Abstract
Background
High-level occupational exposures to some industrial chemicals have been associated with liver diseases, including nonalcoholic fatty liver disease (NAFLD). However, the potential role of low-level environmental pollution on liver disease in the general population has not been evaluated.
Objective
We determined whether environmental pollutants are associated with an elevation in serum alanine aminotransferase (ALT) activity and suspected NAFLD in U.S. adults.
Methods
This cross-sectional cohort study evaluated adult participants without viral hepatitis, hemochromatosis, or alcoholic liver disease from the National Health and Nutrition Examination Survey (NHANES) for 2003–2004. ALT elevation was defined in men as ≥ 37 IU/L (age18–20 years) and ≥ 48 IU/L (age ≥ 21 years) and in women as ≥ 30 IU/L (age 18–20 years) and ≥ 31 IU/L (age ≥ 21 years). Adjusted odds ratios (ORs) for ALT elevation were determined across exposure quartiles for 17 pollutant subclasses comprising 111 individual pollutants present with at least a 60% detection rate. Adjustments were made for age, race/ethnicity, sex, body mass index, poverty income ratio, and insulin resistance. Individual pollutants from subclasses associated with ALT elevation were subsequently analyzed.
Results
The overall prevalence of ALT elevation was 10.6%. Heavy metals and polychlorinated biphenyls (PCBs) were associated with dose-dependent increased adjusted ORs for ALT elevation. Within these subclasses, increasing whole-blood levels of lead and mercury and increasing lipid-adjusted serum levels of 20 PCBs were individually associated with ALT elevation.
Conclusions
PCB, lead, and mercury exposures were associated with unexplained ALT elevation, a proxy marker of NAFLD, in NHANES 2003–2004 adult participants.
Keywords: environmental liver disease, hepatotoxicity, lead, mercury, NAFLD, NASH, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, PCBs, polychlorinated biphenyls, TASH
The burden of liver disease has increased in the United States in parallel with the obesity epidemic, and some cases are believed to be due to nonalcoholic fatty liver disease (NAFLD) or its more advanced form, nonalcoholic steatohepatitis (NASH) (Cave et al. 2007). Serum alanine aminotransferase (ALT) is the most specific of the routinely used biomarkers for hepatocellular liver injury and disease in clinical medicine (Green and Flamm 2002). Currently, there is no serologic biomarker to confirm the diagnosis of NAFLD, but ALT elevation (above normal laboratory reference ranges) is the most common laboratory manifestation of NAFLD, and ALT elevation unexplained by viral hepatitis, ethanol, or iron overload has been used as a surrogate biomarker for NAFLD in the National Health and Nutrition Examination Survey (NHANES) (Clark 2006). Using this approach, Clark et al. (2003) reported that “unexplained ALT elevation” or “suspected NAFLD” was present in 5.4% of adult NHANES III (1988–1994) participants. Since the publication of that study, unexplained ALT elevation has been rapidly adopted by other authors to define subpopulations within NHANES with suspected NAFLD (Clark et al. 2003; Dunn et al. 2008; Liangpunsakul and Chalasani 2004, 2005).
High-level occupational exposures to industrial chemicals have been associated with liver diseases, including NAFLD (Cave et al. 2007; Cotrim et al. 1999, 2004). Recently, insulin resistance and toxicant-associated steatohepatitis (TASH), a form of NASH, were reported in nonobese chemical workers who had been highly exposed to vinyl chloride (Cave et al. 2010). However, the potential influence of low-level environmental pollution on liver disease and suspected NAFLD in the general population has not been determined. Recently, pollutant levels were measured in NHANES participants, and specific pollutants were found to be associated with insulin resistance and metabolic syndrome (Lee et al. 2007a, 2007b), although liver disease was not evaluated.
In the present study, we tested the hypothesis that environmental pollutants are dose-dependently associated with increased risk for ALT elevation and suspected NAFLD in the NHANES adult population.
Materials and Methods
Study design and participants
Adult participants from NHANES 2003–2004, the most recent NHANES for which pollutant data were posted at the time of analysis, were evaluated in this cross-sectional cohort study. NHANES is conducted by the National Center for Health Statistics (NCHS) to evaluate the health and nutrition of Americans, and it is designed to be nationally representative of the noninstitutionalized U.S. civilian population on the basis of a complex multistage probability sample (NCHS 2005). Approval for our analysis of the NHANES data was granted by the University of Louisville Institutional Review Board.
