Abstract
Background
The International Lung Cancer Consortium (ILCCO) was established in 2004, based on the collaboration of research groups leading large molecular epidemiology studies of lung cancer that are ongoing or have been recently completed. This framework offered the opportunity to investigate the role of tobacco smoking in the development of bronchioloalveolar carcinoma (BAC), a rare form of lung cancer.
Methods
Our pooled data comprised seven case–control studies from the United States, with detailed information on tobacco smoking and histology, which contributed 799 cases of BAC and 15,859 controls. We estimated the odds ratio of BAC for tobacco smoking, using never smokers as a referent category, after adjustment for age, sex, race, and study center.
Results
The odds ratio of BAC for ever smoking was 2.47 (95% confidence interval [CI] 2.08, 2.93); the risk increased linearly with duration, amount, and cumulative cigarette smoking and persisted long after smoking cessation. The proportion of BAC cases attributable to smoking was 0.47 (95% CI 0.39, 0.54).
Conclusions
This analysis provides a precise estimate of the risk of BAC for tobacco smoking.
Keywords: Lung cancer, Bronchioloalveolar carcinoma, Tobacco smoking
Introduction
Tobacco smoking is the most important cause of lung cancer, being responsible for an estimated 80–90% of cases in most populations of the world [1]. The carcinogenic effect of tobacco smoking on different histological types has been investigated in several studies, which typically included the most frequent types: a stronger risk has been repeatedly reported for squamous cell carcinoma and small cell carcinoma than for adenocarcinoma [2], while the risk of large cell carcinoma, which has been investigated less frequently, seems intermediate [3]. Data on other, rarer histological types are sparse and often based on a small series of cases leading to imprecise risk estimates.
Bronchioloalveolar carcinoma (BAC) of the lung is a form of adenocarcinoma characterized by growth of neoplastic cells along the alveolar structures with limited invasion of the blood vessels, the stroma, or the pleura. A mucinous and a non-mucinous variants are distinguished, the latter being more frequent. Distinction between BAC and other forms of lung adenocarcinoma is often difficult, and cancers, in particular those of larger size, frequently display mixed histologies [4]. BAC is considered a relatively infrequent form of lung cancer, but population-based data on incidence are scant. In the United States, age-adjusted incidence rates in the 1970s and 1980s were between 1 and 2 per 100,000, with little differences between sexes and races [5]. Reviews of series of patients from single institutions suggested an increase in the proportion of BAC over total lung cancers [6, 7], but the limited data from population-based cancer registries support the lack of a trend in incidence [5, 8]. Specific features of the epidemiology of BAC include a comparable incidence in men and women (in several series the number of female cases outnumber that of male cases), a shift in the distribution toward early-stage lesions, and a better survival rates than for other histological types of lung cancer, which is not fully explained by differences in stage at diagnosis [9].
The rarity of this histological type complicates the analysis of its risk factors. A lower proportion of smokers among BAC cases when compared to other types of lung cancer has been reported in early studies [9–12], but only two epidemiological studies have provided a formal estimate of the risk of BAC among smokers relative to non-smokers. In a study of 87 cases from several US hospitals, Morabia and Wynder [13, 14] reported an odds ratio (OR) of BAC for current smoking of 3.9 (95% confidence interval 1.8, 8.7), an increase in OR for increasing duration and amount of smoking, and a protective effect of quitting. In a study of 21 cases from Louisiana, Falk and colleagues [5] reported an OR of 4.0 (95% CI 0.9, 18.8) for ever smoking. Other risk factors have not been adequately investigated.
Pooled analyses within a consortium of data collected in independently conducted studies represent a cost effective approach in generating a large series of cases and controls. We have therefore conducted a pooled analysis of tobacco smoking and risk of BAC within the framework of the International Lung Cancer Consortium (ILCCO), which includes completed and ongoing studies of molecular and genetic epidemiology of lung cancer [15], with the aim of providing a precise measurement of the association between tobacco smoking and BAC.
