Abstract
Lower respiratory tract infections are one of the leading causes of morbidity and mortality in children worldwide. Recent technological advances in the field of molecular biology have allowed virologists to detect many previously undetected viral pathogens. Two of these, human metapneumovirus (hMPV) and human bocavirus (HBoV), are of particular clinical interest to pediatric health care providers. This review discusses the most common viral respiratory infections in children, explores the role of newly discovered respiratory pathogens, and describes techniques for the diagnosis of viral respiratory infections.
Keywords: pediatric respiratory tract infections; communicable diseases, emerging; human bocavirus; metapneumovirus
Introduction
Lower respiratory tract infections, commonly caused by viruses [1], are one of the leading causes of morbidity and mortality in children worldwide. In the United States, approximately 30,000 children each year are hospitalized during their first year of life due to a viral lower respiratory tract infection [2]. In developing countries, the problem is even more staggering, where up to 1.9 million children die each year due to acute respiratory illnesses [3].
Lower respiratory tract infections are not only difficult for the patient, but they can also be difficult for the medical provider to diagnose [4]. There are many laboratory-based diagnostic criteria that help physicians determine if an infection is due to bacterial or viral etiology, a distinction that guides clinical management of the patient. But even with state-of-the-art diagnostic tools, there are many cases that are simply classified as undiagnosable in terms of their etiology. These cases, hereafter referred to as undiagnosable respiratory infections, have caused many researchers to wonder if there are just many more pathogens out there than are currently known [5-7]. Recent discoveries show that there are new pathogens emerging and that continuing research in respiratory illnesses is an essential component of battling this global issue.
This review will focus on the role of newly discovered viruses in lower respiratory tract infections in children. The review will cover an overview of the most common pediatric viral infections, highlight advances in the treatment of these viruses, review the laboratory techniques for identifying newly discovered respiratory viruses (specifically human metapneumovirus and human bocavirus), and discuss the importance of continued research in the field. Gaining an understanding of respiratory tract infections and remaining up-to-date on the current discoveries and treatments for these illnesses is an important responsibility for every pediatric health care provider.
Viral Respiratory Infections in Children
Until recently, most viral lower respiratory infections in children were attributed to respiratory syncytial virus (RSV), parainfluenza virus, adenovirus, and influenza viruses [8]. However, two newly discovered viruses, human metapneumovirus (hMPV) and human bocavirus (HBoV), have joined the list of significant contributors. Both viruses, discovered in 2001 and 2005 respectively, constitute up to 13 percent of previously undiagnosable respiratory infections in children [9,10]. Additionally, both coronavirus and rhinovirus, traditionally regarded as causes of upper respiratory tract infection, now have been shown to be present in lower respiratory tract infection and, therefore, should be included in studies looking at all respiratory tract infections [11].
Identifying the viral etiology of respiratory infections in children can be challenging, especially due to concurrent infections. A study by Regamey et al. followed 197 children during their first years of life and found that 122 (62 percent) had at least one acute respiratory infection in that first year [12]. Fifteen (17 percent) had dual infections (infections due to two distinct pathogens), and three (3 percent) had triple infections. The findings from Regamey et al. are shown in Figure 1. Together, rhinoviruses, coronaviruses, and the newly discovered hMPV and HBoV accounted for 49 percent of cases, highlighting the role that hMPV and HBoV play in the etiology of respiratory tract infections in children. Gaining a better understanding of these newly discovered viruses is important in gaining a better understanding of respiratory infections in children.
Figure 1.
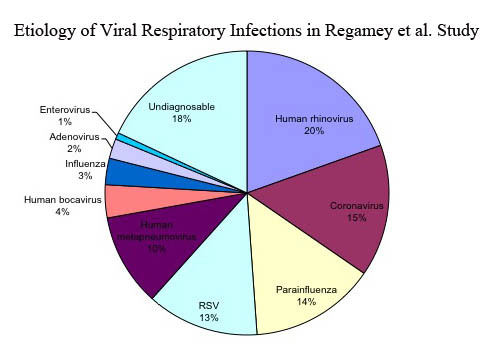
This pie chart summarizes the viral etiology findings of a study published by Regamey et al. The study followed 197 children during the first year of life for viral respiratory infection. One hundred twenty-two children developed respiratory illness during that year. The pie chart summarizes the etiological cause of the respiratory symptoms of the 122 children who developed respiratory tract infection(s).