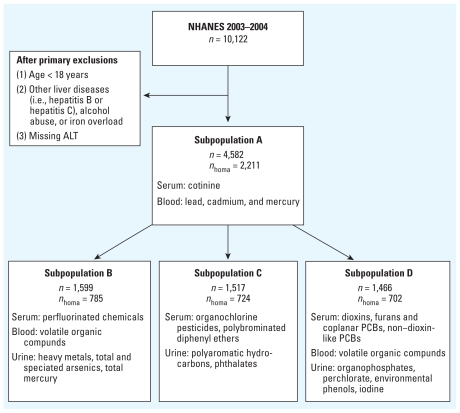
For our study, we used the following exclusion criteria: age < 18 years, positive serum hepatitis B surface antigen, positive serum hepatitis C antibody, elevated transferrin saturation (> 60% for men and > 50% for women), and alcohol consumption ≥ 20 g/day for men and ≥ 10 g/day for women. Consistent with prior studies, these exclusions were used to identify suspected cases of NAFLD based on ALT elevation in the absence of other identifiable causes of liver disease (Clark et al. 2003). A total of 10,122 subjects were evaluated for eligibility, but after applying these exclusion criteria and eliminating another 643 subjects with missing ALT values, the final maximum sample size was 4,582 (Figure 1).
Figure 1.
Schematic diagram depicting adult NHANES 2003–2004 subjects and pollutant subclasses analyzed. nhoma refers to the subset of subjects with HOMA-IR scores, which were required for data adjustment, and represents the maximum number of subjects available for each subpopulation for analysis by the primary model that adjusted for insulin resistance. The actual numbers used varied by analyte primarily because of missing response variables required for the other adjustments. The final numbers used for each analyte or chemical subclass are given in Tables 2–5 and in the Supplemental Material, Tables 2–5 (doi:10.1289/ehp.1002720). Volatile organic compounds were measured in selected subjects from subpopulations B and D.
Pollutants
All pollutant data posted by the NCHS before December 2008 were accessed and downloaded, which yielded 196 pollutants from 17 subclasses as categorized by the NCHS: serum perfluorinated compounds; urinary heavy metals; urinary total arsenic and speciated arsenics; urinary total (elemental plus inorganic) mercury; serum organochlorine pesticides; serum polybrominated diphenyl ethers (PBDEs); urinary polyaromatic hydrocarbons; urinary phthalates; serum polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and coplanar polychlorinated biphenyls (PCBs); serum non–dioxin-like PCBs; urinary organophosphate insecticides; urinary perchlorate; urinary environmental phenols; urinary iodine; blood lead, mercury (total and inorganic), and cadmium; serum cotinine; and blood volatile organic compounds [for the full list of chemicals in each subclass, see Supplemental Material, Table 1 (doi:10.1289/ehp.1002720)]. An additional subclass, coplanar PCBs, was constructed by selecting only these chemicals from the broader “PCDDs, PCDFs, and coplanar PCBs” subclass. A second subclass for total PCBs was then created by combining the non–dioxin-like PCBs and coplanar PCBs subclasses.
All ALT and pollutant levels were measured in biologic samples collected on the same day from each individual participant. We evaluated only pollutants with a ≥ 60% detection rate [111 of 196 pollutants; see Supplemental Material, Table 1 (doi:10.1289/ehp.1002720)] to avoid bias in estimation for those pollutants with levels < the lower limit of detection (Lee et al. 2007a, 2007b). Concentrations of organic pollutants measured in serum (non–dioxin-like PCBs; dioxins, furans, coplanar PCBs; PBDEs; organochlorine pesticides) were lipid adjusted, and concentrations of pollutants measured in urine were adjusted for creatinine [Supplemental Material, Table 1 (doi:10.1289/ehp.1002720)] (Schwartz et al. 2003).
Outcome variables and statistical methods
Serum ALT activity was measured by Collaborative Laboratory Services, LLC (Ottumwa, IA) for NHANES using the Beckman Synchron LX20 (Beckman Coulter, Brea, CA). Elevated ALT was defined as any value greater than this laboratory’s reference range, which was established from data obtained from healthy wellness participants as described in the NHANES Laboratory Procedure Manual (NCHS 2003–2004). Specifically, we used nonparametric techniques to estimate lower and upper bounds of reference ranges (2.5th and 97.5th percentiles) according to age and sex (Reed et al. 1971). ALT above the reference range was classified as elevated (men 18–20 years of age, ALT ≥ 37 IU/L; men ≥ 21 years old, ALT ≥ 48 IU/L; women 18–20 years of age, ALT ≥ 30 IU/L; women ≥ 21 years of age, ALT ≥ 31 IU/L). We determined the prevalence of ALT elevation in 4,582 subjects, and we used the chi-square test to determine statistically significant differences (p < 0.05) in ALT elevation and pollutant exposures according to sex, age, race/ethnicity, and body mass index (BMI).