Methods
This pooled analysis comprises data from seven case–control studies from the United States (Table 1). These studies represent a subset of studies included in the ILCCO collaboration (http://ilcco.iarc.fr). The remaining studies participating in the ILCCO consortium either excluded by design rare histological types of lung cancer or enrolled less than 10 BAC cases. The seven studies included one study each from the University of California at Los Angeles (UCLA) [16], Harvard University (HU) [17], University of Hawaii (UH) [18], two studies from Wayne State University (WSU) [19–21], one study from Mayo Clinic (Mayo) [22], and one multicentric study coordinated by the American Health Foundation (AHF) [23, 24]. Results on tobacco smoking and BAC risk have been reported for part of the AHF study [13, 14]. For some of the studies, the number of subjects included in the pooled analysis was larger than that reported in previous publication because recruitment has continued. Three studies [17, 22, 23] was hospital-based, and the other four were community-based case–control studies. In the UCLA study, newly diagnosed cases were identified using the rapid ascertainment system of the Cancer Surveillance Program for Los Angeles County. All BAC cases were diagnosed pathologically and validated through pathologic records. A similar approach was followed in the WSU studies, based on the Metropolitan Detroit Cancer Surveillance System. In the HU study, cases were enrolled from the Massachusetts General Hospital in Boston, MA, as part of an ongoing case–control molecular epidemiology study of lung cancer. Pathology reports generated by two pulmonary pathologists as part of routine tissue diagnosis were obtained from the clinical information system. The histological confirmed diagnosis of BAC from pathological reports was reviewed by a study pathologist or oncologist. In the UH study, pathology records were obtained from SEER registries; BAC diagnoses were not validated. In the Mayo study, the histological diagnoses were validated. In the AHF study, information on histology was abstracted from the records of the pathology departments of the participating hospitals and was not validated. The proportion of BAC over total lung cancers varied from 3 to 9%. Information on stage of BAC cases was not available.
Table 1.
Characteristics of studies included in the pooled analysis of lung bronchioloalveolar carcinoma and tobacco smoking
| Study, location | Study design | Definition of ever smoker (cigarettes) | Response rate (%) in ca/co | Total number of lung cancer cases | Number of BAC cases | Number of controls | Special features |
|---|---|---|---|---|---|---|---|
| UCLA, Los Angeles [16] | Population-based | >100 | 39/79 | 611 | 39 | 1,040 | |
| HU, Boston [17] | Hospital-based | >100 | 85/55 | 2,253 | 204 | 1,529 | |
| UH, Honolulu [18] | Population-based | >185 | 64/62 | 635 | 38 | 588 | |
| WSU1, Detroit [19] | Population-based | ≥100 | 55/71 | 526 | 50 | 460 | Women |
| WSU2, Detroit [20, 21] | Population-based | ≥100 | 66/93 | 1,006 | 35 | 1,184 | EO |
| Mayo [22] | Hospital-based | ≥100 | 87/84 | 5,698 | 280 | 2,269 | |
| AHF [23, 24] | Hospital-based | ≥100 | 92/93 | 5,130 | 153 | 4,942 |
BAC bronchioloalveolar carcinoma, ca cases, co controls, EO early-onset cases
All studies collected information on lifetime history of tobacco smoking, including age of start smoking, duration, intensity, and time since quitting for the former smokers. All studies collected information on cigarette smoking; in the UCLA, Harvard, and Hawaii studies, cigar and pipe smoking was added to cigarette smoking to calculate cumulative tobacco consumption. For the purposes of the pooled analysis, we generated common variables related to smoking status (never, ever, current, and former smoker, defined as smokers who have quit more than 1 year before diagnosis or interview), daily amount of smoking (1–9, 10–19, 20–29, 30–39, and 40 or more cigarettes/day), duration of smoking (1–9, 10–19, 20–29, 30–39, and 40 or more years), and cumulative smoking (1–9, 10–19, 20–29, 30–39, and 40 or more pack years, one pack year being equivalent to 20 cigarettes/day smoked during 1 year). Former smokers were further categorized according to time since quitting (1–9 years, 10–19, 20 or more years).
Other variables included in the pooled analysis were sex, age at diagnosis or interview (categorized as up to 50, 51–60, 61–70, and more than 70), education (basic or elementary, up to high school, and college or more), study center, and race (Asian, Black, White, Hawaiian, Hispanic, other).