This review will present a brief overview of four important viral respiratory pathogens: RSV, influenza, hMPV, and HBoV.
Respiratory Syncycial Virus (RSV)
RSV is a member of the Paramyxoviridae family, genus Pneumovirus. RSV is described as the single most important viral pathogen causing acute respiratory infections in young children. RSV causes illness in both young infants and in the elderly [13]. In infants, RSV is a common cause of bronchiolitis, a lower respiratory tract infection characterized by wheezing (due to bronchoconstriction), increased secretions, and hypoxia. Additional symptoms may include labored breathing and/or rapid breathing, cough, stuffy nose, and cyanosis (bluish tint to the skin due to lack of oxygen). Serological evidence shows that almost all children are exposed to RSV during the first three years of life [14]. However, previous infection does not prevent subsequent infections, and repeat infections are common in all age groups. Peak infection rates occur in infants aged 6 weeks to 6 months.
RSV takes a heavy toll on infants each year, with up to 500 deaths in the United States [15]. Infants who are younger than 3 months or have a history of premature birth, bronchopulmonary dysplasia, congenital heart disease, cystic fibrosis, or immunosuppression are more pre-disposed to RSV infection.
Treatment for RSV remains controversial [16]. Available treatments include beta-agonists, corticosteroids, racemic epinephrine, and ribavirin (an anti-viral medication used to suppress RSV viral replication). Beta-agnoists, corticosteroids, and racemic epinephrine all target the symptoms of bronchiolitis, with the end goal of dilating the airway and decreasing secretions to allow for adequate oxygen exchange in the lungs. Ribavirin is a seldom-used anti-viral medication that targets RSV viral replication. The end goal of Ribavirin treatment is to decrease the amount of virus in the affected tissue, reducing the virus’ ability to cause disease. However, in a systematic review by King et al., the authors report that none of the above mentioned medications significantly improve clinical outcomes compared to supportive therapy, such as hydration and oxygenation [17].
Currently, no vaccine is available to prevent RSV infection, but several vaccine candidates are in the early stages of clinical trials [18]. Preventive measures include the use of a humanized monoclonal antibody, Palivizumab®, which has been shown to significantly decrease infection in high-risk children when given as prophylaxis during RSV season [19]. Palivizumab’s beneficial effects are due to its role as a monoclonal antibody (IgG) directed against the A antigenic site of the F protein of RSV. It is administered via intramuscular injection and prevents viral infection by binding the virus in situ. While this treatment is effective, it is expensive and reserved for those at high risk, such as premature infants.
Influenza
Influenza viruses are a group of viruses in the Orthomyxoviridae family. While influenza is commonly referred to as the “flu,” influenza is actually a group of viruses in three genuses: Influenza A, Influenza B, and Influenza C. Influenza A is further classified by hemagglutinin (H) and neuraminidase (N) sub-types. For example, the 2009 global pandemic of swine flu was due to influenza A, type H1N1. Infection by Influenza B produces a less serious disease, while Influenza C is not known to cause epidemics. Influenza A and Influenza B are important causes of respiratory tract infection in children of all ages. Common symptoms of these viruses include fever, cough, sore throat, runny or stuffy nose, headache, chills, muscle aches, and, in children, even diarrhea and vomiting.
During the 2009 influenza season, 269 children younger than 18 died from influenza-related symptoms. Approximately 70 percent of the deaths occurred in children with underlying medical conditions, but 30 percent of the deaths occurred in previously healthy children [20]. Vaccines have been successful in reducing morbidity and mortality, yet children continue to suffer from influenza infections each year, in part due to the fact that different strains of influenza are prevalent every year.