Pollutant concentrations were classified according to a common scale that could be aggregated to assess cumulative exposures to multiple pollutants within a subclass. Specifically, we ranked each participant according to their measured concentration of each pollutant and summed the ranks of each one within a given subclass to determine their combined exposure (Lee et al. 2007a, 2007b). Ties were handled by assigning the minimum of the corresponding ranks to each participant, and participants with levels < the lower limit of detection (LLOD) for a pollutant were assigned the LLOD and ranked accordingly. For each pollutant subclass, subjects were stratified into quartiles by their cumulative exposure rank, with the first quartile representing subjects with the lowest levels. We estimated multivariate-adjusted odds ratios (ORs) for unexplained ALT elevation using logistic regression models with the first quartile as the reference group. Models were adjusted for age, race/ethnicity, and poverty income ratio (PIR). We also adjusted for both BMI and homeostasis model assessment of insulin resistance (HOMA-IR), because multiple pollutants have previously been associated with obesity and insulin resistance in NHANES (Lee et al. 2007a, 2007b). However, fasting glucose and insulin were measured in only a subset of NHANES participants, so only 2,211 subjects could be evaluated in this fashion. Further, although lead, cadmium, and mercury measurements were available for all 2,211 of these observations, other pollutant subclasses were measured only in subsets of this sample (perfluorinated chemicals, 785 subjects; organochlorine pesticides and polybrominated diethyl ethers, 724 subjects; dioxins, furans, coplanar PCBs, and non–dioxin-like PCBs, 702 subjects; see Figure 1).
Associations with individual chemicals were estimated if trend tests for the association between the entire subclass and elevated ALT were statistically significant. Subjects with detectable levels of individual pollutants were ranked, placed into quartiles, and compared with a reference group consisting of individuals with levels < LLOD or individuals in the first quartile of exposure (if none of the subjects had levels < LLOD, or if none of the subjects with levels < LLOD had elevated ALT). In the latter situation, subjects with levels < LLOD were still used in calculating the trend statistic for association of exposure level with elevated ALT.
We performed all statistical analyses using SURVEYFREQ and SURVEYLOGISTIC in SAS (version 9.1; SAS Institute Inc., Cary, NC). Estimates were adjusted for age, race/ethnicity, and PIR rather than using sample weights, which is regarded as a good compromise between efficiency and bias (Graubard and Korn 1999; Korn and Graubard 1991). Trend tests for the association between pollutants (both individual and cumulative for a subclass) were performed by modeling ordinal variables with integer scores assigned to each quartile and to the group of individuals with levels < LLOD, when appropriate [see Supplemental Material, Statistical Methods (doi:10.1289/ehp.1002720)]. p-Values were determined, both with (adjusted ptrend) and without (ptrend), for multiple comparisons using the false discovery rate method of Benjamini and Hochberg (1995), and both p-values are reported because adjustment for multiple comparisons has not consistently been performed in the other studies (Lee et al. 2007a, 2007b). Adjustments for multiple comparisons were done separately for subclass analyses and for analyses of individual pollutants within subclasses. We used a p-value ≤ 0.05 to indicate statistical significance.
Results
Demographic information
The full study sample included slightly more women than men (Table 1). The mean age (and corresponding SD) was 47.2 ± 21.2 years (range, 18–85 years). Non-Hispanic whites accounted for 72.3% of the population. Body weights, as defined by National Institutes of Health (1998) guidelines, were fairly evenly distributed between normal weight, overweight, and obese, with very few subjects being underweight (1.7%).
Table 1.
