Abstract
Chronic stress, by initiating changes in the hypothalamic-pituitary-adrenal axis and the immune system, acts as a trigger for anxiety and depression. Both experimental and clinical evidence shows that a rise in the concentrations of proinflammatory cytokines and glucocorticoids, as occurs in chronically stressful situations and in depression, contribute to the behavioural changes associated with depression.
A defect in serotonergic function is associated with hypercortisolaemia and the increase in proinflammatory cytokines that accompany depression. Glucocorticoids and proinflammatory cytokines enhance the conversion of tryptophan to kynurenine. In addition to the resulting decrease in the synthesis of brain serotonin, this leads to the formation of neurotoxins such as the glutamate agonist quinolinic acid and contributes to the increase in apoptosis of astrocytes, oligodendroglia and neurons.
The importance of the inflammation hypothesis of depression lies in raising the possibility that psychotropic drugs that have a central anti-inflammatory action might provide a new generation of antidepressants.
Keywords: proinflammatory cytokines, inflammation, glucocorticoids, serotonin, tryptophan-kynurenine pathway
Introduction
Major depression is a common and sometimes fatal disorder that has been identified by the World Health Organisation as a leading cause of disability worldwide [1]. While antidepressants are undoubtedly effective treatments in about 70% of cases, a substantial proportion of patients remain partially or totally unresponsive to treatment. There is no simple explanation for treatment resistance but there is a possibility that the current antidepressants do not effectively target all of the pathological processes that are responsible for the major symptoms of depression. Thus there is an urgent need to broaden the targets upon which antidepressants are considered to act.
All currently available antidepressants have been developed on the basis of the monoamine hypothesis of depression, a hypothesis which implicates a disorder of biogenic amines in the limbic and cortical circuits as the cause of the main symptoms of depression. Antidepressants are therefore postulated to act by correcting these abnormalities. However, in recent years greater attention has been directed to the inter-relationship between the brain and peripheral organs (the” body-mind” connection) in which changes in the endocrine and immune systems play a major role in the pathological changes that occur in depression. Thus inflammation is beginning to emerge as a major contributing factor not only to depression and other major psychiatric disorders but also to the connection with medical disorders that are frequently associated with mental illness. For example, it is now apparent that, in major depression, there is a relationship between the severity and duration of the disorder and the increased frequency of heart disease, type-2 diabetes, various autoimmune diseases, arthritis and cancer [2]
The concept of a disordered immune system playing a major role in the mental state can be traced back to antiquity. However, it is only in the past 30 years or so that clinical and experimental evidence has been obtained clearly demonstrating that aspects of both cellular and humoral immunity were dysfunctional in major depression [3,4,5]. In the past 20 years, attention has been directed to the role of the immunomodulators and immunotransmitters, in particular the pro- and anti-inflammatory cytokines. Thus Maes and coworkers [6] reported that interleukin-6 (IL-6), a major proinflammatory cytokine, was increased in the blood of depressed patients. It was also apparent that about 45% of patients being therapeutically treated with the proinflammatory cytokine interferon-alpha (IFN) developed major symptoms of depression that terminate when the cytokines was withdrawn [7]. Recently a meta-analysis of 9 cytokines in major depression in which 24 studies were assessed[8] concluded that only the basal levels of IL-6 and TNF were significantly raised. Such clinical observations suggest that proinflammatory cytokines contribute to the major symptoms of depression and now forms the basis of the inflammation, cytokine or inflammatory response hypothesis of depression.
Inter-relationship between cytokines and brain function: relevance to depression?
Until recently, the brain was considered to be an immunologically privileged organ that was protected from the peripheral immune system by the blood-brain-barrier. It is now apparent that this view is incorrect and that the brain is directly influenced by peripherally derived cytokines, chemokines, prostenoids and glucocorticoids, as well as some immune cells, that can access the brain and thereby influence those neuronal networks that appear to be malfunctioning in depression [9]. The influence of large molecules from the periphery on the brain is somewhat surprising as specific transporters for peptides such as the interleukins do not appear to be present at the blood-brain-barrier. Nevertheless, there is now experimental evidence to indicate that such molecules could access the brain a) via a leaky blood-brain-barrier that occurs in major depression, b)by activation of endothelial cells that line the cerebral vasculature and produce inflammatory mediators inside the barrier c)by binding to cytokine receptors associated with the vagus nerve and thereby signalling inflammatory changes in the brain via the nucleus tractus solitarius and hypothalamus [10,11]. Once in the brain, the proinflammatory cytokines activated both neuronal and non-neuronal (for example, the microglia, astrocytes and oligodendroglia) cells via the nuclear factor-kappa-beta (NF-kB) cascade in a similar manner to that occurring in the peripheral inflammatory response [12].
