Abstract
During fiscal year 1986, 40 out of 196 patients (21%) developed hyperamylasemia following orthotopic liver transplantation. The placement of a retropancreatic aortohepatic arterial interposition graft was associated with hyperamylasemia (p < 0.025). Eight patients (20%) developed clinically significant acute pancreatitis and its sequelae; abscesses and pseudocysts each in 2. Pancreatitis was attributable to the retropancreatic arterial graft in 4, viral infection in 2 and obstruction of the pancreatic duct in 1 patient. All 4 patients with arterial graft-related pancreatitis exhibited poor graft function immediately postoperatively, of whom 2 required retransplantation – both of which failed to function. Five patients died (63%); 2 from primary graft non-function, 2 due to sepsis and 1 from systemic cytomegalovirus infection. We conclude that acute pancreatitis after liver transplantation is a life-threatening complication which is often associated with graft non-function.
Keywords: liver transplantation, pancreatitis, pseudocyst, abscess
Introduction
Since first reported in 1964 (1), pancreatitis has been known as a rare but serious complication of organ transplantation. After renal transplantation, the incidence of acute pancreatitis varies from 2 to 7% and a mortality rate ranges from 17 to 70% (2–7). In cardiac transplantation, rates of 19% and 25% each are reported (8). Pancreatic complications following transplantation of these organs have been attributed to multiple factors including hypercalcemia (2–5), uremia (4), gallstones (4, 7), surgical trauma (7, 9), corticosteroids (2–5), azathioprine (3–5), cyclosporine toxicity (8), cytomegalovirus (CMV) or hepatitis B virus (HBV) infection (2, 4, 10) and low perfusion status of the pancreas during cardiopulmonary bypass (8). In liver transplantation, on the other hand, hepatitis B-related liver disease is the only known factor associated with pancreatitis long-term after transplant (11). The purpose of this study was to evaluate the clinical presentation of pancreatitis and its sequelae after orthotopic liver transplantation (OLTx), and to identify factors pertinent to the development of this serious complication.
Patients and methods
Charts of 196 adult patients who underwent 233 OLTx during a 1-yr period between July 1, 1986 and June 30, 1987 at the Presbyterian University Hospital of Pittsburgh were reviewed. Their ages ranged from 19 to 76 yr with a mean of 45 yr, and 85 were male (43%). The indications for OLTx consisted of postnecrotic cirrhosis in 101, (21 alcoholic, 80 non-alcoholic), primary biliary cirrhosis in 41, primary sclerosing cholangitis in 20, neoplasm in 10 and others in 24 patients. None had hyperlipidemia nor evidence of hyperparathyroidism. One patient was excluded from the study due to pre-existing acute pancreatitis at the time of OLTx.
All allografts were preserved in Euro-Collins’ solution (4°C) and OLTx was performed with the use of standard techniques: gastroduodenal artery was ligated routinely, while the splenic artery was left intact. When the inflow artery was judged to be inadequate, an aortohepatic interposition graft (AHIG) was placed through a retropancreatic tunnel from the infrarenal aorta (12). If the portal vein was thrombosed, a venous graft was placed between the donor portal vein and the recipient mesosplenic confluence as an interposition graft (13). Veno-venous bypass was used routinely in all but 2 cases. Bile duct reconstruction consisted of choledocho-choledochostomy (114 transplants, 49%) or Roux-en-Y choledocho-jejunostomy (110 transplants, 47%), while this could not be completed in the other 9 due to various technical problems. An intraoperative cholangiograms were taken routinely after completion of a choledocho-choledochostomy. Although a retained and impacted cystic duct stone was found in 1 patient, none of the intra- or postoperative cholangiograms showed evidence of retained common duct stones. The allograft gallbladder was excised routinely following bile duct reconstruction.
Posttransplant immunosuppression consisted of cyclosporine and steroids. Steroid therapy consisted of i.v. bolus of methylprednisone (1 gram, immediately before reperfusion) and postoperative rapid tapering of i.v. methylprednisone, from 200 mg/d to 20 mg/d in 6 d, followed by maintenance with methylprednisone i.v. or prednisone p.o. at 20 mg/d. Rejection episodes were treated with a bolus of methyl prednisone, 1 g i.v., followed by the afore-mentioned recycle of steroids. Steroid-resistant rejection was treated with Orthoclone OKT3® (Ortho Pharmaceutical Co., Raritan, NJ).