Prevalence of unexplained ALT elevation (suspected NAFLD) and association with elevated total PCB levels in adult NHANES 2003–2004, by demographic groups.
| Demographic variable | Population distribution (%) | Prevalence of unexplained ALT elevation |
PCBs > 75th percentilea |
||||
|---|---|---|---|---|---|---|---|
| Percent | SE | p-Value | Percent | SE | p-Value | ||
| Sex | 0.020 | 0.182 | |||||
| Male | 47.8 | 9.2 | 0.8 | 18.5 | 2.1 | ||
| Female | 52.2 | 11.9 | 0.5 | 22.8 | 1.8 | ||
| Race/ethnicity | < 0.001 | 0.002 | |||||
| Non-Hispanic White | 72.3 | 10.0 | 0.5 | 21.7 | 1.4 | ||
| Non-Hispanic Black | 10.8 | 5.6 | 0.8 | 29.2 | 2.9 | ||
| Hispanic | 11.7 | 18.6 | 1.5 | 8.2 | 2.2 | ||
| Other | 5.1 | 10.9 | 2.9 | 19.3 | 8.3 | ||
| Age (years) | 0.005 | < 0.001 | |||||
| < 30 | 21.5 | 9.1 | 1.2 | 2.2 | 1.2 | ||
| 30–40 | 19.5 | 11.8 | 1.3 | 4.2 | 1.5 | ||
| 40–50 | 20.4 | 13.2 | 1.4 | 12.0 | 2.1 | ||
| 50–60 | 16.0 | 13.0 | 1.2 | 24.6 | 5.1 | ||
| 60–70 | 10.5 | 10.6 | 2.0 | 45.0 | 5.2 | ||
| ≥ 70 | 12.1 | 3.7 | 0.9 | 71.7 | 4.4 | ||
| BMI (kg/m2) | < 0.001 | 0.525 | |||||
| < 18.5 | 1.7 | 7.1 | 3.1 | 17.7 | 9.9 | ||
| 18.5–24.9 | 31.5 | 5.1 | 0.8 | 17.6 | 1.9 | ||
| 25–29.9 | 34.3 | 10.7 | 1.3 | 23.1 | 2.7 | ||
| ≥ 30 | 32.5 | 15.7 | 1.0 | 21.6 | 2.4 | ||
SE, standard error of the mean. p-Values were calculated by chi-square tests.
Percentage of subjects within a demographic group having lipid-adjusted serum total PCB levels in the highest exposure quartile.
Prevalence of unexplained ALT elevation and PCB exposure
Of the 4,582 adult subjects remaining after applying the exclusion criteria, 436 had unexplained ALT elevation (i.e., suspected NAFLD), which corresponds to 10.6% of the U.S. adult population or 19.4 million people (after accounting for NHANES sampling weights). ALT elevation was more common in women than in men (11.9% vs. 9.2%; p = 0.020) (Table 1). ALT elevation was more common in Hispanics than in non-Hispanic whites (18.6% vs. 10.0%), whereas non-Hispanic blacks had a lower prevalence of ALT elevation (5.6%; p = 0.001). ALT elevation was most prevalent during the fifth and sixth decades and was more prevalent in overweight and obese participants than in normal-weight participants (10.7%, 15.7%, and 5.1%, respectively; p = 0.001).
Older age and non-Hispanic black race, but not BMI or sex, were significantly associated with total PCB levels in the highest quartile (Table 1). Age had the most pronounced association: 71.7% of participants age ≥ 70 years had PCB levels in the highest quartile, compared with only 2.2% of subjects < 30 years of age (p < 0.001). Non-Hispanic blacks (29.2%) were more likely to be in the highest quartile of total cumulative PCB exposure than were non-Hispanic whites (21.7%) and Hispanics (8.2%; p = 0.002).
Pollutant subclass results
We estimated significant positive trends for adjusted ORs for 3 of the 17 NHANES pollutant subclasses investigated (Table 2). Specifically, the adjusted ORs and 95% confidence intervals (CIs) for the highest versus lowest quartiles of exposure were for serum dioxins, furans, and coplanar PCBs, 5.8 (95% CI, 1.1–30.2; ptrend = 0.024); for serum non–dioxin-like PCBs, 4.5 (95% CI, 2.0–10.0; ptrend < 0.001); and for blood lead, mercury, and cadmium, 1.6 (95% CI, 1.1–2.3; ptrend = 0.015). After adjusting for multiple comparisons, the trend test for non–dioxin-like PCBs remained statistically significant (ptrend-adj = 0.001). In general, results were comparable when estimated without adjustment for BMI or HOMA-IR [see Supplemental Material, Tables 3 and 4, respectively (doi:10.1289/ehp.1002720)], although trend tests for associations with creatinine-adjusted urine polyaromatic hydrocarbons and serum lipid-adjusted PBDEs indicated significant positive and negative trends in associations with ALT based on models without adjustment for HOMA-IR.
Table 2.