There is also evidence from clinical studies that peripherally administered cytokines can enter the brain. Thus the therapeutic administration of IFN to patients with hepatitis results in an increase in the cerebrospinal fluid (CSF) not only of IFN but also IL-6 and monocyte chemoattractant protein (MCP-1) [13]. In experimental studies it has been shown that MCP-1 activates microglia to release IL-1 and TNF [14] and as the microglia are the primary source of proinflammmatory cytokines in the brain this could be an important means whereby peripheral inflammatory mediators activate the inflammatory response in the brain. In addition, the proinflammatory cytokines modulate the release of biogenic amine neurotransmitters [15]. Recently much attention has been paid to the activation of the tryptophan-kynurenine pathway by these cytokines whereby tryptophan is shunted from the synthesis of serotonin to that of kynurenine. The importance of this pathway will be discussed in more detail later and clearly this is an important mechanism whereby serotonergic function is decreased in depression. The activity of the dopaminergic system is also reduced in response to inflammation. For example, IFN reduces the synthesis of dopamine by decreasing the concentration of the co-factor tetrahydrobiopterin (BH4), thereby reducing the synthesis of dihydroxyphenylalanine (DOPA), the immediate precursor of dopamine, from tyrosine [16]. As IFN increases the synthesis of nitric oxide by activating the BH4 dependent enzyme nitric oxide synthase in the microglia it seems likely that the reduction in dopaminergic function is linked to the increase in nitric oxide. This gaseous neurotransmitter is known to activate the glutamatergic system which, when this exceeds physiologically limits, enhances apoptosis and neurodegeneration [15,16].
Cytokines, and their signalling pathways, have been shown to enhance the re-uptake of monoamine neurotransmitters and thereby reduce their functionally important inter-synaptic concentrations in the brain [16,17]. For example,IL-1 and TNF have been shown to activate the serotonin transporter on neurons by stimulating the p38 mitogen activated protein kinase pathway [17]
In addition to the modulation of neurotransmitter function, proinflammatory cytokines contribute to the major symptoms of depression by activating the HPA axis by increasing the release of CRF, thereby contributing to hypercortisolaemia, a feature of major depression [18,19,20]. The mechanism whereby the cytokines induce hypercortisolaemia involves a decreased sensitivity of the glucocorticoid receptors thereby leading to glucocorticoid resistance; both the brain and the peripheral receptors become insensitive to glucocorticoid activation. The precise mechanism whereby the proinflammatory cytokines cause glucocorticoid receptor insensitivity is uncertain but it is known that the cytokines activate the inflammatory cascade. Thus the NF-kB, p38MAPK and the 5-STATS (signal transducer and activator of transcription 5) pathway is activated and leads to a disruption of the translocation of glucocorticoid receptors from the cytoplasm to the nucleus (21), thereby decreasing the active form of the receptor. While it would appear that glucocorticoid receptor resistance is correlated with the increase in the serum concentration of the proinflammatory cytokines, it seems unlikely that glucocorticoid resistance is directly related to the psychopathology of depression. Thus an increase in proinflammatory cytokines leading to glucocorticoid resistance also occurs in nondepressed individuals without any major change in the mood state [22]. However, such observations do throw light on the fact that many aspects of cellular and humoral immunity are not suppressed in patients with major depression despite the elevation of the plasma glucocorticoid concentration that is a common feature of the disorder.
The role of stress and proinflammatory cytokines
An important conceptual shift in the possible cause of depression has occurred recently with the discovery that inflammation plays a crucial role in the psychopathology of the disorder. However, as major depression is often accompanied by inflammatory diseases (such as irritable bowel syndrome, type 2 diabetes, arthritis and autoimmune disorders) that can activate the peripheral and central inflammatory response, it is possible that such inflammatory disorders initiate the inflammatory changes that precipitate depression. Although this is plausible, it is evident that inflammation also occurs in depressed patients who are not suffering from concurrent inflammatory disorders. Thus the increased vulnerability of depressed patients to psychosocial stress is probably the key factor that leads to the activation of the immune and endocrine axes in depression. It is known, for example, that even the relatively mild acute stress of public speaking causes an increase in NF-kB activity, a key element in the induction of the inflammatory cascade [23]. In this regard, it is also known that patients with major depression frequently show an enhanced responsiveness of IL-6 and NF-kB to an antigen challenge [24]. However, chronic stress, as experienced by caregivers or individual subject to marital discord but who are not suffering from depression also show an increase in plasma C-reactive protein, IL-6 and other inflammatory mediators [24,25]. Whatever the cause, such changes appear to be associated with activation of the microglia thereby suggestion that the inflammatory changes are also occurring in the brain [26].
The mechanism whereby psychological stress influences both the peripheral and central inflammatory cascade is co-ordinated by the autonomic nervous system. Thus the release of noradrenaline and adrenaline following the activation of the sympathetic system results in the activation of both alpha and beta adrenoceptors on immune cells thereby initiating the release of proinflammatory cytokines, via the activation of the NF-kB cascade, particularly on macrophages and monocytes in peripheral blood; antagonists of these adrenoceptors block the stress induced rise in these cytokines [27]. Conversely stimulation of the parasympathetic system has the opposite effect on the stress induced inflammatory response. Thus stimulation of the vagus nerve results in release of acetylcholine that activates the alpha-7 sub-unit on nicotinic receptors thereby reduces the activation of NF-kB [28]. It is possible that the anti-depressant-like action of vagal nerve stimulation, occasionally used to treat resistant depression, is associated with such an anti-inflammatory action.
