Nov 2006. In the University Medical Center Groningen, the post-implementation period on the general internal medicine ward was from Aug through Dec 2006. Post-implementation data collection on the gastroenterology/rheumatology ward was planned from Sep 2006 through Jan 2007, but, due to the delay in implementation of CPOE/CDSS, this period was postponed to Jan through May 2008. The CPOE was implemented per ward, that is, simultaneously for all hospital beds in that ward. Post-implementation data collection for each ward started 8 weeks after CPOE was implemented and lasted for 5 months for all beds in each ward.
Figure 1.
Study planning.
Preimplementation
In both hospitals, the conventional process of medication ordering during the baseline period was paper-based; physicians wrote handwritten medication orders on charts and nurses transcribed these medication orders onto the administration charts. From these administration charts nurses read what medication should be administrated to which patients. There was no decision support for the physicians at the moment of prescribing.
During the conventional process, central order entry by the pharmacy was performed in the TweeSteden Hospital only. As a result, it was only in the TweeSteden Hospital that medication orders were reviewed by pharmacists during the baseline period.
Intervention
The intervention was the introduction of the CPOE/CDSS system. This is a computer-based system by which physicians order medication electronically in a standardized way. In this study, the hospitals used the CPOE/CDSS system only for ordering medication. In the system, medication can be selected from menus in which medication from the local ward stock or from the pharmacy drug database is shown. Physicians are obliged to complete fields with key prescription characteristics (such as frequency and administration route). Moreover, standardized prescriptions and medication protocols (a set of prescriptions belonging to one protocol) can be programmed. In this system, transcription of medication orders by both the nurses and the pharmacy staff was no longer necessary. The CDSS system used was basic: safety alerts were rather straightforward and were only generated in case of drug–drug interactions, overdosing, and allergies. 13 This medication control was based on a national drug database for community pharmacies (the Z-index of the Royal Dutch Association of Pharmacists [KNMP]). More advanced CDSS systems currently do exist, which perform more complex functions (e.g., adjustment for renal impairment), 13 but these more advanced CDSS systems are still in an experimental stage in the Netherlands.
Physicians receive safety alerts in real time when prescribing drugs that, for example, interact with already prescribed drugs or when the dosage is too high. When an alert is shown, physicians can continue prescribing by accepting the order (while knowing there is a safety issue) or they can cancel the order. The safety alerts for the accepted medication orders are seen by pharmacists who can contact the physicians and nurses if necessary. In both hospitals, different types of CPOE/CDSS systems were in use. The commercially available system used in the University Medical Center Groningen was Medicator® (iSOFT, Leiden, the Netherlands). In this system, only the process of ordering medication is computerized, the process of dispensing and administering the medication is still paper-based. After the medication orders are entered into the computer, labels are printed out, which nurses then stick onto the administration charts. This is in contrast to the partly homegrown system used in the TweeSteden Hospital in Tilburg, Theriak® (Theriak evf, Tilburg, the Netherlands), a system in which the process of patient identification and medication administration is also automated (i.e., through a closed loop system) by scanning barcodes on patients' wristbands and barcodes on the packaging of medication. As mentioned before, the CDDS system in both Medicator® and Theriak® is quite basic.
Data Collection
Prospectively, the following patient data were collected by two research pharmacists: patients' characteristics (gender, age, height, weight, duration of stay in the ward), medical history, diseases (reasons for admission and diagnoses during hospital stay), medication (medication orders [MOs] during hospital stay), laboratory values and adverse events. Adverse events were defined as any untoward medical occurrences during hospital stay, which do not necessarily need to be related to medication use. Data were extracted from the hospital information system, medical charts, and the medication order and administration charts, and, during the post-intervention period, from the CPOE/CDSS system as well. Data from periods before and after the patient's admission period were not included (e.g., outpatient information or data from a stay on a ward other than the one included in this study).