Adjusted ORsa (95% CIs) for ALT elevation by exposure quartile for pollutant subclasses in adults, NHANES 2003–2004.
| Pollutant subclass | n | Pollutants screened/analyzedb | Quartile [cases/total, OR (95% CI)] |
p-Value |
||||
|---|---|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Trend | Adjusted trendc | |||
| Coplanar PCBs (serum) | 535 | 10/9 | 12/133 | 12/137 | 18/141 | 15/129 | < 0.001 | < 0.001 |
| Referent | 2.2 (0.9–5.2) | 4.4 (1.8–10.5) | 7.6 (2.8–20.7) | |||||
| Non–dioxin-like PCBs (serum) | 532 | 26/25 | 18/125 | 9/145 | 15/131 | 17/131 | < 0.001 | 0.001 |
| Referent | 0.8 (0.3–2.0) | 2.4 (1.1–5.2) | 4.5 (2.0–10.0) | |||||
| Total PCBs (serum) | 535 | 36/34 | 18/119 | 8/129 | 15/138 | 15/119 | 0.002 | 0.010 |
| Referent | 0.8 (0.3–2.3) | 2.2 (0.9–5.3) | 4.3 (1.8–10.1) | |||||
| Dioxins, furans, coplanar PCBs (serum) | 535 | 29/17 | 13/138 | 12/136 | 17/135 | 13/126 | 0.024 | 0.137 |
| Referent | 1.7 (0.7–4.4) | 3.6 (1.2–10.8) | 5.8 (1.1–30.2) | |||||
| Lead, cadmium, mercury (blood) | 2,051 | 4/3 | 57/520 | 45/505 | 49/518 | 54/508 | 0.015 | 0.126 |
| Referent | 0.9 (0.6–1.4) | 1.1 (0.8–1.7) | 1.6 (1.1–2.3) | |||||
| Environmental phenols (urine) | 643 | 4/3 | 19/145 | 22/162 | 11/165 | 17/171 | 0.333 | 0.811 |
| Referent | 1.2 (0.5–2.7) | 0.5 (0.2–1.3) | 0.9 (0.4–2.1) | |||||
| Polyaromatic hydrocarbons (urine) | 563 | 21/10 | 14/147 | 10/139 | 15/142 | 17/135 | 0.335 | 0.811 |
| Referent | 0.8 (0.3–2.6) | 1.0 (0.4–2.7) | 1.6 (0.6–4.6) | |||||
| PBDEs (serum) | 614 | 11/6 | 20/169 | 19/149 | 18/149 | 13/147 | 0.354 | 0.811 |
| Referent | 1.0 (0.5–1.9) | 0.9 (0.4–1.9) | 0.7 (0.3–1.5) | |||||
| Volatile organic compounds (blood) | 433 | 33/6 | 10/108 | 16/97 | 13/111 | 14/117 | 0.427 | 0.811 |
| Referent | 2.0 (0.8–4.7) | 1.3 (0.6–2.9) | 1.6 (0.8–3.4) | |||||
| Perfluorinated compounds (serum) | 694 | 12/4 | 18/189 | 13/169 | 12/167 | 15/169 | 0.460 | 0.811 |
| Referent | 0.8 (0.3–2.1) | 1.0 (0.3–3.0) | 1.6 (0.5–5.1) | |||||
| Perchlorate (urine) | 638 | 1/1 | 13/161 | 17/163 | 21/166 | 18/148 | 0.533 | 0.811 |
| Referent | 1.2 (0.6–2.3) | 1.4 (0.7–2.8) | 1.2 (0.5–2.9) | |||||
| Phthalates (urine) | 655 | 13/9 | 23/177 | 17/158 | 13/150 | 19/170 | 0.553 | 0.811 |
| Referent | 0.9 (0.3–2.3) | 0.6 (0.3–1.6) | 0.9 (0.5–1.6) | |||||
| Organophosphate insecticides (urine) | 631 | 6/4 | 26/159 | 10/150 | 13/157 | 18/144 | 0.561 | 0.811 |
| Referent | 0.4 (0.2–0.7) | 0.5 (0.2–1.0) | 0.8 (0.5–1.3) | |||||
| Iodine (urine) | 645 | 1/1 | 18/158 | 16/160 | 18/180 | 16/147 | 0.572 | 0.811 |
| Referent | 0.7 (0.4–1.2) | 0.7 (0.4–1.2) | 0.8 (0.4–1.5) | |||||
| Total and speciated arsenics (urine) | 710 | 8/3 | 15/188 | 14/187 | 17/170 | 13/165 | 0.725 | 0.901 |
| Referent | 0.8 (0.3–2.0) | 1.3 (0.7–2.3) | 0.9 (0.5–1.9) | |||||
| Cotinine (serum) | 2,050 | 1/1 | 54/527 | 49/515 | 58/504 | 43/504 | 0.783 | 0.901 |
| Referent | 0.9 (0.6–1.4) | 1.3 (0.9–1.9) | 1.0 (0.6–1.7) | |||||