Postoperatively, a serum amylase was measured daily in the intensive care unit, and then twice weekly in the ward. Hyperamylasemia was defined as a serum amylase level over 400 IU/l (normal 0–100 IU/l). None of the patients developed clinical evidence of parotitis postoperatively. When pancreatitis was suspected, computed tomography (CT) of the abdomen was performed.
The diagnosis of pancreatitis was made when the following criteria were satisfied: 1) direct evidence of pancreatitis, i.e., swollen pancreas with saponification of the adipose tissue on exploratory laparotomy or retransplantation, 2) autopsy findings, and 3) hyperamylasemia associated with symptoms and signs of pancreatitis as well as findings of pancreatitis on CT. In patients with pancreatitis, CT was performed periodically to evaluate the changes in the pancreas, and to rule out the development of its sequelae.
Primary non-function was defined as inability of the graft to sustain metabolic homeostasis of the recipient during the 1st postoperative wk, as manifested by grade III or IV coma, coagulopathy with the prothrombin time over 20 sec, high transaminases, progressive or persistent hyperbilirubinemia and rapid development of renal failure, which resulted in retransplantation or death of the recipient. Poor graft function was defined as SGOT over 3500 IU/l, SGPT over 2500 IU/l or prothrombin time over 20 sec during the first 5 postoperative d but without the need for retransplantation.
In patients with fever of undetermined origin or with leukocytopenia, viral studies were performed, which consisted of viral titers, buffy coat, urine and throat cultures as well as a needle liver biopsy for a hematoxylin-eosin stain and immunohistochemical stains for viral antigens.
Chi-square test and one-way analysis of variance were used for the statistical analyses in this study.
Results
Of the 40 patients with posttransplant hyperamylasemia, 8 (20%) developed clinically significant pancreatitis (Table 1). The diagnosis of pancreatitis was established on exploratory laparotomy or retransplantation in 6 patients (Case 1–4, 6, 8), at autopsy in 1 (Case 7), and by hyperamylasemia associated with typical presentation of pancreatitis and positive CT findings in 1 patient (Case 5). Their ages varied from 26 to 65 yr (mean 46), and 4 were male (50%). Their peak serum amylase levels varied from 536 to 2900 IU/l, with a mean of 2303 IU/l. In 4 patients (Case 1–4), the occurrence of pancreatitis was attributable to the placement of the AHIG (Fig. 1). Other causes included obstruction of the pancreatic duct by the distal limb of a migrated T-tube (Case 5) and viral infection due to CMV (Case 7) or HBV (Case 6). In case 8, who showed hyperamylasemia immediately postoperatively and developed pancreatic pseudocysts, no obvious causative factor was identified.
Table 1.
Clinical data of patients who developed pancreatitis after OLTx
| Case | Age/ Sex |
POD# on Diagnosis |
Cause | Highest Amylase (IU/l) |
Clinical Presentation |
Sequelae | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 37/M | 2 | IAG | 1.764 | PGNFN | none | OLTx IV | died of PGNFN |
| 2 | 62/F | 2 | IAG | 535 | PGNFN | pancreatic abscesses (14)* |
drainage, OLTx II | died of PGNFN |
| 3 | 26/M | 1 | IAG | 1.193 | poor GFN, fever, hypocal- cemia |
pseudocysts (32)*, in- fected pseudocysts (80*) |
drainage | alive and well |
| 4 | 43/F | 2 | IAG | 7.121 | poor GFN ARF, semicoma | retroperitoneal abscess (15)* |
drainage | died of MOF |
| 5 | 49/F | 71 | migrated T-tube |
2.900 | fever, back pain | none | removal of T-tube | alive and well |
| 6 | 58/M | 108 | HBV | 1.889 | stupor, acute abdomen | none | NPO, NG suction IV fluid | died of MOF |
| 7 | 31/M | 41 (au- topsy) |
CMV | 1.072 | systemic CMV infection | none | died of systemic CMV in- fection |
|
| 8 | 65/F | 56 | unknown | (1.952)+ | pancreatic pseudocysts | infected pseudocysts (62)* |
drainage | alive and well |
=number of postoperative day;
=data on the 3rd postoperative d;
OLTx=orthotopic liver transplantation; POD#=postoperative day; IAG=iliac artery graft PGNFN=primary graft non-function; GFN graft-function; ARF=acute renal failure; MOF=multiple organ failure; HBV=hepatitis B virus; CMV=cytomegalovirus.