Classification of Prescribing and Transcribing Errors
After collecting the data, the two research pharmacists, in parallel, individually reviewed the medication orders and identified medication errors according to the classification scheme for medication errors developed by The Netherlands Association of Hospital Pharmacists. 14 They were not blinded as to whether they assessed data before or after the introduction of CPOE/CDSS. The two research pharmacists were thoroughly trained in the classification scheme before the data collection. Moreover, in the first period of the study the research pharmacists discussed their findings weekly so as to guarantee that they were using the scheme in the same way. They also individually assessed ten pilot patients and afterwards discussed differences in classification. In this scheme, a distinction was made between prescribing, transcribing, dispensing, administering, and “across setting” errors. Because CPOE/CDSS was expected to have the largest effect on the number of prescribing and transcribing errors, only these two types of medication errors were taken into account. Prescribing errors are those errors made in the process of prescribing medication. These errors were subdivided into administrative and procedural errors (errors in readability, patient data, ward and prescriber data, drug name, dosage form, and route of administration), dosing errors (errors in strength, frequency, dosage, length of therapy, and directions for use) and therapeutic errors (drug–drug interactions, contra-indications, incorrect mono-therapy, duplicate therapy, and errors in therapeutic drug monitoring or laboratory monitoring; inappropriate drug choices were not actively assessed and were only taken into account when these were obvious). Transcribing errors are errors that occur in the process of the interpreting, verifying, and transcribing of medication orders. Transcribing errors were not subdivided into any sub-categories.
Classification of the Severity of Medication Errors/Incidence of pADEs
For the assessment of the severity of the identified prescribing and transcribing errors (including whether a related pADE had occurred), the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) scheme 15 and the simplified Yale algorithm 16 were combined into a new assessment tool. 17 The NCC MERP scheme categorizes MEs into nine categories (A through I) based on the severity of the related patient outcomes. Category A is a category for “circumstances or events that have the potential to cause an error”, for example, a drug–drug interaction that seems not to be relevant in a specific patient. In our study, we did not include this kind of circumstance as belonging to MEs. Categories B through D are associated with the absence of a preventable ADE, and Categories E through I are associated with the presence of a pADE (▶). In order to define whether an ME was categorized in the first group (B through D) or the second group (E through I), a causality assessment needed to be performed between the ME and an adverse event. Therefore, we adopted the first three items of the Yale algorithm in the new assessment tool (knowledge about the relationship between this drug and the event, influence of other clinical conditions, and the time relationship between drug and event). The causal relationship could be assessed as unlikely (score < 0), possible (score ≥ 0 and ≤ 3), and probable (score = 4). When the relationship was possible or probable, the ME was categorized as E, F, G, H, or I and was defined as a pADE. When the relationship was unlikely, the ME was categorized as B, C, or D, and was not associated with a pADE.
Table 1.
Table 1 NCC MERP Scheme
| Category | Content |
|---|---|
| A ∗ | Circumstances or events that have the capacity to cause error |
| B† | An error occurred, but the error did not reach the patient |
| C† | An error occurred that reached the patient, but did not cause patient harm |
| D† | An error occurred that reached the patient, and required monitoring to confirm that it resulted in no harm to the patient and/or required intervention to preclude harm |
| E‡ | An error occurred that may have contributed to or resulted in temporary harm to the patient, and required intervention |
| F‡ | An error occurred that may have contributed to or resulted in temporary harm to the patient, and required initial or prolonged hospitalization |
| G‡ | An error occurred that may have contributed to or resulted in permanent patient harm |
| H‡ | An error occurred that required intervention necessary to sustain life |
| I‡ | An error occurred that may have contributed to or resulted in the patient's death |
∗ No error.
† Error: no harm (no preventable adverse drug event [pADE]).
‡ Error: harm (pADE).
NCC MERP = National Coordinating Council for Medication Error Reporting and Prevention.
The assessment procedure (on severity of medication errors and incidence of pADEs) was carried out by five pharmacists. After individual assessment by the pharmacists, consensus meetings took place where consensus was reached for all cases of causality, between error and adverse event, as well as for severity of the error. The use of a consensus method was based on our findings in another study in which we showed that agreement between individual assessors was low (kappa in range “fair”), irrespective of their professional background (pharmacists and physicians). 17
Outcomes
The two primary outcome measurements were defined as: (1) percentage of MOs with one or more MEs; and (2) percentage of admitted patients with one or more preventable adverse drug events (pADEs).
Data Analysis
All data were processed using MS Access 2003. The SPSS version 14 (SPSS Inc., Chicago, IL) was used for the analysis. For the baseline period and the post-intervention period, the frequencies of the different types of MEs and pADEs were calculated, as well as the percentage of medication orders with one or more MEs and the percentage of patients with one or more pADEs. Segmented linear regression analysis was used to assess level and trend for: (1) the percentage of medication orders with one or more MEs at baseline; and (2) the percentage of patients with one or more pADEs at baseline; and to assess to what extent the intervention changed these levels. Separate analyses were performed for the different types of medication errors.