The question arises why should inflammation occur in depressed patients despite the frequently observed increase in glucocortioids? The most parsimonious explanation is that glucocorticoid receptor resistance in the brain and periphery contribute to the lack of suppression of most types of immune cells with the possible exception of the natural killer cells. One possible explanation is that the stress induced increase in the sympathetic nervous system, combined with steroid resistance, leads to the activation of the microglia in the brain, and macrophages and monocytes in the periphery, thereby leading to the inflammatory state.
Serotonin, stress and depression
Of the numerous neurotransmitters that have been postulated to be dysfunctional in major depression, serotonin has been widely implicated for its contributory role in the symptoms of the disorder (sleep disturbance, depressed mood, anorexia, loss of libido and anxiety). Serotonin modulates the stress axis by activating the corticotrophin releasing factor pathways in the para ventricular nucleus thereby increasing the release of adrenocorticotrophic hormone from the anterior pituitary gland [29] There is a close relationship between the plasma cortisol concentration and the serotonergic system. Thus the stress induced rise in cortical is associated with an increased turnover of serotonin, a change that is linked to the stimulation of the rate limiting enzyme, tryptophan hydroxylase, in the pathway leading to the synthesis of serotonin from tryptophan [30]. Chronic stress that results in a sustained rise in cortisol has the opposite effect and sertotonin release is decreased. This is associated with the glucocorticoid activation of tryptophan dioxygenase in the liver whereby tryptophan is diverted from serotonin synthesis down the tryptophan-kynurenine pathway [31].
The increase in anxiety, and the impairment if adaptation to chronic stress that has been observed in both animals and man can be explained by the changes in the functional activity of the somatodendritic 5HT 1A receptors located on the median raphe nucleus and the hippocampus [32]. Experimental studies have shown that rats raised in a stressful, overcrowded environment show an increase in anxiety which is correlated with a decrease in the functional activity of the 5HT1A receptors. These receptors are influenced by the mineralocorticoid receptors that inhibit the 5HT1A receptor activity under conditions of chronic stress [33] In contrast to the 5HT1A receptors, the 5HT2 receptors are activated by chronic stress [34] while the 5HT1B receptors, that act as autoreceptors and thereby control the release of the transmitter, are activated by chronic stress [35]. Thus the stress induced changes in the circulating glucocorticoids can help to explain the decrease in the functional activity of the serotonergic system in depression.
However, this evidence has been mainly provided from experimental studies. What is the evidence from clinical studies? In some depressed patients during remission, the administration of a tryptophan deficient amino acid drink triggers an acute depressive response; this change is associated with the hypersecretion of cortisol [36]. The elevation in plasma prolactin and cortisol in depressed patients following the acute administration of the serotonin releasing agent fenfluramine is also reduced in depressed patients [37], as is the secretion of growth hormone, in response to a tryptophan challenge [38]. These observations add additional evidence to the importance of serotonin in depression not only for its mood modulating role but also for its direct effect on the endocrine axis. Thus the results of experimental and clinical studies clearly demonstrate that chronic stress, as a results of hypercortisolaemia, initiates changes in the serotonergic system that appear to play a critical role in the onset of anxiety and depression.
Stress, depression and neurodegeneration
The emphasis in this review is on the adverse effects of the proinflammatory cytokines that, in pathological concentrations in the brain and periphery, are likely to cause cellular injury. However, it must be remembered that at physiological concentrations, these same cytokines provide trophic support for neurons, enhance neurogenesis and contribute to normal cognitive function [39]. Such effects are severely compromised when the cytokines are present in pathological concentrations and result in changes that are important in the psychopathology of depression. Thus in major depression, the prolonged activation of the inflammatory network in the brain results in a decrease in neurotrophins, leading to reduced neuronal repair, a decrease in neurogenesis, and an increased activation of the glutamatergic pathway that contributes to neuronal apoptosis, oxidative stress and the induction of apoptosis in astrocytes and oligodendrocytes [40,41,42,43].
In addition to the proinflammatory cytokines, nitric oxide and the glucocorticoids, glutamate plays a crucial role in the pathological processes that are associated with depression. The proinflammatory cytokines, and inflammatory mediators such as nitric oxide, increase glutamate release and decrease the expression of glutamate transporters on astrocytes and oligodendroglia thereby decreasing glutamate reuptake and enhancing the inter-synaptic concentration [44].
Stimulation of the extra-synaptic N-methyl-D-aspartate (NMDA) glutamate receptor not only causes excitotoxic damage to the neurons and astrocytes but also results in a decrease in synthesis of brain derived neurotrophic factor (BDNF), a key neurotrophic factor governing neuronal repair [45]. To add to the potential neurotoxic changes, IL-1 and TNF, that are generally raised in depression, trigger the release of reactive oxygen and nitrogen species from activated microglia and astrocytes; these are toxic to both neurons and oligodendroglia [46,47]. The net result of these changes is a loss of astrocytes and oligodendroglia, and neuronal apoptosis particularly in the subgenual prefrontal cortex, the amygdala and the hippocampus, brain regions that are thought to be crucially involved in the genesis of the symptoms of depression.[48].