Fig. 1.
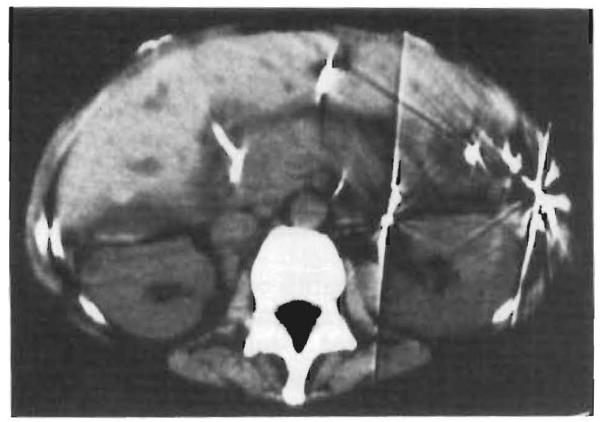
Computed tomogram of Case 4 on the posttransplant d 8. The entire pancreas is swollen.
Of the 4 patients (Case 1–4) in whom acute pancreatitis was diagnosed on post-transplant d 1 or 2, 2 (Case 1, 2) developed primary graft non-function, while the other 2 (Case 3, 4) exhibited extremely poor allograft function with a high prothrombin time, SGOT and SGPT as well as persistent hyperbilirubinemia. The development of renal failure was another feature among these patients. Case 1 and 2 underwent retransplantation but both again failed to function.
The sequelae of pancreatitis consisted of the formation of pseudocysts in 2 patients (Case 3, 8), a pancreatic abscess in 1 (Case 2) and a large retroperitoneal abscess in 1 (Case 4). The pseudocysts in both patients became infected. Overall, infectious complications of the pancreatitis occurred in 4 patients (50%), the pathogen of which consisted of Staphylococcus aureus in 1, and Pseudomonas aeruginosa and P. maltophilia in another. On the other two occasions, the pus was culture-negative.
Treatment of pancreatitis consisted of bowel rest and intravenous hyperalimentation. In Case 6, removal of the migrated T-tube resulted in rapid disappearance of the symptoms and normalization of the serum amylase. The abscesses and infected pseudocysts were drained surgically or under CT guidance. Five patients died (63%); 2 from primary graft non-function (Case 1, 2), 2 from multiple organ failure (Case 4, 6), and 1 from systemic CMV infection (Case 7).
All 8 patients with clinically proven pancreatitis in our series received no azathioprine, showed no evidence of common duct stones, and none demonstrated evidence of cyclosporin toxicity as manifested by a high blood cyclosporin level in combination with mental confusion, severe tremor and renal failure. Furthermore, none of these patients has had chronic renal failure prior to OLTx. One patient (Case 5) underwent splenectomy for patent Warren shunt during OLTx.
In order to identify the cause of hyperamylasemia in 40 patients following OLTx, the following perioperative variables were correlated with hyperamylasemia; the placement of an artery or portal vein graft, methods of bile duct reconstruction and operative blood loss (Table 2). A statistically significant correlation was identified between hyperamylasemia and the placement of an AHIG during OLTx (p < 0.025, χ2 = 6.38), while the placement of a portal vein graft or methods of bile duct reconstruction did not. The operative blood loss tended to be higher among the patients with hyperamylasemia but this did not achieve statistical significance.
Table 2.