The data points for the time-series data represent the percentage of medication orders with MEs aggregated per week (i.e., 20 data points before and after the intervention) and the percentage of patients with one or more pADEs aggregated per month (i.e., 5 data points before and after the intervention). The MEs were analyzed using weeks as data points due to their high incidence, while pADEs were analyzed using months as data points. The low incidence of pADEs and the limited number of admissions (<30) per week that was expected would otherwise lead to an unstable baseline. Durbin-Watson statistics and visual inspection of the residuals versus time were used to check for possible autocorrelation (correlation between error terms of consecutive observations). In the case of non-significant trends in pADEs, a more parsimonious statistical analysis of mean pADE rate pre- and post-implementation with a Student's t-test was also performed.
Power Analysis
The study design met the criteria for a robust ITS, that is, 3 data points pre- and post-intervention, each consisting of at least 30 admissions. 18 To detect an assumed 50% decrease in the primary endpoint of medication orders with one or more medication errors (assuming a baseline prevalence of 10%) with a power of 80% and α = 5%, 474 medication orders, counted two times, would be required for the Student's t-test. By the same token, to detect a decrease in the number of pADEs per 100 admissions from 15 to 7.5 (rate ratio < 0.5) resulting from the intervention, a sample of 496 admissions equally distributed over pre- and post-intervention periods achieved 80% power at an α 0.05 significance level.
To estimate the level and trend of the percentages of medication orders with one or more MEs, and of the percentages of patients with one or more pADEs before the implementation of CPOE/CDSS, and to estimate the changes in level and trend after the implementation of CPOE/CDSS, the following linear regression model was used: 12
Yt = β0 + β1 * timet + β2 * interventiont + β3 * time after interventiont + et
Y0 = mean percentage at time is 0 = β0
-
β1 = baseline trend
β2 = immediate change after intervention
β3 = change in trend
Results
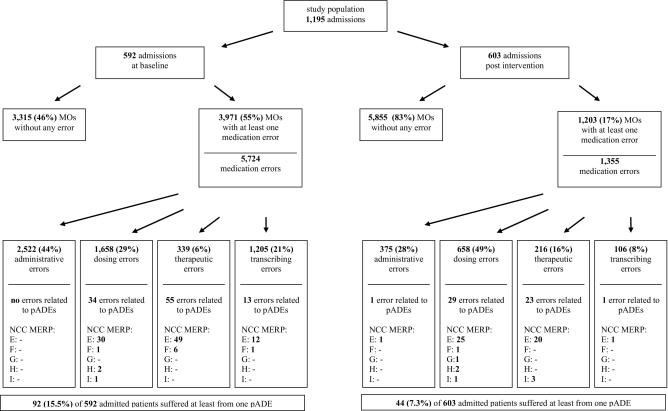
Five hundred and ninety-two patients during the baseline period and 603 patients during the post-intervention period were included (▶). Four patients did not provide consent and were excluded from the study. The mean age of the patients included in both periods was rather high (±65 years), which can be explained by the inclusion of a geriatric ward from one hospital in this study. During both periods, the mean number of MOs per hospital stay was 12 (baseline 12.3 ± 7.8, intervention 11.7 ± 8.7).
Table 2.
Table 2 Descriptives of the Study Population
| Study Period |
Hospital |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | p Value ∗ | UMCG | Twee-Steden Hospital | p Value ∗ | |
| Age (mean ± SD) | 65.5 ± 19.2 | 65.1 ± 19.1 | 0.74 | 58.2 ± 19.1 | 73.0 ± 16.0 | < 0.001 |
| Female (%) | 54.7 | 56.6 | 0.53 | 55.7% | 55.6% | 0.96 |
| MOs per hospital stay (mean ± SD) | 12.3 ± 7.8 | 11.7 ± 8.7 | 0.21 | 11.1 ± 8.4 | 13.0 ± 8.1 | < 0.001 |
| Patients (n) | 0.04 | NA† | ||||
| Internal medicine | 251 | 235 | 200 | 286 | ||
| Geriatrics | 153 | 135 | — | 288 | ||
| Gastroenterology/rheumatology | 188 | 233 | 421 | — | ||
| Total | 592 | 603 | 621 | 574 | ||
∗ Continuous variables are analyzed with a t test and categorical with a χ2 test.