The American Academy of Pediatrics (AAP) and the U.S. Center for Disease Control’s Advisory Committee on Immunization Practices (ACIP) currently recommend that all children older than 6 months receive the influenza vaccine each year [21]. Recent studies also have suggested that giving the vaccine to pregnant mothers offers protection to the newborn during the first six months of life, presumably via passive transfer of immunity from the mother [22,23].
Treatment for influenza includes four currently available anti-viral medications: amantadine, rimantadine, anamivir, and oseltamivir [16]. Oseltamivir (trade name Tamiflu®) and zanamivir (trade name Relenza®) are neuraminidase inhibitors that are often effective against Influenza A and B. Neuraminidase inhibitors block the neuraminidase enzyme that cleaves budding viral progeny just prior to release, therefore effectively blocking viral replication. Amantadine (trade name Symmetrel®) and rimantadine (trade name Flumadine®) target a viral ion channel necessary in the uncoating process of Influenza A, thereby preventing the virus from infecting host cells. Amantadine and rimantadine are effective against Influenza A only and reach maximum benefit when begun early in infection. Influenza viruses have a high rate of mutation, and anti-viral resistance has been an ongoing issue with the development of influenza anti-viral medications. The CDC provides recommendations on the use of anti-virals to minimize the risk of viral resistance to the medications and currently only recommends the use of oseltamivir and zanamivir for treatment in the United States.
Influenza anti-virals are most effective when started early in infection (within 48 hours) [24]. The AAP recommends antiviral treatment be considered in any child with influenza who is also at high risk of influenza complications, regardless of vaccination status, and any otherwise healthy child with moderate-to-severe influenza infection who might benefit from the decrease in duration of clinical symptoms documented to occur with antiviral therapy [25]. However, the use of influenza anti-virals is always a clinical balancing act, as the anti-virals are expensive, have side effects (such as headache, sore throat, nausea, vomiting, and, rarely, allergic reaction), and the overuse of the medications can lead to influenza strains resistant to anti-virals.
Human metapneumovirus
In June 2001, researchers in the Netherlands identified a new respiratory pathogen isolated from respiratory secretions in children with respiratory tract disease [26]. Further analysis showed the new pathogen to be a paramyxovirus, the first known non-avian pathogen of the Metapneumovirus genus. This new pathogen, termed hMPV, was found to be closely related to RSV.
Esper et al. showed that hMPV is a significant contributor to respiratory illnesses in children, responsible for up to 8 percent of previously undiagnosable viral respiratory infections [9]. The clinical presentation of hMPV is similar to that of other respiratory viruses, such as the closely associated RSV. Both upper and lower respiratory tract infections have been shown to be caused by hMPV. Clinical symptoms include coughing, wheezing, hypoxia, and fever [27]. Infection occurs in all age groups, but young children and the elderly are most commonly affected.
Serological studies have been undertaken to examine the extent of hMPV in the general population and have shown that almost all people are infected with hMPV by the age of 5 [28]. For most, the symptoms are similar to a mild cold. Yet in some children, hMPV can be the cause of severe respiratory infection requiring hospitalization due to respiratory failure.
hMPV appears to have a seasonal distribution, with most infections occurring during the winter and spring [29]. There are no antiviral treatments for hMPV infection currently available or in clinical trials. Treatment options include supportive care for respiratory infections, bronchodilators, and inhaled corticosteroids.
Human Bocavirus
HBoV is a parvovirus first described in 2005 by Allander and colleagues in respiratory samples from Sweden [30]. Allander’s research team identified HBoV by random polymerase chain reaction (PCR) analysis of samples from patients with pneumonia. The team identified a parvovirus-like sequence by sequencing all possible DNA/RNA material in the samples and comparing them to known viral, bacterial, and human sequences. Phylogenetic analysis showed that this novel parvovirus was most closely related to bovine parvovirus and a canine minute virus. The only other known parvovirus with clinical implications is parvovirus B19, which causes fifth disease or erythema infectiosum (a common rash of childhood) and can have serious complications such as aplastic crisis in hemolytic syndromes (such as sickle cell disease) and hydrops fetalis in fetuses.