The question now arises regarding the possible link between the neurotoxic effects of the proinflammatory cytokines, excess glutamate and the tryptophan-kynurenine pathway that, in depression, produces neurotoxic end-products that contribute to neurodegeneration in depression. Tryptophan is metabolised through two main pathways, one of which leads to the synthesis of serotonin and the other to kynurenine and kynurenic acid. In the latter pathway, tryptophan is metabolised by indoleamine 2,3 dioxygenase (IDO), an enzyme that is quite widely distributed in peripheral tissues and the brain, and by tryptophan 2,3 dioxygenase (TDO) that is primarily located in the liver [49]. IDO is activated by proinflammatory cytokines while TDO is activated by glucocorticoids. As both the cytokines and cortisol are raised in major depression, it is not surprising to find that the tryptophan-kynurenine pathway is increased [49,50]. Anti-inflammatory cytokines reduce the activity of this pathway [51]. There are two main pathways that lead to the metabolism of tryptophan following the formation of kynurenine. Kynurenine hydroxylase metabolises kynurenine first to 3-hydroxykynurenine and then to 3-hydroxyanthranilic acid and quinolinic acid. This pathway is increased in depression and dementia [49,50]. In glia and neurons 3-hydroxykynurenine increases the formation of reactive oxygen species while quinolinic acid activates NMDA glutamate receptors and thereby enhances apoptosis. By contrast kynurenine can be metabolised by kynurenine aminotransferase to for the neuroprotective end product, kynurenic acid, an antagonist of NMDA receptors [51]. In the brain,the metabolism of tryptophan by IDO occurs both in the microglia and astrocytes [52]. The microglia synthesise both 3-hydroxyanthranilic acid and quinolinic acid while the astrocytes produce mainly kynurenic acid. Astrocytes also metabolise quinolinic acid and therefore under physiological conditions can reduce the impact of the neurotoxins [53]. In chronic depression however, the activated microglia produce an excess of the neurotoxin that cannot be adequately metabolised by the astrocytes. Furthermore quinolinic acid can cause apoptosis of the astrocytes. This results in a reduction in the metabolic and physical buffer to the neurons that is usually provided by the astrocytes and thereby further exposes the neurons to the neurodegenerative actions of quinolinic acid [54].
The clinical evidence supporting the hypothesis that the tryptophan-kynurenine pathway is activated comes from two major studies. Wichers and coworkers [55] showed that the concentration of kynurenic acid was reduced in patients being treated with IFN for the treatment of hepatitis while Myint and colleagues [56] reported evidence that components of the neurodegerative pathway was increased in the blood of depressed patients before antidepressant treatment. Effective treatment for 8 weeks only partially reversed these changes in patients being treated for their first major episode of depression but had no effect in those patients who had suffered several episodes. This suggests that more permanent changes may occur in the brain of those with a chronic depression.
The structural changes observed in the brain of patients with chronic depression lends support to the neurodegenerative hypothesis of depression [57]. It is known that there is a shrinkage of the hippocampus in patients with major depression [58] and a decrease in the number of astrocytes and a neuronal loss in the prefrontal cortex [59] and in the striatum. Such changes could be the consequence of chronic low grade inflammation in which the proinflammatory cytokines, nitric oxide, prostaglandin E2 and other inflammatory mediators play key roles; the cytokines are known to induce the cyclo-oxygenase and nitric oxide sythase pathways in the brain and thereby increase the inflammatory insult [60]. The inhibition of neurotrophin synthesis in the brain by glucocorticoids [61], and the neurotoxic action of quinolinic acid, add further to the impact of the inflammatory changes.
Could the inflammation hypothesis of depression contribute to the development of novel antidepressants?
If inflammation plays a significant role in the pathogenesis of depression, it would be anticipated that effective antidepressant treatments would attenuate the inflammatory changes. Indeed, there is now substantial evidence that antidepressants not only inhibit the release of proinflammatory cytokines from whole blood cultures in vitro but also stimulate the release of anti-inflammatory cytokines such as IL-10 [62]. Clinically, different strategies for treating depression, that include ECT and psychotherapy in addition to antidepressants, have been shown to attenuate the inflammatory changes that correlate with the improvement in the mood state [63,64]. Such findings suggest that a reduction in inflammation is causally related to the treatment response.
Further evidence for the relationship between inflammation and depression is provided by the observation that depressed patients with a history of partial or lack of response to antidepressant treatments have elevated plasma concentrations of IL-6 and acute phase proteins that persist despite antidepressant treatment [65]. It has been suggested that patients who are resistant to conventional antidepressant treatment possess abnormal alleles of the IL-1 and TNF genes, and possibly for T-cell function [66].