All analyses were performed using the STATA statistical package [25]. Study-specific OR of lung cancer and their 95% CI for smoking intensity, duration, cumulative smoking, and time since quitting were estimated using unconditional logistic regression. All the estimates were adjusted for age at diagnosis, sex, race and study center.
We explored the heterogeneity between study–specific results using the Q statistic at the significance level of p < 0.05. Galbraith plot where the ratio of the log odds ratio to the standard error (the Z statistic) for each study is plotted against the reciprocal of the standard error [26]. Further, the influence of each study on the overall meta-analysis estimate was analyzed by an influence analysis, where the meta-analysis estimates are computed after omitting each study. Pooled OR estimates stratified by sex, age at diagnosis, education level, and study design were also calculated.
Linear trends for the amount, duration, cumulative smoking, and time since quitting were tested according to Greenland and Longnecker [27], using the ‘glst’ command on STATA, which utilizes the generalized least squares for trend estimation of summarized dose–response data. Population attributable fractions were calculated using ‘aflogit’ on STATA, which estimates the adjusted measures of population attributable fraction from a logistic regression model [28].
Results
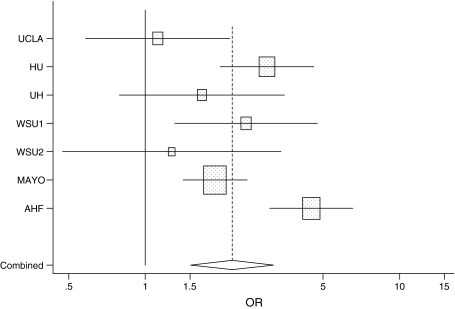
Overall, 799 cases of BAC and 15,859 controls were included in the pooled analysis. Their demographic characteristics are summarized in Table 2. A total of 514 cases (64.3%) and 5,779 controls (48.1%) were women. The median age of cases was 65 years (interquartile range 56–73 years) and that of controls was 56 years (interquartile range: 45–65 years). Table 3 reports the results of the analysis of tobacco smoking. The OR for ever smoking was 2.47 (95% CI 2.08, 2.93); it was 2.51 (95% CI 2.09, 3.01) for current smoking and 2.35 (95% CI 1.90, 2.92) for former smoking. The study-specific ORs for ever smoking are presented in Fig. 1: they ranged from 1.12 in the UCLA study to 3.90 in the AHF study; the p-value of the test for between-study heterogeneity in OR was 0.01. The summary estimate of OR for BAC based on a meta-analysis fixed effects model was 2.38 (2.00, 2.85); the summary OR based on a random-effects model was 2.20 (95% CI 1.51, 3.20). A linear trend was observed for amount of smoking (OR for an increase in 10 cigarettes/day 1.32, 95% CI 1.27, 1.38; p-value of test for between-study heterogeneity 0.07), duration of smoking (OR for a 10-year increase 1.25, 95% CI 1.20, 1.30; between-study heterogeneity p-value 0.01), and cumulative smoking (OR for an increase in 10 pack years 1.29, 95% CI 1.24, 1.34).
Table 2.
Demographic characteristics of lung bronchioloalveolar carcinoma cases and controls included in the pooled analysis
| Characteristic | Cases | Controls | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Men | 285 | 35.7 | 6,233 | 51.9 |
| Women | 514 | 64.3 | 5,779 | 48.1 |
| Age | ||||
| <50 years | 95 | 11.9 | 3,015 | 25.1 |
| 51–60 years | 158 | 19.8 | 3,114 | 25.9 |
| 61–70 years | 281 | 35.2 | 3,606 | 30.0 |
| >70 years | 265 | 33.2 | 2,277 | 19.0 |
| Ethnicity | ||||
| White | 697 | 87.6 | 10,144 | 84.5 |
| Black | 35 | 4.4 | 1,002 | 8.3 |
| Asian | 32 | 4.1 | 341 | 2.8 |
| Other | 29 | 3.9 | 521 | 4.4 |
| Education | ||||
| Lower than high school | 60 | 8.1 | 900 | 7.8 |
| High school | 308 | 41.5 | 5,004 | 43.6 |
| College or higher | 374 | 50.4 | 5,563 | 48.6 |
Table 3.