HBoV is a small, but significant contributor to respiratory tract infections in children. In a study at Yale University, researchers examined respiratory isolates from children with respiratory illness that were negative for all other known viral pathogens. Of these previously undiagnosable specimens, 5.2 percent tested positive for HBoV [10]. In serological studies, researchers have found that almost all children are exposed to HBoV by the time they are 5 years old [31,32].
HBoV infection is clinically similar to other respiratory virus infections. Common symptoms include fever, rhinorrhea, cough, and wheezing [10]. To date, there are no medications available or in clinical trials for HBoV. Mainstays of treatment include supportive care, inhaled bronchodilators, and corticosteroids.
Methods to Identify the Viruses causing Infection
Rapid Diagnosis
Rapid diagnoses of viral infections can be obtained using immunofluorescence and PCR techniques. Direct immunofluorescence assays, which utilize an antibody labeled with an immunofluorescent tag to identify the presence of live virus in a sample, can offer a rapid diagnosis. Direct immunofluorescence tests generally have a sensitivity of 95 percent to 100 percent [33].
The advent of PCR has greatly enhanced identification and classification of viruses. PCR has proven to be a very sensitive and specific method for the detection of respiratory viruses [34], and the recent development of real-time PCR has allowed PCR to be used in rapid diagnostics.
Serological Diagnosis and Viral Culture
Serological diagnosis can be performed using an enzyme-linked immunosorbent assay (ELISA). An ELISA can identify antibodies in human serum against a specific virus and can be used to detect current infection as well as past viral exposure. Although viral culture remains the gold standard for viral detection, PCR and newer technologies in molecular biology are likely to replace viral culture in the future. Many laboratories will run a rapid diagnostic test and follow up with viral culture.
Human Metapneumovirus
hMPV PCR
hMPV has five main viral genes that can be identified by PCR: the nucleoprotein (N), phosphoprotein (P) matrix (M), fusion (F), and polymerase (L). hMPV is difficult to grow in culture, so PCR is the predominant method used to detect the virus. In a comparative evaluation of Real-Time PCR Assays, Coté and colleagues found that the N and L genes, which code for two internal hMPV viral proteins, were most suitable for hMPV diagnosis. The sequences for the primers used can be found in their paper [35].
hMPV ELISA
An hMPV ELISA technique was developed at Yale University by Leung et al [28]. These researchers cloned the fusion (F) gene of hMPV into vesicular stomatitis virus (VSV), and then infected BHK or HEp-2 (human larynx carcinoma cells) with the VSV-hMPV F viral construct. After multiple rounds of replication in the BHK cells, the VSV-hMPV F viral construct was removed and purified to be plated as antigen in an ELISA assay. The ELISA assay was used to detect antibody to hMPV from human serum. A more detailed explanation of their techniques, along with sequences for viral primers can be found in their paper [28].
Human Bocavirus
HBoV PCR
Allander et al. included the protocol for PCR and sequence for HBoV primers in their paper [30]. Forward and reverse primers along with PCR protocol to target a portion of the HBoV NP-1 gene are published by Kahn et al [31]. The primers can also be accessed on GenBank.
HBoV ELISA
A HBoV specific ELISA was developed by Kahn et al. at Yale University using virus-like particles (VLP) [31]. Nucleic acids from a respiratory specimen positive for HBoV by PCR were extracted, and the HBoV VP2 gene was amplified and cloned sequentially into a pFastBac vector (Invitrogen). A recombinant baculovirus was generated using the Bac-to-Back Expression System (Invitrogen) by transforming into competent, bacmid-containing DH10 E. coli cells and transfection of the high-molecular-weight DNA that this generated into Sf9 insect cells. The resulting baculovirus was expanded and then titered by plaque assay on Sf9 monolayer cultures and plated on a 96-well plate for use in the ELISA assay. Human serum from patients was used as primary antibody in the ELISA assay to screen for antibodies to HBoV.