If effective treatment with conventional antidepressants is associated with the attenuation of the inflammatory response, it may be argued that there is little urgency to develop new types of antidepressants that act by mechanisms other than modulating the brain monoamines. However, there is abundant clinical evidence that the available antidepressants, though largely effective in attenuating the depressed mood state and associated symptoms of anxiety, are far less effective in treating the memory and cognitive dysfunction (fatigue, psychomotor retardation) that commonly affect middle aged and elderly depressed patients [67]. This not only emphasises the need to develop better antidepressants but also to consider anti-inflammatory drugs as possible candidates. There are already indications from the clinical literature that TNF antagonists, such as etanercept and infliximab, reduce the symptoms of depression in a variety of patients with autoimmune diseases (for example, rheumatoid arthritis and psoriasis), the mood state of the patients improving before the signs of improvement of the autoimmune disorder [68.69].
Anti-inflammatory cytokines have also been shown to block the depressive-like state in rodents that was induced by TNF or following the mitogen activation of macrophages by lipopolysaccharide. Thus IL-10, and insulin-like growth factor that has prominent anti-inflammatory activity, have been shown to attenuate the depressive-like behaviour in rodents induced by an inflammatory challenge [70]. Another novel method for targeting inflammation lies in reducing the activity of the glutamatergic system that is activated by the neurotoxic end products of the tryptophan-kynurenine pathway (for example, quinolinic acid) and by nitric oxide whose synthesis is enhanced by proinflammatory cytokines. Riluzole, used in the treatment of the symptoms of amyotropic lateral sclerosis (ALS, motor neuron disease), acts by enhancing the uptake of glutamate into astrocytes via the excitatory amino acid transporter and thereby reduces the activation of neurons by glutamate. Clinical observations also shows that riluzole has antidepressant-like activity and could therefore act as a prototype for glutamate modulating antidepressants [71].
Perhaps the most obvious step to the reduction of inflammation both centrally and peripherally is to reduce the activity of the prostenoid pathway and thereby reduce the synthesis of inflammatory prostaglandins such as PGE2. There is already some clinical evidence that a reduction in the activity of cyclo-oxygenase 2 (COX-2) by celecoxib has a beneficial effect in depressed patient who were only partial responders to reboxetine [72]. Even aspirin has been reported to have similar effects to celecoxib in a pilot study in depression [73] These are important “ proof of concept” studies. However, it must be cautioned that several COX-2 inhibitors have already been withdrawn because of cardiac complications. Furthermore, some prostenoids and eicosanoids can have opposing roles in different tissues and may even change from pro-to anti-inflammatory substances during the course of an inflammatory disease[74]. It must also be remembered that proinflammatory prostenoids such as PGE2 have physiological actions in the brain and periphery that are not directly connected with the inflammatory response [75]. Thus it is important to be cautious in developing anti-inflammatory drugs as antidepressants particularly when they only target one aspect of the inflammatory cascade.
Inflammation is also a protective mechanism against invading bacteria, viruses, oncogenes and an important component of the stress response. Inflammation starts as a time- and site specific defence mechanism aimed not only at protecting the organism from pathogenic microorganisms but also at removing damaged neurons and, under physiological conditions, repairing damaged neuronal networks. It is only in situations where the inflammatory mediators occur in concentrations above the physiologically relevant range that they play a pathological role. While it is now evident that this situation applies to patients with major depression, it must be remembered that inflammation plays a vital role in protection against infection. Caution must therefore be exercised in developing immunomodulators that, if they are to become clinically effective antidepressants, will have to be administered for months, or even years, to patients in order to maintain remission from depression.
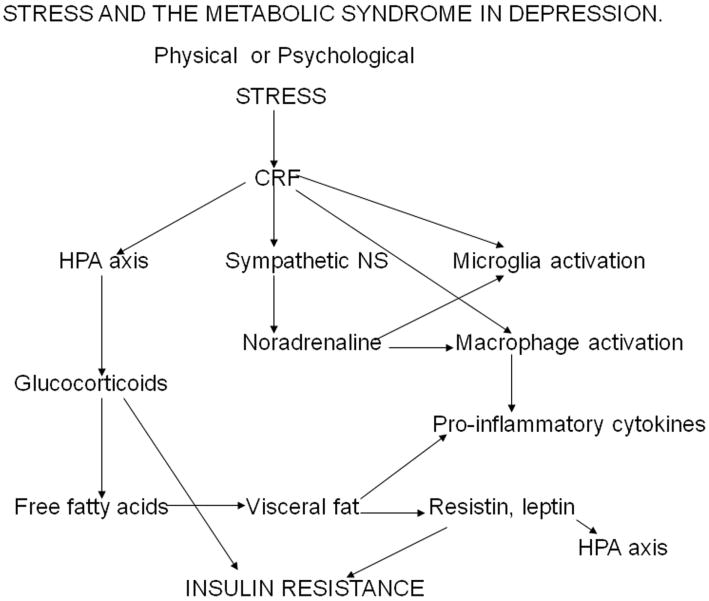
Figure 1.
