Introduction
The development of de novo malignancies is a well-recognized complication of the immunosuppressed state that follows organ transplantation (1-6). In the cyclosporine era, lymphomas, skin cancer and Kaposi’s sarcoma account for more than half of the reported cases (6). Other tumors of mesenchymal origin are uncommon.
We describe a case of a primary smooth muscle tumor arising in a liver allograft. The tumor was of donor origin. To our knowledge this complication has not been seen before.
Case Report
An 18-month old white female underwent orthotopic liver transplantation for biliary atresia in April of 1986. Immunosuppression was with cyclosporine and prednisone. She had previously undergone a Kasai procedure at the age of 2 months. The donor was a healthy 22-month-old black male who died of a closed head injury. A complete autopsy was subsequently performed, which revealed only bilateral subdural hematomas. The kidneys were also harvested, and transplanted en bloc into a 53-year-old female.
The patient’s post-operative course was initially complicated by steroid-resistant rejection, which was successfully treated with OKT3. She later developed cortical blindness of unknown etiology, following an episode of grand mal seizure, but this resolved gradually over the next several months. Abdominal ultrasounds were performed over the next several weeks (4, 12, 21, and 29 days post-transplant). None of these showed a hepatic parenchya lesion. An abdominal CT scan performed on post-op d 25 also showed a normal liver. She was discharged on cyclosporine and prednisone (60 mg/kg and 0.75 mg/kg respectively), with normal liver function. In May of 1989 a routine follow-up abdominal ultrasound revealed a single, hypoechoic lesion in the left lobe of the liver measuring 3 × 2 cm. A CT scan of the abdomen confirmed this finding, but was otherwise unremarkable. At the time she was completely asymptomatic, and her physical examination was also unremarkable.
Total bilirubin was 0.2 mg/dl, alkaline phosphatase was 267 u/l, GGTP 53 u/l, SGOT 31 u/l, and SGPT 22 u/l. Prothrombin time was 12.2 seconds. Alpha fetoprotein was < 2 ng/ml and CEA 1.2 ng/ml.
An ultrasound-guided biopsy showed fibroadipose tissue, plus a moderately cellular minute portion of tissue composed of wavy, thin, elongated cells that stained positively with muscle-specific actin.
The patient underwent a left lateral segmentectomy. Thorough exploration of the abdominal cavity failed to reveal any additional pathology.
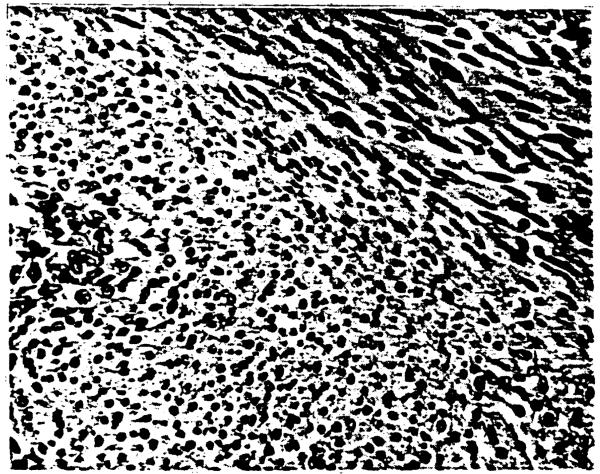
The resection specimen measured 10 × 7 × 3.5 cm and contained a 3 cm tumor with clean lines of resection. The tumor was oval and encapsulated except for two small satellite nodules adjacent to it, each measuring 0.7 cm in diameter. It was composed of interlacing bundles of spindle cells (Fig. 1) tightly packed in some areas with some cellular pleomorphism focally. The nuclei were long, blunt-ended and sometimes wavy. It had focal areas of necrosis, possibly corresponding to a previous biopsy site. Mitoses were less than 1/10 HPF. Tumor cells stained with desmin focally and with muscle-specific actin, but not with S100 protein, suggesting a muscle origin. This was proven by electron microscopy (Fig. 2). The tumor cells had a well-defined basement membrane, masses of cytoplasmic filaments with dense bodies, scattered electron-dense condensations along the plasma membrane and numerous pinocytotic vesicles as well as abundant glycogen. The liver and tumor tissues, as well as positive and negative controls, were studied with a probe complementary to a repeated sequence on the Y-chromosome labeled with biotinylated d-UTP by the random priming method. The tumor showed a male pattern indicative of its donor origin.
Fig. 1.
Interlacing bundles of elongated cells showing some nuclear variation, perinuclear clearing and distinct cell borders. H&E stain.
Fig. 2.
Electron micrograph illustrating tumor cells with features of smooth muscle: basement membrane, pinocytoxic vesicles, masses of cytoplasmic filaments with scattered electron densities and glycogen.
The patient made an uneventful recovery. Post-operatively a CT scan of the chest was obtained that showed only left lower lobe atelectasis, which was attributed to her recent surgery. A bone scan was negative. She was discharged with normal liver function. She is currently doing well 8 months following the resection.
Discussion
Any state of immunodeficiency, whether congenital acquired or iatrogenic, is complicated by an increased incidence of certain neoplasms (1-6). Since the early days of renal transplantation malignant disease has been recognized as a significant hazard, with an incidence ranging from 1% to 16%, and an average of 6.1% (6).