† NA not appropriate: clearly the distribution per ward was different across the hospital as different wards were included.
MO = month; NA = not appropriate; SD = standard deviation; UMCG = University Medical Center Groningen, Groningen, The Netherlands.
The mean length of hospital stay for our total study population decreased significantly after the introduction of CPOE/CDSS: 14.6 ± 12.5 days pre-implementation versus 12.1 ± 11.6 days post-implementation.
During the baseline period, 55% of all MOs contained at least one error, whereas during the post-intervention period this was 17% (▶). In the baseline period, 15.5% of admitted patients experienced patient harm (pADE), as opposed to 7.3% after CPOE/CDSS was implemented (post-intervention) (▶).
Figure 2.
Flow chart of study population, medication orders (MOs), medication errors (MEs) and preventable adverse drug events (pADEs).
Effect of CPOE/CDSS
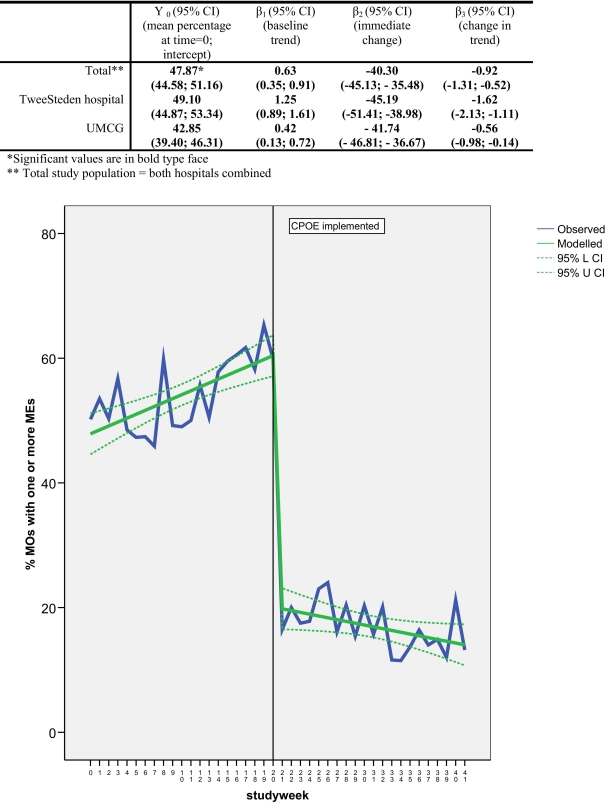
▶ ▶ ▶ show the medication error and pADEs patterns during the study period. The introduction of CPOE/CDSS led to a significant immediate absolute reduction of 40.3% (95% CI: 45, −36%) of medication orders with one or more errors (β2), and a change in trend of −0.92% (95% CI: −1.3, −0.5%) per week (β3) (▶). A trend of + 0.63% (95% CI: 0.35, 0.91%) of ME/MO per week was observed at baseline. Similar effect sizes in both trend and immediate change were observed in both hospitals (▶).
Figure 3.
Impact of CPOE/CDSS on percentage of medication orders with one or more medication errors (total study population).
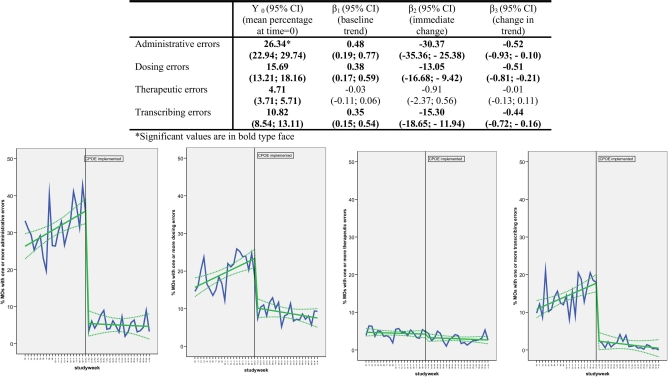
Figure 4.