Odds ratio of bronchioloalveolar carcinoma for tobacco smoking in the pooled analysis
| Cases | Controls | OR | 95% CI | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Smoking status* | ||||||
| Ever smokers | 600 | 75.1 | 7,229 | 60.3 | 2.47 | 2.08, 2.93 |
| Current smokers | 180 | 22.5 | 2,718 | 22.7 | 2.51 | 2.09, 3.01 |
| Former smokers | 415 | 51.9 | 4,431 | 36.9 | 2.35 | 1.90, 2.92 |
| Amount of smoking* | ||||||
| 1–9 cpd | 71 | 9.0 | 1,139 | 9.6 | 1.57 | 1.18, 2.08 |
| 10–19 cpd | 115 | 14.5 | 1,717 | 14.5 | 1.93 | 1.51, 2.46 |
| 20–29 cpd | 208 | 26.2 | 2,213 | 18.7 | 2.93 | 2.38, 3.61 |
| 30–40 cpd | 85 | 10.7 | 898 | 7.6 | 3.15 | 2.39, 4.14 |
| 40+ cpd | 115 | 14.5 | 1,110 | 9.4 | 3.79 | 2.94, 4.90 |
| p-value for linear trend | <0.001 | |||||
| Duration of smoking* | ||||||
| 1–9 years | 33 | 4.3 | 865 | 7.4 | 0.96 | 1.53, 1.75 |
| 10–19 years | 82 | 10.7 | 1,241 | 10.6 | 1.98 | 1.30, 3.03 |
| 20–29 years | 106 | 13.8 | 1,623 | 13.8 | 1.83 | 1.23, 2.71 |
| 30–40 years | 157 | 20.5 | 1,618 | 13.8 | 2.87 | 2.10, 3.94 |
| 40+ years | 189 | 24.7 | 1,631 | 13.9 | 2.89 | 2.66, 3.55 |
| p-value for linear trend | <0.001 | |||||
| Cumulative smoking* | ||||||
| 1–9 pack years | 70 | 9.1 | 1,606 | 13.6 | 1.26 | 0.95, 1.67 |
| 10–19 pack years | 74 | 9.6 | 1,195 | 10.1 | 1.89 | 1.43, 2.50 |
| 20–29 pack years | 90 | 11.7 | 1,055 | 8.9 | 2.60 | 1.99, 3.38 |
| 30–40 pack years | 85 | 11.0 | 982 | 8.3 | 2.81 | 2.14, 3.68 |
| 40+ pack years | 254 | 32.9 | 2,195 | 18.6 | 3.48 | 2.84, 4.26 |
| p-value for linear trend | <0.001 | |||||
| Time since quitting** | ||||||
| 1–9 years | 87 | 11.3 | 1,046 | 8.8 | 1.12 | 0.90, 1.53 |
| 10–19 years | 138 | 17.9 | 1,367 | 11.5 | 1.06 | 0.82, 1.46 |
| 20–29 years | 84 | 10.9 | 1,041 | 8.8 | 0.89 | 0.70, 1.14 |
| 30+ years | 84 | 10.9 | 910 | 7.7 | 0.83 | 0.63, 1.04 |
| Never smokers | 199 | 25.8 | 4,763 | 40.2 | 0.42 | 0.34, 0.52 |
| p-value for linear trend | <0.001 | |||||
CI confidence interval, cpd, cigarettes per day, n number, OR odds ratio, adjusted for age, gender, race, and study center
* Reference category: never smokers (199 cases, 4,763 controls)
** Reference category: current smokers (180 cases, 2,718 controls)
Fig. 1.
Study-specific odds ratios (and 95% confidence intervals) of lung bronchioloalveolar carcinoma for ever tobacco smoking. The combined odds ratio is based on a random-effects model meta-analysis
Table 4 presents the pooled results for ever smoking stratified by sex, age at diagnosis, and education level. No heterogeneity was suggested with respect to age sex or education level. The proportion of BAC attributable to ever smoking tobacco was 0.47 (95% CI 0.39, 0.54). It was 0.74 (95% CI 0.17, 0.66) in men and 0.37 (95% CI 0.24, 0.48) in women.
Table 4.