Correlation between hyperamylasemia and pertinent clinical variables
| Serum amylase (IU/l) |
|||
|---|---|---|---|
| Variable | ≥ 400 | < 400 | Total |
| Aortohepatic interposition graft | |||
| yes* | 12 (34.3%) | 23 (65.7%) | 35 (100%) |
| no* | 32 (16.2%) | 166 (83.8%) | 198 (100%) |
| Portal vein graft | |||
| yes | 2 (18.2%) | 9 (81.8%) | 11 (100%) |
| no | 38 (17.1%) | 184 (82.9%) | 222 (100%) |
| Method of bile duct reconstruction | |||
| C-C | 93 (81.6%) | 21 (18.4%) | 114 (100%) |
| C-J | 87 (79.1%) | 23 (20.9%) | 110 (100%) |
| Operative blood loss (U) | |||
| mean±S.D. | 26.7±33.3† | 18.2±24.7† | |
| range | 3–250 | 1–155 | |
p < 0.025 (χ2=6.38);
p=0.06 (One-way analysis of variance);
C-C=choledochocholedochostomy; C-J = choledochojejunostomy.
Among the 32 patients who developed asymptomatic hyperamylasemia following OLTx, the elevation of the amylase was attributable to accidental injury of the portal vein and the pancreas during portal vein cannulation for veno-venous bypass in 1 (3%) and acute renal failure in 3 patients (9%). In the remaining 32 grafts of 28 patients (88%), no definitive causative factor could be identified.
Discussion
Acute pancreatitis in this series can be classified into two categories, i.e., early (Case 1–4) and late (Case 5–8). Pancreatitis late after OLTx seems to be attributable to causes which are not specific for OLTx. On the other hand, a peculiar feature of pancreatitis early after OLTx was related to the placement of the AHIG and presented with compromised graft function.
Factors for the development of acute pancreatitis early after OLTx include direct compression of the pancreas for the exposure of the hepatic hilus, suboptimal splanchnic decompression in venovenous bypass, splenectomy mainly for patent Warren shunt (14), the placement of AHIG, intraoperative cholangiography and intra- or postoperative immunosuppression.
In our series, all 4 instances of pancreatitis early after OLTx were associated with the placement of AHIG. During the period studied, the AHIG was placed exclusively retropancreatic, by blunt finger dissection of the plane between the pancreas and the left renal vein. It seems highly likely that the manipulation of the pancreas in such a maneuver is at least partly responsible for the development of aucte pancreatitis. The high correlation between hyperamylasemia and the placement of AHIG in this series seems to reinforce such speculation.
For the arterial reconstruction in OLTx, anatomical reconstruction has always been the first choice. When the arterial flow after reconstruction is in question, it should be objectively evaluated with the use of a flowmeter (15). When the flow is suboptimal, less than 200 ml/min in adults, the use of a AHIG is indicated to avoid hepatic artery thrombosis (16). For the placement of AHIG, we now place AHIG antepancreatic, rather than retropancreatic, as a “jump” graft through the mesocolon (17). Since we adopted this technique, we have encountered far fewer incidences of acute pancreatitis early after OLTx.
Although acute pancreatitis is a known sequela of splenectomy, we have not experienced such a complication (14). Reflux of a radiopaque dye into the pancreatic duct is frequently observed on an intraoperative cholangiogram after choledochocholedochostomy. This, however, does not seem to be a predisposing factor for post-OLTx hyperamylasemia.
As to the strong correlation between acute pancreatitis early after OLTx and poor allograft function, the pancreas has been known as a key organ to secrete hepatotrophic factors, of which insulin seems to be the most important (18). Furthermore, the severity of acute pancreatitis has been known to correlate with elevated SGOT and hyperglycemia (19). We believe that acute pancreatitis immediately after OLTx is a clinical entity which can cause posttransplant graft non- or poor function.
As to asymptomatic hyperamylasemia after OLTx, the incidence was 14.3% (24/196 patients), which is comparable to cardiac (19%) or non-cardiac (10%) surgical procedures (20, 21). Since acute pancreatitis early after OLTx is associated with a very high mortality, it is extremely important to differentiate clinical pancreatitis from asymptomatic hyperamylasemia. When hyperamylasemia is encountered early after OLTx, renal function should be taken into consideration, and if normal or near normal, immediate measurement of serum lipase and emergency CT should be performed.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM 29961 from the National Institute of Health, Bethesda, Maryland.