Impact of CPOE/CDSS on percentage of medication orders with one or more subtypes of medication errors. Panels (from left to right): administrative errors, dosing errors, therapeutic errors, transcribing errors.
Figure 5.
Impact of CPOE/CDSS on percentage of admitted patients with one or more preventable Adverse Drug Events (pADEs).
The introduction of CPOE/CDSS led to an immediate decrease in level (β2) and trend (β3) for all types of MEs, except for therapeutic errors (▶). The introduction of CPOE/CDSS had the largest impact on the number of administrative and procedural errors (a significant immediate change of −30% [95% CI: −35%, −25%]). The immediate change in dosing and transcribing errors was about the same (−13% respectively −15%). With the introduction of CPOE/CDSS, the incidence of transcribing errors was not reduced to zero as in the University Medical Center Groningen transcribing errors still occurred in the post-intervention period, for example, labels fixed in the wrong place or on the wrong chart, or MOs still prescribed by hand instead of by CPOE/CDSS.
In contrast to the medication errors, the introduction of CPOE/CDSS did not lead to a significant change in level and trend of pADEs (▶). The observed underlying negative trend at baseline −4.0% pADEs per admission per month [95% CI: −7.70%, −0.38%] negated the obvious reduction in pADEs that was observed in the descriptive analysis (▶).
No autocorrelation was detected for any of the outcome parameters presented. Visual inspection of residuals versus time also did not indicate the presence of any autocorrelation.
Discussion
In our study, the introduction of CPOE/CDSS led to a large reduction in the incidence of medication errors in line with findings in earlier studies. 3–9 All types of errors were reduced with the exception of therapeutic errors. However, this substantial reduction in errors was not followed by a significant reduction in the incidence of pADEs.
The lack of effect on pADEs may be explained by the lack of effect on therapeutic errors due to the fact that, as we have demonstrated earlier, this is the very type of medication error most strongly associated with an increased risk of pADEs. 19 Another reason for not finding an effect may be that the CDSS in both hospitals was basic: only in case of overdosing, drug–drug interactions and allergies were alerts generated. To prevent other types of therapeutic errors, more advanced decision-making support would be needed such as, for example, adaptive dose support for patients with clinical chemical parameters that are outside the normal range (e.g., renally excreted medication in patients with renal failure), support when drugs are contraindicated (e.g., in case of the frail elderly) or support for drug choice by linking the system to formularies and disease guidelines that could lead to more optimal pharmacotherapy. A further reason could lie in the inappropriateness of the CDSS in respect to the clinical setting, since the CDSS is based on a national drug database for community pharmacies and not for hospital pharmacies. The standard drug safety alerts that are generated may not always be relevant for the particular hospital setting, for example, an alert for the combination of an ACE-inhibitor and a diuretic that gives rise to a risk of orthostatic hypotension or an alert for a high dose of furosemide, both very commonly found in the hospital. This may lead to an overload of irrelevant alerts and may cause alert fatigue. 20 One undesirable effect is that physicians not only override irrelevant alerts but also relevant ones. It is possible that other measurements of decision-making support are needed such as clinical pharmacists attending physicians meetings 21 at the medical ward or more intensive education in prescribing skills for junior physicians. 22,23
On average, fewer patients experienced a pADE in the post-intervention period than in the baseline period (a reduction approximately by half). However, because of the underlying negative trend at baseline, this decrease cannot be attributed to the introduction of CPOE/CDSS. In four recent reviews of the effect of CPOE/CDSS on medication safety, only a few studies evaluated the impact on pADEs or ADEs; this is possibly due to the labor-intensive way the data needed to determine (p)ADEs must be collected and assessed. 3,7,8 The evidence from these studies was inconclusive due to the fact that only half of the studies showed any significant effect on (p)ADEs and those studies that did show an impact primarily used a pre/post analysis. 9,24–26 Our ITS study design with segmented linear regression analysis was more robust because it evaluated the longitudinal effect of an intervention and controlled for trends appearing in the outcome. 12 Thus, differences in the findings between our study and other studies may be explained by the study design chosen and by the data analysis. Although there was no effect on the incidence of pADEs and therapeutic errors, it should be emphasized that the decrease in medication errors in the post-intervention period is likely to contribute to a decreased risk of preventable harm, because medication errors can be considered as process measurements, while pADEs are patient outcome measurements.