| Heavy metals (urine) | 709 | 12/9 | 15/184 | 17/197 | 11/179 | 16/149 | 0.807 | 0.901 |
| Referent | 0.9 (0.4–1.9) | 0.5 (0.2–1.2) | 1.1 (0.5–2.3) | |||||
| Organochlorine pesticides (serum) | 587 | 13/8 | 15/136 | 15/140 | 21/155 | 12/156 | 0.848 | 0.901 |
| Referent | 0.9 (0.5–1.6) | 1.5 (0.5–4.2) | 0.9 (0.2–3.7) | |||||
| Total mercury (urine) | 707 | 1/1 | 11/180 | 20/167 | 11/169 | 16/191 | 0.918 | 0.918 |
| Referent | 2.3 (0.8–6.8) | 0.9 (0.3–2.8) | 1.5 (0.5–4.7) | |||||
Detectable values of each pollutant were individually ranked, and the rank orders of the individual pollutants in each subclass were summed to arrive at the subclass value. All nondetectable values were ranked as one. The summary values were categorized by cutoff points of 25th, 50th, and 75th values of the sum of ranks.
ORs were adjusted for age, sex, race, PIR, HOMA-IR, and BMI.
In any given pollutant subclass, only chemicals with at least a 60% detection rate were included in the analysis.
Additionally adjusted for multiple comparisons.
Significant positive trends were also evident for associations with coplanar PCBs and total PCBs (Table 2). The highest quartile of cumulative coplanar PCB exposure, compared with the lowest quartile, was associated with a significantly increased adjusted OR for ALT elevation of 7.6 (95% CI, 2.8–20.7; ptrend-adj < 0.001). Likewise, the adjusted OR for the highest versus lowest quartile of exposure to cumulative total PCB subclass (coplanar PCBs plus non–dioxin like PCBs) was 4.3 (95% CI, 1.8–10.1; ptrend-adj = 0.01).
Individual pollutant results
We also estimated associations with 45 individual pollutants from subclasses with significant trend tests. Blood lead (99.6%) and total mercury (92.5%) had extremely high detection rates and were positively associated with ALT elevation, but cadmium exposure was not associated with ALT elevation (Table 3).
Table 3.
Adjusted ORsa (95% CIs) for ALT elevation by exposure quartile for lead, cadmium, and mercury in adults, NHANES 2003–2004.
| Pollutant | Detection rate (%) | Not detectable (cases/total) | Detectable [median concentration, cases/total, OR (95% CI)] |
p-Value |
||||
|---|---|---|---|---|---|---|---|---|
| First quartile | Second quartile | Third quartile | Fourth quartile | Trend | Adjusted trendb | |||
| Lead (μg/dL) | 99.6 | 0.80 | 1.30 | 1.90 | 3.30 | 0.006 | 0.014 | |
| 0/6 | 55/579 | 48/494 | 53/498 | 49/474 | ||||
| Referent | 1.2 (0.9–1.7) | 1.5 (1.0–2.1) | 1.6 (1.1–2.3) | |||||
| Mercury, total (μg/L) | 92.5 | 0.40 | 0.80 | 1.40 | 3.10 | 0.010 | 0.014 | |
| 12/158 | 40/500 | 64/540 | 50/395 | 39/458 | ||||
| Referent | 1.1 (0.7–1.8) | 2.0 (1.3–3.2) | 2.2 (1.4–3.5) | 1.6 (1.1–2.4) | ||||
| Cadmium (μg/L) | 82.8 | 0.30 | 0.40 | 0.60 | 1.10 | 0.503 | 0.503 | |
| 38/345 | 73/672 | 21/257 | 38/406 | 35/371 | ||||
| Referent | 1.1 (0.7–1.7) | 0.9 (0.6–1.4) | 1.2 (0.8–1.7) | 1.2 (0.7–2.0) | ||||
ORs were adjusted for age, sex, race, PIR, HOMA-IR, and BMI.
Additionally adjusted for multiple comparisons.