Odds ratio of bronchioloalveolar carcinoma for ever tobacco smoking in the pooled analysis, stratified by sex, age at diagnosis, and education
| Stratum | OR | 95% CI |
|---|---|---|
| Sex | ||
| Men | 2.10 | 1.61, 2.91 |
| Women | 2.52 | 2.01, 3.08 |
| Test for heterogeneity, p-value | 0.87 | |
| Age | ||
| ≤60 years | 1.48 | 0.97, 2.26 |
| 50–60 years | 2.65 | 1.78, 3.95 |
| 60–70 years | 2.39 | 1.77, 3.23 |
| >70 years | 2.73 | 1.99, 3.70 |
| Test for heterogeneity, p-value | 0.12 | |
| Education | ||
| Lower than college | 3.05 | 2.35, 3.98 |
| College of higher | 2.39 | 1.88, 3.05 |
| Test for heterogeneity, p-value | 0.18 | |
CI confidence interval, OR odds ratio, adjusted for age, gender, race, and study center
Discussion
Our analysis adds strong evidence to the notion that BAC is a form of lung cancer associated with tobacco smoking. The current analysis also provides strong evidence on a beneficial effect of quitting tobacco smoking, as it has been shown for lung cancer in general and for the major histological types [29].
Our results are quantitatively consistent with those of the two epidemiological studies of BAC available in the literature [5, 13, 14]: a meta-analysis of the results for current smoking for the three studies resulted in a p-value for heterogeneity of 0.7.
We acknowledge several limitations to our study. We were not able to perform a systematic pathology review of the cases, and the possibility of misdiagnosis remains open for some cases. However, misclassification is most likely to occur with other forms of adenocarcinoma [4], a type of lung cancer that is less strongly associated with tobacco smoking than other types, and diagnoses of well-differentiated lung cancer have been found to be reproducible [30, 31]. The proportion of BAC over total lung cancer cases varied from over 9% in the HU and WSU1 studies to 3% in the AHF study. A validation of BAC diagnosis was performed only in some of studies included in this pooled analysis; however, the study-specific results presented in Fig. 1 do not suggest an important role for diagnostic misclassification, since the magnitude of the excess risk did not appear to correlate with validation of pathological diagnosis.
The lack of histological verification also pre-empted us from distinguishing between the two main types of BAC, namely mucinous and non-mucinous. This is unfortunate because the molecular alterations seem to differ between the two types, with a higher proportion of codon 12 KRAS mutations in the mucinous type and, conversely, a higher proportion of TP53 mutations in the non-mucinous type [32–35]. The high proportion of EGFR mutations in BAC, when compared to other types of lung adenocarcinoma [36], is consistent with a comparatively lesser etiologic role of tobacco smoking in the former type of neoplasm.
An additional potential limitation, which is common to retrospective epidemiological studies, is recall bias, i.e., lung cancer cases over-reporting tobacco smoking when compared to controls, either because of better recall or because they are trying to explain their disease. However, the internal consistency of our results (dose–response relation with amount and duration of smoking, effect of long-term quitting, lack of heterogeneity by sex, age, or education) argues against a strong role of recall bias.
The main strength of our study lies in its size, as it included more than seven times the total number of BAC cases included in previous epidemiological studies on the effect of tobacco smoking on this form of lung cancer. The high quality of participating studies and the validity of the information on exposure and outcome are confirmed by the consistency of inter-study risk estimates.
No results have been reported on risk of BAC after cessation of smoking. The available evidence points toward a more rapid decrease in risk with increasing time since cessation for squamous cell carcinoma than for adenocarcinoma [29]. The similarities between BAC and adenocarcinoma might explain the relatively weak effect of quitting we observed in our analysis.
In conclusion, our pooled study resulted in a twofold increased risk of lung BAC in ever smokers when compared to never smokers, and in evidence of a dose–response relation according to duration and amount of smoking. Forty-seven percent of BAC cases in the study population would be attributable to the habit. This is the most precise estimate of the risk of BAC for tobacco smoking available in the literature and supports the notion of an association between tobacco smoking.