References
- 1.Starzl TE. Experience in renal transplantation. W. B. Saunders Co.; Philadelphia: 1964. [Google Scholar]
- 2.Johnson WC, Nabseth DC. Pancreatitis in renal transplantation. Ann Surg. 1970;171:309. doi: 10.1097/00000658-197002000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez JA, Rosenberg JC. Post-transplantation pancreatitis. Surg Gynecol Obstet. 1976;143:795. [PubMed] [Google Scholar]
- 4.Corrodi P, Knoblauch M, Binswanger U, Scholzel E, Largiader F. Pancreatitis after renal transplantation. Gut. 1975;16:285. doi: 10.1136/gut.16.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frick TW, Fryd DS, Sutherland DER, Goodale RL, Simmons RL, Najarian JS. Hypercalcemia associated with pancreatitis and hyperamylasemia in renal transplant recipients: data from the Minnesota randomized trial of cyclosporine versus antilymphoblast azathioprine. Am J Surg. 1987;154:487. doi: 10.1016/0002-9610(87)90260-1. [DOI] [PubMed] [Google Scholar]
- 6.Lorber MI, Van Buren CT, Flechner SM, Williams C, Cahan BD. Hepatobiliary and pancreatic complications of cyclosporine therapy in 466 renal transplant recipients. Transplantation. 1987;43:35. doi: 10.1097/00007890-198701000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Penn I, Durst AL, Machado M, et al. Acute pancreatitis and hyperamylasemia in renal homograft recipients. Arch Surg. 1972;105:167. doi: 10.1001/archsurg.1972.04180080021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colon R, Frazier OH, Kahan BD, et al. Complications in cardiac transplant patients requiring general surgery. Surgery. 1988;103:32. [PubMed] [Google Scholar]
- 9.Starzl TE, Porter KA. Use of heterologous anti-lymphocytic globulin in human renal homotransplantation. In: Wolstenholme GE, O’Connor M, editors. Antilymphocytic serum (Chiba Foundation Study Group Book #29) Vol. 4. Churchhill; London: 1967. [Google Scholar]
- 10.Parham DM. Post-transplantation pancreatitis associated with cytomegalovirus (report of a case) Human Pathology. 1981;12:663. doi: 10.1016/s0046-8177(81)80053-6. [DOI] [PubMed] [Google Scholar]
- 11.Alexander JA, Demetris AJ, Gavaler JS, Makowka L, Starzl TE, Van Thiel DH. Pancreatitis following liver transplantation. Transplantation. 1988;45:1062. doi: 10.1097/00007890-198806000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw BW, Jr, Iwatsuki S, Starzl TE. Alternative methods of arterialization of the hepatic graft. Surg Gynecol Obstet. 1984;159:490. [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw BW, Jr, Iwatsuki S, Bron K, Starzl TE. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985;161:66. [PMC free article] [PubMed] [Google Scholar]
- 14.Esquivel EO, Klintmalm GBG, Iwatsuki S, et al. Liver transplantation in patients with patent splenorenal shunts. Surgery. 1987;101:430. [PMC free article] [PubMed] [Google Scholar]
- 15.Yanaga K, Makowka L, Starzl TE. Is hepatic artery thrombosis after liver transplantation really a surgical complication? Transplant Proc. 1989;21:3511. [PMC free article] [PubMed] [Google Scholar]
- 16.Tzakis AG, Gordon RD, Shaw BW, Iwatsuki S, Starzl TE. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporin era. Transplantation. 1985;40:667. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein RM, Secrest CL, Klintmalm GB, Husberg BS. Problematic vascular reconstruction in liver transplantation. Part I. Arterial. Surgery. 1990;107:540. [PubMed] [Google Scholar]
- 18.Starzl TE, Porter KA, Kashiwagi N, Lee IY, Russel WJI, Putnam CW. The effect of diabetes mellitus on portal blood hepatotrophic factors in dogs. Surg Gynecol Obstet. 1975;140:549. [PMC free article] [PubMed] [Google Scholar]
- 19.Ranson JHC, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69. [PubMed] [Google Scholar]
- 20.Rattner DW, Gu ZY, Vlahakes GJ, Warshaw AL. Hyeramylasemia after cardiac surgery: incidence, significance and management. Ann Surg. 1989;209:279. doi: 10.1097/00000658-198903000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrissey R, Berk JE, Fridhandler L, Pelot D. The nature and significance of hyperamylasemia following operation. Ann Surg. 1974;180:67. doi: 10.1097/00000658-197407000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