Ten coplanar PCBs were present at detection rates ranging from 68% to 100%, and nine of these were positively associated with elevated ALT (Table 4). However, none of the four PCDDs or three PCDFs was associated with ALT elevation [see Supplemental Material, Table 5 (doi:10.1289/ehp.1002720)]. Twenty-five non–dioxin-like PCBs were present, with detection rates ranging from 65.5% to 100%, and 11 of these were positively associated with ALT elevation with significant trends (Table 5).
Table 4.
Adjusted ORsa (95% CIs) for ALT elevation by exposure quartile for coplanar PCBs in adults, NHANES 2003–2004.
| Pollutant (lipid adjusted) | Detection rate (%) | Not detectable (cases/total) | Detectable [median concentration, cases/total, OR (95% CI)] |
p-Value |
||||
|---|---|---|---|---|---|---|---|---|
| First quartile | Second quartile | Third quartile | Fourth quartile | Trend | Adjusted trendb | |||
| PCB-28 (ng/g) | 100.0 | 2.75 | 4.28 | 5.75 | 8.77 | 0.740 | 0.839 | |
| 17/140 | 12/151 | 14/141 | 18/139 | |||||
| Referent | 0.5 (0.2–1.4) | 0.8 (0.4–1.6) | 1.0 (0.4–2.3) | |||||
| PCB-66 (ng/g) | 98.9 | 0.74 | 1.18 | 1.67 | 3.00 | 0.003 | 0.011 | |
| 0/8 | 12/135 | 8/150 | 25/154 | 18/134 | ||||
| Referent | 0.6 (0.2–1.5) | 2.9 (1.6–5.4) | 1.9 (0.9–4.0) | |||||
| PCB-74 (ng/g) | 100.0 | 1.76 | 3.32 | 6.82 | 17.88 | < 0.001 | 0.004 | |
| 14/142 | 15/150 | 19/157 | 15/132 | |||||
| Referent | 2.2 (1.0–5.1) | 3.0 (1.5–6.0) | 6.0 (2.4–14.9) | |||||
| PCB-105 (ng/g) | 98.0 | 0.52 | 0.93 | 1.51 | 4.46 | 0.015 | 0.031 | |
| 0/11 | 13/142 | 12/150 | 21/144 | 17/131 | ||||
| Referent | 1.2 (0.5–3.0) | 2.8 (1.2–6.5) | 3.4 (1.1–10.9) | |||||
| PCB-118 (ng/g) | 100.0 | 2.30 | 4.26 | 8.16 | 22.80 | 0.006 | 0.016 | |
| 12/142 | 14/158 | 22/144 | 15/135 | |||||
| Referent | 1.8 (0.7–4.8) | 3.8 (1.3–11.1) | 4.4 (1.4–13.7) | |||||
| PCB-126 (pg/g) | 94.8 | 8.70 | 13.80 | 22.00 | 50.50 | < 0.001 | < 0.001 | |
| 0/33 | 9/134 | 12/127 | 22/146 | 18/123 | ||||
| Referent | 1.5 (0.6–3.5) | 3.3 (1.5–7.2) | 4.3 (2.0–9.4) | |||||
| PCB-156 (ng/g) | 91.7 | 0.90 | 2.74 | 6.11 | 12.40 | < 0.001 | 0.004 | |
| 4/49 | 15/130 | 14/131 | 16/139 | 14/130 | ||||
| Referent | 1.8 (0.6–5.5) | 3.4 (1.3–8.7) | 5.0 (1.5–17.2) | 9.4 (2.5–36.2) | ||||
| PCB-157 (ng/g) | 74.9 | 0.41 | 1.00 | 1.79 | 3.30 | 0.006 | 0.016 | |
| 15/152 | 11/111 | 17/105 | 8/100 | 11/105 | ||||
| Referent | 1.5 (0.8–2.8) | 4.1 (1.8–9.2) | 2.1 (0.6–7.6) | 7.1 (2.2–22.4) | ||||
| PCB-167 (ng/g) | 68.0 | 0.50 | 1.10 | 1.93 | 3.81 | 0.003 | 0.011 | |
| 19/188 | 9/103 | 12/104 | 11/90 | 11/90 | ||||
| Referent | 0.9 (0.4–2.2) | 2.2 (1.0–4.8) | 2.7 (1.0–7.0) | 5.0 (1.9–13.3) | ||||
| PCB-169 (pg/g) | 70.3 | 7.50 | 13.50 | 22.40 | 39.65 | 0.032 | 0.061 | |
| 14/164 | 15/107 | 11/94 | 17/111 | 4/89 | ||||
| Referent | 2.4 (0.9–7.0) | 3.5 (1.3–9.7) | 5.0 (1.8–14.0) | 2.4 (0.4–12.8) | ||||
Coplanar PCBs were measured in serum and are reported as lipid-adjusted values.