Although the inadvertent transplantation of malignant tumors continues to occur sporadically despite careful screening of donors (7), most of these tumors arise de novo. In the cyclosporine era, immunosuppression therapy is complicated by a relatively high incidence of non-Hodgkin’s lymphoma, skin cancer, and Kaposi’s sarcoma (6).
In the particular case of liver transplantation, the Cambridge group reported a series of 122 patients who survived for 3 months or more, among whom 7 de novo tumors were found (8). These included a patient described as having a reticulum cell sarcoma of host origin that developed in the grafted liver (8, 9). The remaining 6 patients had extrahepatic neoplasms, including a case of small bowel leiomyoma. To the best of our knowledge our case represents the first instance of a primary liver tumor, that is, of donor origin, to be reported in a liver transplant patient.
Primary smooth muscle tumors of the liver are rare entities and we were only able to find 21 reported cases in the literature (10–13). Four were leiomyomas and the rest were primary hepatic leiomyosarcomas. Clinical information was provided in 20 of these cases. There were 13 females and 7 males, with an age range of 12 to 86 yr. Most presented with an abdominal mass or hepatomegaly (10, 11).
Smooth muscle tumors seem to represent a biological continuum in which one is forced to draw certain arbitrary lines in designating some as “benign” and others as “malignant”. A number of features such as size, cellularity, atypia, and necrosis correlate to some extent with malignancy, with mitotic activity being the most accurate and reproducible. Even this last criterion, however, is subordinate to the organ of origin (14). Because of their rarity it is very difficult to set forth criteria that will distinguish benign from malignant variants in cases of primary hepatic smooth muscle tumors. Leiomyosarcomas are described as having cells with hyperchromatic nuclei, with considerable variation in size, and frequent mitoses (one or more per high-power field) (15). In the 2 reported cases of leiomyoma that have addressed this issue, mitoses were rare (less than 1/10 high-power fields) (16).
By analogy with other gastrointestinal smooth muscle tumors it is to be expected that, although a combined assessment of size, cellularity, and cellular atypia conveys some impression of the aggressive potential of these neoplasms, absolute criteria for the diagnosis of malignancy cannot be established.
The case we present, with its low mitotic rate, focally highly cellular, and areas of necrosis, falls into an indeterminate category. Treatment consists of resection with a margin of normal liver. The unpredictability of these tumors mandates a careful and life-long follow-up.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Doyle H, Tzakis AG, Yunis E, Starzl TE. Smooth muscle tumor arising de novo in a liver allograft. A case report. Clin Transplantation 1990: 4: 000-000.
References
- 1.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalesnik MA, Makowka L, Starzl TE. The diagnosis and treatment of post transplant lymphoproliferative disorders. Curr Prob Surg. 1988;25:365–472. doi: 10.1016/0011-3840(88)90011-1. [DOI] [PubMed] [Google Scholar]
- 4.Penn I. Malignancies associated with immunosuppressive or cytotoxic therapy. Surgery. 1978;83:492–502. [PubMed] [Google Scholar]
- 5.Penn I. The price of immunotherapy. Curr Prob Surg. 1981;18:682–751. doi: 10.1016/s0011-3840(81)80011-1. [DOI] [PubMed] [Google Scholar]
- 6.Penn I, Brunson ME. Cancers after cyclosporine therapy. Transplant Proc. 1988;20(suppl. 3):885–892. [PubMed] [Google Scholar]
- 7.Marsh JW, Jr., Esquivel CO, Makowka L, et al. Accidental transplantation of malignant tumor from a donor to multiple recipients. Transplantation. 1987;44:449–450. doi: 10.1097/00007890-198709000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polson RJ, Neuberger J, Forman D, Calne R, Williams R. De Novo malignancies after liver transplantation. Transplant Proc. 1988;20:94–97. [PubMed] [Google Scholar]
- 9.Portmann B, Schindler AM, Murray-Lyon IM, Williams R. Histological sexing of a reticulum cell sarcoma arising after liver transplantation. Gastroenterology. 1976;70:82–84. [PubMed] [Google Scholar]
- 10.Maki HS, Hubert BC, Sajjad SM, Kirchner JP, Kuehner ME. Primary hepatic leiomyosarcoma. Arch Surg. 1987;122:1193–1196. doi: 10.1001/archsurg.1987.01400220103020. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins EP, Jordan GL, McGavran MH. Primary leiomyoma of the liver. Successful treatmemt by lobectomy and presentation of criteria for diagnosis. Am J Surg Path. 1980;4:301–304. [PubMed] [Google Scholar]
- 12.Iwatsuki S, Todo S, Starzl TE. Right trisegmentectomy with a synthetic vena cava graft. Arch Surg. 1988;123:1021–1022. doi: 10.1001/archsurg.1988.01400320107023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rummeny E, Weissleder R, Stark DD, et al. Primary liver tumors: diagnosis by MR imaging. AJR. 1989;152:63–72. doi: 10.2214/ajr.152.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Brook JJ. Disorders of soft tissues. In: Sternberg S, editor. Diagnostic Surgical Pathology. Vol. 1. Raven Press; 1989. pp. 166–168. Chap. 5. [Google Scholar]
- 15.Ishack KG. Mesenchymal tumors of the liver. In: Okude K, Peters RL, editors. Hepatocellular Carcinoma. John Wiley and Sons, Inc.; New York: 1976. pp. 268–275. [Google Scholar]
- 16.Hawkins EP, Jordan GL, McGavran MH. Primary leiomyoma of the liver. Am J Surg Pathol. 1980;4:301–304. [PubMed] [Google Scholar]