Stress causes the activation of the corticotrophin releasing factor (CRF) in the corticolimbic regions of the brain. CRF activates the hypothalamic-pituitary-adrenal axis resulting in an elevation in circulating glucocorticoids. In addition, CRF activates the locus coeruleus which results in an increase in central and peripheral sympathetic activity. Stress increases the release of pro-inflammatory cytokines from microglia in the brain and from macrophages in the blood. In addition noradrenaline and adrenaline, from the sympathetic system, also activate the macrophages and microglia thereby contributing to the inflammatory response. Chronic hypercortisolaemia that results from the lowered stress threshold in depression, combined with the activation of the anterior pituitary by the pro-inflammatory cytokine interleukin-6, desensitizes the glucocorticoid type 2 receptors on immune cells, and in the pituitary, hypothalamus and on neurons. This causes glucocorticoid resistance. The increase in the mobilization of fat by the glucocorticoids contributes to the increased deposition of visceral fat. Visceral fat acts as an extra-endocrine organ and liberates pro-inflammatory cytokines together with the peptides leptin and resistin. Leptin contributes to the activation of the HPA axis while resistin, together with the increased glucocorticoids, contributes to insulin resistance.
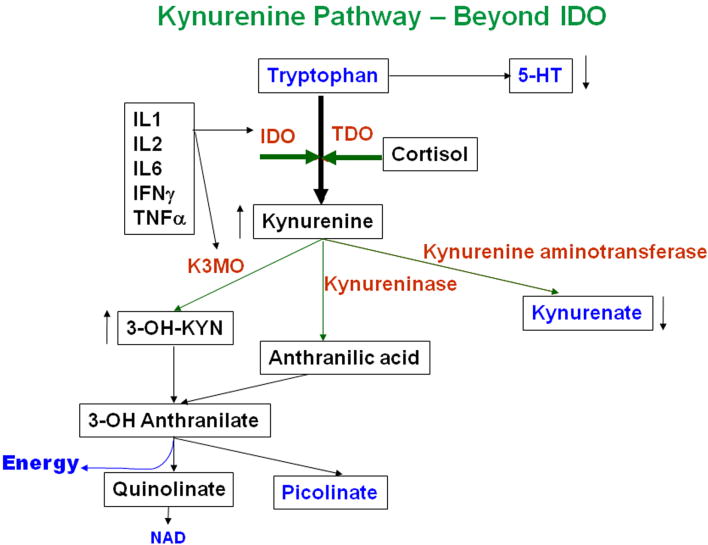
Figure 2.
In depression, tryptophan is preferentially metabolized through the kynurenine pathway. The two key enzymes that convert tryptophan to kynurenine are indoleamine 2,3 dioxygenase (IDO) and tryptophan dioxygenase (TDO). IDO, that is quite widely distributed in the peripheral tissues and the brain, is induced by pro-inflammatory cytokines while TDO, which is confined to the liver, is induced by glucocorticoids. In depression, kynurenine is further metabolized to 3-hydroxykynurenine by kynurenine 3 mono-oxygenase, an enzyme that is also induced by pro-inflammatory cytokines. The most important end product of this inflammatory pathway is the neurotoxin quinolinic acid which activates N-methyl-D-aspartate glutamate receptor on neurons leading to neuronal apoptosis. Activated microglia synthesise quinolinic acid in the brain while astrocytes can metabolise the toxin to nicotinamide-adenine dinucleotide (NAD) when present in low concentrations. High concentrations of quinolinic acid, thought to occur in chronic depression, cause apoptosis of astrocytes thereby increasing the vulnerability of the neurons to neurotoxic damage.
References
- 1.Moussavi S, Chatterji E, Verdes A, et al. Depression, chronic disease and decrements in health: results from WHO surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality and the combination of risk factors: global burden of disease study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 3.Maes M, Meltzer H, Bosmans E, et al. Increased plasma concentrations of IL-6,sIL-6R,sIL-2R and transferring receptor in major depression. J Affect Dis. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 4.Raison CL, Capuron L, Miller AH. Cytokines sing the blues : inflammation and pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two major cardiac events in men after acute coronary syndromes. Biol Psychiat. 2007;62:302–308. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation tosickness and depression:when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiat. 2009 doi: 10.1016/j.biopsych.2009.09.033. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopheral attenuates NF-kB activation and proinflammatory cytokine production in brain improves recovery from lipopolysaccharide induced sickness behaviour. J Neuroimmunol. 2005;169:97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, Borisov AS, Majer M, et al. Activation of CNS inflammatory pathways by interferon-alpha : relationship of monoamines an depression. Biol Psychiat. 2008:64. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Si Q, Cosenza M, Kim MO, et al. A novel action of minocycline: inhibition of human immunodeficiency virus type 1 infection in microglia. J Neurovirol. 2004;10:284–292. doi: 10.1080/13550280490499533. [DOI] [PubMed] [Google Scholar]
- 12.Anisman H, Merali Z, Hayley S. Neurotransmitters, peptides and cytokines process in relation to depressive disorder: comorbidity between depression and neurodegenerative disorder. Prog Neurobiol Biol Psychiat. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Kitagami T, Yamada K, Miura H, et al. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats:role of nitric oxide as a signal crossing the blood-brain-barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- 14.Zielasek J, Hartung HP. Molecular mechanisms of microglia activation. Adv Neuroimmunol. 1996;6:191–222. doi: 10.1016/0960-5428(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 15.Miller AH, Maletic V, Raison CL. Inflammation and its discontents:the role of cytokines in the pathophysiology of major depression. Biol Psychiat. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moron JA, Zakharova I, Ferrer JV, et al. Mitogen activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines IL-1 beta and TNF-alpha activate serotonin transporters. Neuropsychopharmacol. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 18.Besedovsky HO, del Rey A, Klusman I, et al. Cytokines as modulators of the HPA axis. J Steroid Biochem Mol Biol. 1991;40:613–618. doi: 10.1016/0960-0760(91)90284-c. [DOI] [PubMed] [Google Scholar]