Acknowledgments
The original studies were supported by the following grants: WSU1 and WSU2 studies: NIH R01CA60691, R01CA87895, N01PC35145, and P30CA22453; HU study: NIH R01CA074386, R01CA092824, and P20CA090578; UCLA study: NIH R01DA11386 and R01CA90833; UH study: NIH R01CA55874; Mayo study: NIH-CA 77118, NIH-CA 80127, and NIH-CA 84354; AHF study: NIH CA-32617, CA-68384, CA-29580, and CA-17613.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Rayjean J. Hung, David C. Christiani authors contributed equally to this work.
References
- 1.The Health Consequences of Smoking (2004) A report of the Surgeon General. Office on Smoking and Health, DHHS Washington, DC
- 2.International Agency for Research on Cancer . Tobacco smoke. In: IARC Monographs of the Evaluation of Carcinogenic Risks to Humans, editor. Tobacco smoke and involuntary smoking. Lyon: IARC; 2004. pp. 51–1187. [PMC free article] [PubMed] [Google Scholar]
- 3.Muscat JE, Stellman SD, Zhang ZF, Neugut AI, Wynder EL. Cigarette smoking and large cell carcinoma of the lung. Cancer Epidemiol Biomarkers Prev. 1997;6:477–480. [PubMed] [Google Scholar]
- 4.Colby TV, Noguchi M, Henschke C, Vazquez MF, Geisinger K, Yokose T, et al. Adenocarcinoma. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, et al., editors. World health classification of tumours pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. pp. 35–44. [Google Scholar]
- 5.Falk RT, Pickle LW, Fontham ET, Greenberg SD, Jacobs HL, Correa P, et al. Epidemiology of bronchioloalveolar carcinoma. Cancer Epidemiol Biomarkers Prev. 1992;1:339–344. [PubMed] [Google Scholar]
- 6.Auerbach O, Garfinkel L. The changing pattern of lung carcinoma. Cancer. 1991;68:1973–1977. doi: 10.1002/1097-0142(19911101)68:9<1973::AID-CNCR2820680921>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Barsky SH, Cameron R, Osann KE, Tomita D, Holmes EC. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer. 1994;73:1163–1170. doi: 10.1002/1097-0142(19940215)73:4<1163::AID-CNCR2820730407>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 8.Read WL, Page NC, Tierney RM, Piccirillo JF, Govindan R. The epidemiology of bronchioloalveolar carcinoma over the past two decades: analysis of the SEER database. Lung Cancer. 2004;45:137–142. doi: 10.1016/j.lungcan.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Vincent RG, Pickren JW, Lane WW, Bross I, Takita H, Houten L, et al. The changing histopathology of lung cancer: a review of 1682 cases. Cancer. 1977;39:1647–1655. doi: 10.1002/1097-0142(197704)39:4<1647::AID-CNCR2820390439>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Schraufnagel D, Peloquin A, Pare JAP, Wang NS. Differentiating bronchioloalveolar carcinoma from adenocarcinoma. Am Rev Respir Dis. 1982;15:74–79. doi: 10.1164/arrd.1982.125.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Greco RJ, Steiner RM, Goldman S, Cotler H, Patchefsky A, Cohn HE. Bronchoalveolar cell carcinoma of the lung. Ann Thorac Surg. 1986;41:652–656. doi: 10.1016/S0003-4975(10)63082-2. [DOI] [PubMed] [Google Scholar]
- 12.Grover FL, Piantadosi S. The Lung Cancer Study Group. Recurrence and survival following resection of bronchioloalveolar carcinoma of the lung: The Lung Cancer Study Group experience. Ann Surg. 1989;209:779–790. doi: 10.1097/00000658-198906000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morabia A, Wynder EL. Relation of bronchioloalveolar carcinoma to tobacco. BMJ. 1992;304:541–543. doi: 10.1136/bmj.304.6826.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morabia A, Wynder EL, Correspondence re: Falk RT et al (1992) Epidemiology of bronchioloalveolar carcinoma. Cancer Epidemiol Biomarkers Prev 1:339–344. Cancer Epidemiol Biomarkers Prev 1993;2:89–90 [PubMed]
- 15.ilcco.iarc.fr (accessed 12 December 2009)