With respect to the other types of errors, the largest impact was seen on the rate of administrative and procedural errors due to an improvement in readability and due to the fact that key characteristics of a prescription had to be filled in (required fields), which led to more complete medication orders. Although these types of errors do not frequently lead to patient harm, 19 we would argue that it is worthwhile preventing them; when nurses and pharmacy technicians must correct these errors, a substantial amount of valuable time is wasted, which could be better spent on primary patient care. In hospitals with paper-based systems that do not include nurse transcription—a potential source of MEs—the introduction of CPOE/CDSS might lead to a less impressive reduction in MEs. The same may be the case for hospitals that do include pharmacy review in their paper-based systems, which might lead to a lower number of MEs in the baseline than hospitals that have no pharmacy review. In our study the TweeSteden Hospital made use of pharmacy review. The similar reduction in MEs found in both hospitals would indicate that pharmacy review in itself does not explain the observed reduction. In the baseline, probably other factors might be as or more important than the presence of this kind of pharmacy review, such as the illegibility and incompleteness of MOs.
The significant upward trend observed in MOs with one or more MEs in the baseline period is surprising. This might well be an artifact stemming from a learning effect for both observers in terms of detecting medication errors. When they were assessing data, the observers were not blinded, neither before nor after the introduction of CPOE/CDSS. It was not feasible, in view of the time constraints, to begin to classify errors only after all data (pre- and post-CPOE/CDSS) had been collected, and therefore we could not blind our data. This is thus one limitation of our study. At the start of the study, the observers individually assessed ten pilot patients and then discussed differences in classification. Despite this pilot period and the use of a strict classification scheme, interpretation of medication errors is subjective and a learning curve cannot be excluded. Another explanation could be that, due to the limited number of data points, the baseline was unstable. Although we have adequately fulfilled the Cochrane criteria of 3 data points before and after the intervention, 18 longer time periods and more data points may well result in a more stable and reliable baseline. One-year data collection before and after CPOE implementation would facilitate a correction for seasonality. However, there is no evidence that pADEs are subject to seasonal influences. Longer data collection was not feasible in our case because of the labor-intensive assessment of pADEs, along with financial constraints.
The delay in implementation on the gastroenterology/rheumatology ward was due to management issues and strategic interests. The eventual implementation process on this ward took as long as on the other ward in the University Medical Center Groningen (17 weeks). As on the other wards, data collection started 8 weeks after finishing the implementation process. In another study, we concluded that physicians and nurses were positive about the way CPOE/CDSS was introduced as well as about the system itself. 27 In addition, the CPOE/CDSS users on the gastroenterology/rheumatology ward were also satisfied and did not show any resistance to the system. These findings suggest that the delay would not have had any effect on the results of CPOE/CDSS on MEs and pADEs.
One strength of our study is that we evaluated the impact of CPOE/CDSS in two different types of hospitals with one home-grown and one commercial package. Although these circumstances are considered potential sources of bias, similar effects for medication errors were demonstrated even despite different baseline rates. This emphasizes the robustness of our study findings and implies that our results could be applicable to a wider range of settings than those of studies simply evaluating one type of CPOE system in a single hospital.
Our study was performed in adult-based general medical wards, and findings should not be extrapolated to special-care settings such as intensive care wards. Future research may clarify the effect of CPOE/CDSS in these settings. Since investigating the effect of CPOE/CDSS on the readmission rate would have been interesting, future research is also needed into this effect.
Conclusions
Based on our findings, it can be concluded that CPOE with basic CDSS decreased medication errors and thus possibly might contribute to a decreased risk of preventable harm. However, we were not able to confirm any effect on actual patient harm. Implementing a CPOE with basic CDSS is simply not enough to prevent pADEs in a general internal medicine/geriatric setting. More effort is needed, such as more advanced CDSS or other forms of clinical decision support.
Acknowledgments
The authors thank Y. Chahid, A. Dequito, V. Tanaydin and J. Wolters for their assistance in data collection. The authors also thank all the physicians, nurses, and patients who cooperated in this study.
Footnotes
This work (file Number 94504109) was funded by an unconditional grant from the Netherlands Organization for Health Research and Development (ZonMw). This agency played no role in the collection, analysis and interpretation of the data or in the decision to submit the manuscript for publication.