- 19.Pariante CM, Miller AM. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiat. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 20.Pace TW, Hu F, Miller AH. Cytokine effects on glucocorticoid receptor function:relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vedder H, Schreiber W, Schuld A, et al. Immune-endocrine host response to endotoxin in major depression. J Psychiat Res. 2007;41:280–289. doi: 10.1016/j.jpsychires.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Bierhaus A, Wolf J, Andrassy N, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pace TW, Mietzko TC, Alagbe O, et al. Increased stress induced inflammatory response in male patients with major depression and increased early life stress. Am J Psychiat. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 24.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health,aging and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 25.Kielcolt-Glaser JK, Loving TJ, Stowell JR, et al. Hostile marital interactions, proinflammatory cytokine production and wound healing. Arch Gen Psychiat. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 26.Frank MG, Baratta MV, Sprunger LR, et al. Microglia serve a neuroimmune substrate for stress-induced potentiation of CNS proinflammatory cytokine response. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Mazzeo RS, Donovan D, Fleshner M, et al. IL-6 response to exercise and high altitude exposure: influence of alpha-adrenergic blockade. J appl Physiol. 2001;91:2143–2149. doi: 10.1152/jappl.2001.91.5.2143. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Calogero AE, Bagdy G, Moncada ML, D’Agata R. Effect of SSRI agonists on basal CRH and VP induced ACTH release in vitro from rat pituitary cells. J Endocrinol. 1993;136:381–387. doi: 10.1677/joe.0.1360381. [DOI] [PubMed] [Google Scholar]
- 30.Davis S, Heal DJ, Stanfort SC. Long lasting effects of acute stress on the neurochemistry and function of 5HT neurons in mouse brain. Psychopharmacol. 1995;118:267–272. doi: 10.1007/BF02245954. [DOI] [PubMed] [Google Scholar]
- 31.Myint A-M, Kim Y-K, Verkerk R, et al. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Dis. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Meijer OC, De Kloet ER. Corticosterone and serotonergic neurotransmission in hippocampus: functional implications of central corticosteroid receptor diversity. Crit Rev Neurobiol. 1998;12:1–20. [PubMed] [Google Scholar]
- 33.Chaouloff F. Regualtion of 5HT receptors by corticosteroids: where do we stand? Fund Clin Pharmacol. 1995;9:219–233. doi: 10.1111/j.1472-8206.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 34.McKittrick CR, Blanchard DC, Blanchard RJ, et al. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiat. 1995;37:383–395. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 35.Neumeier JC, Petty F, Kramer GL, et al. Learned helplessness increases 5HT1B receptor mRNA levels in the rat dorsal nucleus. Biol Psychiat. 1997;41:668–674. doi: 10.1016/S0006-3223(96)00114-X. [DOI] [PubMed] [Google Scholar]
- 36.Aberg-Wistedt A, Husselmark L, Stain-Malingren R, et al. Serotonergic vulnerability in affective disorder. Acta Psychiat Scand. 1998;97:374–380. doi: 10.1111/j.1600-0447.1998.tb10017.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Keane V, Dinan TG. Prolactin and cortisol responses to d-fenfluramine in major depression: evidence for diminished responsivity of central serotonergic function. Am J Psychiat. 1991;148:1009–1015. doi: 10.1176/ajp.148.8.1009. [DOI] [PubMed] [Google Scholar]
- 38.Porter R, McAllister-Williams RH, Lunn B, Young A. 5HT receptor function in man is reduced with susceptibility to major depression. Psychpharmacol. 1998;139:243–250. doi: 10.1007/s002130050711. [DOI] [PubMed] [Google Scholar]
- 39.Bernardino L, Agasse F, Silva R, et al. TNF-alpha modulates survival, proliferation and neural differentiation in neonatal sunventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- 40.Goshen T, Kreisel H, Ounallah-Saad P, et al. A dual role for IL-1 in hippocampal dependent memory processes. Psychoneuroimmunol. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Barrientos RM, Sprunger DB, Campeau S, et al. BF mRNA down regulataion produced by social isolation is blocked by intrahippocampal IL-1R antagonist. Neurosci. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 42.Tilleux S, Hermans E. Neuroinflammation and regulation of glia glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 43.Gavillet M, Allamen I, Magistretti PJ. Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia. 2008;56:975–989. doi: 10.1002/glia.20671. [DOI] [PubMed] [Google Scholar]