- 16.Hashibe M, Morgenstern H, Cui Y, Tashkin DP, Zhang ZF, Cozen W, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case–control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–1834. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- 17.Ter-Minassian M, Zhai R, Asomaning K, Su L, Zhou W, Liu G, et al. Apoptosis gene polymorphisms, age, smoking and the risk of non-small cell lung cancer. Carcinogenesis. 2008;29:2147–2152. doi: 10.1093/carcin/bgn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. JNCI. 2000;92:154–160. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 19.Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, Schwartz AG. Regular adult aspirin use decreases risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2008;17:148–157. doi: 10.1158/1055-9965.EPI-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz AG, Yang P, Swanson GM. Familial risk of lung cancer among non-smokers and their relatives. Am J Epidemiol. 1996;144:554–562. doi: 10.1093/oxfordjournals.aje.a008965. [DOI] [PubMed] [Google Scholar]
- 21.Cote ML, Wenzlaff AS, Bock CH, Land SJ, Santer SK, Schwartz DR, et al. Combinations of cytochrome P-450 genotypes and risk of early-onset lung cancer in Caucasians and African Americans: a population-based study. Lung Cancer. 2007;55:255–262. doi: 10.1016/j.lungcan.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, Thibodeau SN, Katzmann JA, Allen MS, Midthun DE, Marks RS, de Andrade M. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med. 2008;168:1097–1103. doi: 10.1001/archinte.168.10.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stellman SD, Muscat JE, Hoffmann D, Wynder EL. Impact of filter cigarette smoking on lung cancer histology. Prev Med. 1997;26:451–456. doi: 10.1006/pmed.1997.0212. [DOI] [PubMed] [Google Scholar]
- 24.Wynder EL, Stellman SD. Comparative epidemiology of tobacco-related cancers. Cancer Res. 1977;37:4608–4622. [PubMed] [Google Scholar]
- 25.StataCorp LP . Intercooled Stata 9.1 for Windows. College Station: StataCorp LP; 2005. [Google Scholar]
- 26.Galbraith R. Graphical display of estimates having differing standard errors. Technometrics. 1988;30:271–281. doi: 10.2307/1270081. [DOI] [Google Scholar]
- 27.Greenland SC, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. doi: 10.2307/2532206. [DOI] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer (2007) IARC handbooks of cancer prevention. Tobacco control, vol. 11. Reversal of risk after quitting smoking. IARC, Lyon
- 30.Stanley KE, Matthews MJ. Analysis of a pathology review of patients with lung tumors. JNCI. 1981;66:989–992. doi: 10.1093/jnci/66.6.989. [DOI] [PubMed] [Google Scholar]
- 31.Ives JC, Buffler PA, Greenberg SD. Environmental associations and histopathologic patterns of carcinoma of the lung: the challenge and dilemma in epidemiologic studies. Am Rev Respir Dis. 1983;128:195–209. doi: 10.1164/arrd.1983.128.1.195. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti A, Buttitta F, Pellegrini S, Chella A, Bertacca G, Filardo A, et al. Bronchioloalveolar lung carcinoma: K-ras mutations are constant events in the mucinous subtype. J Pathol. 1996;179:254–259. doi: 10.1002/(SICI)1096-9896(199607)179:3<254::AID-PATH589>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Marchetti A, Pellegrini S, Bertacca G, Buttitta F, Gaeta P, Carnicelli V, et al. FHIT and p53 gene abnormalities in bronchioloalveolar carcinomas. Correlations with clinicopathological data and K-ras mutations. J Pathol. 1998;184:240–246. doi: 10.1002/(SICI)1096-9896(199803)184:3<240::AID-PATH20>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 34.Koga T, Hashimoto S, Sugio K, Yoshino I, Mojtahedzadeh S, Matsuo Y, et al. Clinicopathological and molecular evidence indicating the independence of bronchioloalveolar components from other subtypes of human peripheral lung adenocarcinomas. Clin Cancer Res. 2001;7:1730–1738. [PubMed] [Google Scholar]
- 35.Maeshima A, Sakamoto M, Hirohashi S. Mixed mucinous-type and non-mucinous-type adenocarcinoma of the lung: immunohistochemical examination and K-ras gene mutation. Virchows Arch. 2002;440:598–603. doi: 10.1007/s00428-002-0629-6. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]