References
- 1. To Err Is Human: Building a Safer Health SystemWashington, DC: National Academies Press; 1999. [PubMed]
- 2.Kaushal R, Bates DW. Information technology and medication safety: What is the benefit? Qual Saf Health Care 2002;11(3):261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfstadt JI, Gurwitz JH, Field TS, et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: A systematic review J Gen Intern Med 2008;23(4):451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhry B, Wang J, Wu S, et al. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care Ann Intern Med 2006;144(10):742-752. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors J Am Med Assoc 1998;280(15):1311-1316. [DOI] [PubMed] [Google Scholar]
- 6.Shamliyan TA, Duval S, Du J, Kane RL. Just what the doctor ordered. Review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res 2008;43(1 Pt 1):32-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eslami S, de Keizer NF, Abu-Hanna A. The impact of computerized physician medication order entry in hospitalized patients—A systematic review Int J Med Inform 2008;77(6):365-376. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: A systematic review Arch Intern Med 2003;163(12):1409-1416. [DOI] [PubMed] [Google Scholar]
- 9.Ammenwerth E, Schnell-Inderst P, Machan C, Siebert U. The Effect of Electronic Prescribing on medication Errors and adverse drug Events: A systematic review J Am Med Inform Assoc 2008;15(5):585-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors J Am Med Assoc 2005;293(10):1197-1203. [DOI] [PubMed] [Google Scholar]
- 11.Han YY, Carcillo JA, Venkataraman ST, et al. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system Pediatrics 2005;116(6):1506-1512. [DOI] [PubMed] [Google Scholar]
- 12.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research J Clin Pharm Ther 2002;27(4):299-309. [DOI] [PubMed] [Google Scholar]
- 13.Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: A review J Am Med Inform Assoc 2007;14(1):29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bemt van den Pmla EACG. Drug-related problems: Definitions and classifications EJHP 2006;12(suppl.):10-12. [Google Scholar]
- 15.National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) NCC MERP index for categorizing medication errorshttp://www.nccmerp.org 2006(Accessed September 30, 2008).
- 16.Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the Operational assessment of adverse drug reactions J Am Med Assoc 1979;242:623-632. [PubMed] [Google Scholar]
- 17.van Doormaal JE, Mol PG, van den Bemt PM, et al. Reliability of the assessment of preventable adverse drug events in daily clinical practice Pharmacoepidemiol Drug Saf 2008;17(7):645-654. [DOI] [PubMed] [Google Scholar]
- 18.The Cochrane Effective Practice and Organisation of Care Group (EPOC)http://www.epoc.cochrane.org/Files/Website/Reviewer%20Resources/inttime.pdf 2008. Accessed September 2008.
- 19.van Doormaal JE, van den Bemt PMLA, Mol PGM, et al. Medication Errors: The Impact of Prescribing and Transcribing Errors on Preventable Harm in Hospitalised Patients Qual Saf Health Care February2009;18(1):22-27. [DOI] [PubMed] [Google Scholar]
- 20.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry J Am Med Inform Assoc 2006;13(2):138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: A systematic review Arch Intern Med 2006;166(9):955-964. [DOI] [PubMed] [Google Scholar]
- 22.Heaton A, Webb DJ, Maxwell SR. Undergraduate preparation for prescribing: The views of 2413 UK medical students and recent graduates Br J Clin Pharmacol 2008;66(1):128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coombes ID, Mitchell CA, Stowasser DA. Safe medication practice: Attitudes of medical students about to begin their intern year Med Educ 2008;42(4):427-431. [DOI] [PubMed] [Google Scholar]
- 24.Upperman JS, Staley P, Friend K, et al. The impact of hospitalwide computerized physician order entry on medical errors in a pediatric hospital J Pediatr Surg 2005;40(1):57-59. [DOI] [PubMed] [Google Scholar]
- 25.Evans RS, Pestotnik SL, Classen DC, et al. Preventing adverse drug events in hospitalized patients Ann Pharmacother 1994;28(4):523-527. [DOI] [PubMed] [Google Scholar]
- 26.Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents N Engl J Med 1998;338(4):232-238. [DOI] [PubMed] [Google Scholar]
- 27.van Doormaal JE, Mol PGM, Zaal RJ, et al. Computerised Physician Order Entry (CPOE) system: Expectations and experiences of users. J Eval Clin Pract (in press). [DOI] [PubMed]