- 44.Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: implications for excitotoxicity in multiple sclerosis. Neurol. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 45.Hardingham GE, Fukinaga Y, Badinmg H. Extrasynaptic NMDAR’s oppose synaptic NMDAR’s by triggering CREB shut-off and cell death pathway. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 46.Buntinx M, Moreels M, Vandenabeele I, et al. Cytokine induced cell death in human oligodendroglia cell lines: synergistic effects of IFN gamma and TNF-alpha on apoptosis. Neurosci Res. 2004;76:834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- 47.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glia pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontalo cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myint A-M, Kim Y-K. Cytokine-serotonin interactions through indoleamine 2,3 dioxygenase : a neurodegenerative hypothesis of depression. Med Hypoth. 2003;61:519–525. doi: 10.1016/s0306-9877(03)00207-x. [DOI] [PubMed] [Google Scholar]
- 50.Guillemin GJ, Kerr SJ, Smyth GA, et al. The kynurenine pathway metabolism in human astrocytes:pathway for neuroprotection. J Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 51.Musso T, Gusella GL, Brooks A, et al. IL-4 inhibits IDO expression in human monocytes. Blood. 1994;83:1408–1411. [PubMed] [Google Scholar]
- 52.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 53.Perkins MN, Stone TW. An iontophoretic investigation of actions of convulsant kynurenines and their interaction with endogenous quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 54.Grant RC, Kapoor V. Murine glial cells regenerate NAD after peroxide induced depletion, using nicotinic acid, nicotinamide or quinolinic acid as substrates. Ann N Y Acad Sci. 1998;697:53–60. doi: 10.1046/j.1471-4159.1998.70041759.x. [DOI] [PubMed] [Google Scholar]
- 55.Wichers MC, Kock GH, Robays G, et al. IDO and IFN-alpha induced depressive symptoms : a shift in hypothesis from tryptophan depletion. Mol Psychiat. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 56.Myint A-M, Kim Y-K, Verkerk R, et al. Kynurenine pathway in major depression:evidence of impaired neuroprotection. J Affect Dis. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 57.Sheline YI, Sanghavi MA, Mintum M, Gado MH. Depression duration, but not age, predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ongur D, Katz IR, Meyers BS, et al. Glia reduction in subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neural and glia prefrontal cell pathology in major depression. Biol Psychiat. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 60.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 61.Nibuya M, Takahashi M, Russell DS, Duman RS. Regualtion of BDNF and trkB mRNA in rat brain by chronic ECTseizures in rat hippocampus. Neurosci Lett. 1999;267:81–84. doi: 10.1016/s0304-3940(99)00335-3. [DOI] [PubMed] [Google Scholar]
- 62.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmac. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 63.Lanquillon S, Krieg JC, Bening-Abu-Shach U. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacol. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 64.Leonard BE, Song C. Stress, Depression and the role of cytokines. In: Dantzer R, editor. Cytokines,Stress and Depression. Academic Plenum; New York, Kluwer: 1999. pp. 251–265. [DOI] [PubMed] [Google Scholar]
- 65.Sluzewska A, Rybakowski J, Bosmans E, et al. Indicators of immune activation in major depression. Psychait Res. 1996;64:162–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 66.Wong M-L, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation related genes and susceptibility to major depression and antidepressants. Mol Psychiat. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrow GR, et al. Differential effects of paroxetine on fatigue and depression: a randomized,double-blind trial from University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 68.Lichtenstein GR. Infliximab improves quality of life in patients with Crohn’s disease. Inflam Bowel Dis. 2002;8:237–243. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Matthias SD. Health related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Therap. 2000;22:128–139. doi: 10.1016/s0149-2918(00)87984-9. [DOI] [PubMed] [Google Scholar]
- 70.Bluthe RM, Dantzer R, Kelley KW. Central mediation of the effects of IL-1 on social exploration and body weight in mice. Psychoneuroendocrinol. 1997;22:1–11. doi: 10.1016/s0306-4530(96)00042-x. [DOI] [PubMed] [Google Scholar]
- 71.Sancora G, Gueorguieva R, Epperson CN, et al. Subtype specific alterations of GABA and glutamate in patients with major depression. Arch Gen Psychiat. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 72.Mueller N, Schwarz MJ, Dehning A, et al. The COX 2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, controlled, add-on pilot study to reboxetine. Mol Psychiat. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 73.Mendlewicz J, Kriwin P, Oswald D, et al. Shortened onset of action of antidepressants in major depression using acetylsalicyclic acid augmentation. A pilot open-label study. Int clin Psychopharmacol. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Yedgar S, Krimsky M, Cohen Y, Flower RJ. Treatment of inflammatory disease by selective eicosanoid inhibition:a double-edged sword? Tends Pharmacol Sci. 2007;28:459–464. doi: 10.1016/j.tips.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Marchetti B, Abbracchio MP. To be or not to be (inflammed) –is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]