Conclusion
Recent developments in biotechnology have allowed research scientists to identify many new respiratory viruses. Two of these, hMPV and HBoV, account for a significant portion of previously undiagnosable respiratory infections. The current advances in laboratory techniques are important for clinicians, who must be up to date on current treatment and diagnostics in respiratory infections. Pediatricians must pay particular attention due to the high morbidity and mortality caused by respiratory infections in children.
Viral respiratory infections continue to be a worldwide health issue. Scientific advances in the field have increased diagnostics and treatment. Studies suggest there may be more viruses yet to be discovered [5-7]. Additional research into understanding these viruses and the developments of vaccines for viral respiratory infections will prove to be essential for fighting this worldwide health issue.
Abbreviations
- hMPV
human metapneumovirus
- HBoV
human bocavirus
- RSV
respiratory syncytial virus
- H
hemagglutinin
- N
neuraminidase
- AAP
American Academy of Pediatrics
- ACIP
US Center for Disease Control’s Advisory Committee on Immunization Practices
- PCR
polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- VSV
vesicular stomatitis virus
- VLP
virus-like particles
- RSV
respiratory syncycial virus
References
- van Woensel JBM, van Aalderen WMC, Kimpen JLL. Viral lower respiratory tract infection in infants and young children. BMJ. 2003;327(7405):36–40C. doi: 10.1136/bmj.327.7405.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- Mulholland K. Global burden of acute respiratory infections in children: Implications for interventions. Pediatr Pulmonol. 2003;36(6):469–474. doi: 10.1002/ppul.10344. [DOI] [PubMed] [Google Scholar]
- Meissner HC. Uncertainty in the management of viral lower respiratory tract disease. Pediatrics. 2001;108(4):1000–1004. doi: 10.1542/peds.108.4.1000. [DOI] [PubMed] [Google Scholar]
- Davies HD, Matlow A, Petric M, Glazier R, Wang EEL. Prospective comparative study of viral, bacterial and atypical organisms identified in pneumonia and bronchiolitis in hospitalized Canadian infants. Pediatr Infect Dis J. 1996;15(4):371–375. doi: 10.1097/00006454-199604000-00017. [DOI] [PubMed] [Google Scholar]
- Louie JK, Hacker JK, Gonzales R, Mark J, Maselli JH, Yagi S. et al. Characterization of viral agents causing acute respiratory infection in a San Francisco University medical center clinic during the influenza season. Clin Infect Dis. 2005;41(6):822–828. doi: 10.1086/432800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Childrens Respiratory Study. II. Lower Respiratory-Tract Illness In The 1st Year Of Life. Am J Epidemiol. 1989;129(6):1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- Henrickson KJ. Viral Pneumonia in Children. Semin Pediatr Infect Dis. 1998;9(3):217–233. doi: 10.1016/S1045-1870(98)80035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F, Martinello RA, Boucher D, Weibel C, Ferguson D, Landry ML. et al. A 1-year experience with human Metapneumovirus in children aged < 5 years. J Infect Dis. 2004;189(8):1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML. et al. Human bocavirus infection in young children in the United States: Molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194(9):1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital — Role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23(11):1003–1007. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- Regamey N, Kaiser L, Roiha HL, Deffernez C, Kuehni CE, Latzin P. et al. Viral etiology of acute respiratory infections with cough in infancy — A community-based birth cohort study. Pediatr Infect Dis J. 2008;27(2):100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- Simoes EAF. Respiratory syncytial virus infection. Lancet. 1999;354(9181):847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- Ukkonen P, Hovi T, Vonbonsdorff CH, Saikku P, Penttinen K. Age-Specific Prevalence of Complement-Fixing Antibodies to 16 Viral-Antigens — A Computer-Analysis of 58,500 Patients Covering a Period of 8 Years. J Med Virol. 1984;13(2):131–148. doi: 10.1002/jmv.1890130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. J Infect Dis. 2001;183(1):16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- Klig JE, Shah NB. Office pediatrics: current issues in lower respiratory infections in children. Curr Opin Pediatr. 2005;17(1):111–118. doi: 10.1097/01.mop.0000150599.31091.f0. [DOI] [PubMed] [Google Scholar]
- King VJ, Viswanathan M, Bordley C, Jackman AM, Sutton SF, Lohr KN. et al. Pharmacologic treatment of bronchiolitis in infants and children - A systematic review. Arch Pediatr Adolesc Med. 2004;158(2):127–137. doi: 10.1001/archpedi.158.2.127. [DOI] [PubMed] [Google Scholar]
- Schickli JH, Dubovsky F, Tang RS. Challenges in developing a pediatric RSV vaccine. Human Vaccines. 2009;5(9):582–591. doi: 10.4161/hv.9131. [DOI] [PubMed] [Google Scholar]
- Lieberthal AS, Bauchner H, Hall CB, Johnson DW, Kotagal U, Light MJ. et al. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- Jhung M, Brammer L, Epperson S, Blanton L, Dhara R, Wallis T. et al. Update: Influenza activity — United States, August 20, 2009-March 27, 2010, and Composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59(14):423–430. [PubMed] [Google Scholar]
- Committee on Infectious Diseases. Policy Statement. Recommendations for Prevention and Control of Influenza in Children, 2010-2011. Pediatrics. 2010;126(4):816–826. doi: 10.1542/peds.2010-2216. [DOI] [PubMed] [Google Scholar]
- Benowitz I, Esposito D, DePeau K, Shapiro ED, Vazquez M. Effectiveness of Influenza Vaccine Given to Pregnant Women in Preventing Hospitalization in their Infants [abstract]; Proceedings of the 47th Annual Meeting of the Infectious Disease Society of America; 2009 Oct 29-Nov 1; Philadelphia, PA. 2009. Abstract 704. [Google Scholar]
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E. et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC. et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Infectious Diseases. Antiviral therapy and prophylaxis for influenza in children. Pediatrics. 2007;119(4):852–860. doi: 10.1542/peds.2007-0224. [DOI] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong J, Groen J, Kuiken T, de Groot R, Fouchier RAM. et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: Clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111(6):1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- Leung J, Esper F, Weibel C, Kahn JS. Seroepidemiology of human metapneumovirus (hMPV) on the basis of a novel enzyme-linked immunosorbent assay utilizing hMPV fusion protein expressed in recombinant vesicular stomatitis virus. J Clin Microbiol. 2005;43(3):1213–1219. doi: 10.1128/JCM.43.3.1213-1219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xepapadaki P, Psarras S, Bossios A, Tsolia M, Gourgiotis D, Liapi-Adamidou G. et al. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30(3):267–270. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JS, Kesebir D, Cotmore SF, D’Abramo A, Cosby C, Weibel C. et al. Seroepidemiology of human bocavirus defined using recombinant virus-like particles. J Infect Dis. 2008;198(1):41–50. doi: 10.1086/588674. [DOI] [PubMed] [Google Scholar]
- Hustedt J, Esposito D, Christie-Samuels C, Hustedt M, Vazquez M. Sero-epidemiology of Human Bocavirus Infection in Young Children in Kingston, Jamaica [abstract]; Proceedings of the 48th Annual Meeting of the Infectious Disease Society of America; 2010 Oct. 21-24; Vancouver, BC, Canada. 2010. Abstract 566. [Google Scholar]
- Grandien M, Pettersson CA, Gardner PS, Linde A, Stanton A. Rapid Viral Diagnosis Of Acute Respiratory-Infections — Comparison Of Enzyme-Linked Immunosorbent-Assay And The Immunofluorescence Technique For Detection Of Viral-Antigens In Nasopharyngeal Secretions. J Clin Microbiol. 1985;22(5):757–760. doi: 10.1128/jcm.22.5.757-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl SC, Henrickson KJ, Hua WM, Fan J. Evaluation of the Hexaplex assay for detection of respiratory viruses in children. J Clin Microbiol. 2001;39(5):1696–1701. doi: 10.1128/JCM.39.5.1696-1701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote S, Abed Y, Boivin G. Comparative evaluation of real-time PCR assays for detection of the human metapneumovirus. J Clin Microbiol. 2003;41(8):3631–3635. doi: 10.1128/JCM.41.8.3631-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