ORs were adjusted for age, sex, race, PIR, HOMA-IR, and BMI.
Additionally adjusted for multiple comparisons.
Table 5.
Adjusted ORsa (95% CIs) for ALT elevation by exposure quartile for non–dioxin-like PCBs in adults, NHANES 2003–2004.
| Pollutant (lipid adjusted) | Detection rate (%) | Not detectable (cases/total) | Detectable [median concentration, cases/total, OR (95% CI)] |
p-Value |
||||
|---|---|---|---|---|---|---|---|---|
| First quartile | Second quartile | Third quartile | Fourth quartile | Trend | Adjusted trendb | |||
| PCB-44 (ng/g) | 99.9 | 1.00 | 1.70 | 2.49 | 4.00 | 0.587 | 0.734 | |
| 17/146 | 9/133 | 21/162 | 16/136 | |||||
| Referent | 0.6 (0.2–2.1) | 1.2 (0.7–2.0) | 1.0 (0.4–2.7) | |||||
| PCB-49 (ng/g) | 99.4 | 0.63 | 1.10 | 1.60 | 2.55 | 0.779 | 0.928 | |
| 1/3 | 17/146 | 10/131 | 17/155 | 18/139 | ||||
| Referent | 0.2 (0.0–3.2) | 0.1 (0.0–2.1) | 0.2 (0.0–2.4) | 0.2 (0.0–4.0) | ||||
| PCB-52 (ng/g) | 100.0 | 1.27 | 2.17 | 3.40 | 5.40 | 0.571 | 0.734 | |
| 18/150 | 11/128 | 11/159 | 23/143 | |||||
| Referent | 0.7 (0.3–1.8) | 0.5 (0.2–1.0) | 1.5 (0.6–3.8) | |||||
| PCB-87 (ng/g) | 83.5 | 0.57 | 0.90 | 1.20 | 2.01 | 0.354 | 0.520 | |
| 11/95 | 16/120 | 5/118 | 13/119 | 18/128 | ||||
| Referent | 0.9 (0.5–1.7) | 0.3 (0.1–0.7) | 0.9 (0.5–1.7) | 1.3 (0.6–2.7) | ||||
| PCB-99 (ng/g) | 100.0 | 1.73 | 3.15 | 5.40 | 12.90 | 0.149 | 0.286 | |
| 16/139 | 13/156 | 19/146 | 15/135 | |||||
| Referent | 1.1 (0.5–2.6) | 2.1 (0.6–7.9) | 2.4 (0.7–8.9) | |||||
| PCB-101 (ng/g) | 96.6 | 0.76 | 1.42 | 2.20 | 4.00 | 0.210 | 0.336 | |
| 2/18 | 14/148 | 12/127 | 12/144 | 23/144 | ||||
| Referent | 0.3 (0.1–1.8) | 0.3 (0.1–1.5) | 0.4 (0.1–2.1) | 0.7 (0.1–3.9) | ||||
| PCB-110 (ng/g) | 98.4 | 0.51 | 1.00 | 1.59 | 3.03 | 0.427 | 0.593 | |
| 1/7 | 17/152 | 12/138 | 12/146 | 21/136 | ||||
| Referent | 0.5 (0.1–5.4) | 0.4 (0.0–4.4) | 0.4 (0.0–4.4) | 0.8 (0.1–6.9) | ||||
| PCB-138 and PCB-158 (ng/g) | 100.0 | 4.58 | 11.07 | 25.10 | 57.98 | 0.001 | 0.009 | |
| 15/148 | 14/140 | 15/159 | 19/133 | |||||
| Referent | 1.9 (0.8–4.1) | 2.5 (1.0–6.0) | 6.7 (2.1–21.5) | |||||
| PCB-146 (ng/g) | 99.2 | 0.61 | 1.70 | 4.00 | 8.90 | 0.004 | 0.019 | |
| 0/7 | 14/140 | 18/148 | 14/147 | 17/139 | ||||
| Referent | 2.2 (1.0–4.5) | 2.7 (1.1–6.9) | 6.8 (1.8–25.5) | |||||
| PCB-149 (ng/g) | 95.8 | 0.31 | 0.52 | 0.79 | 1.33 | 0.215 | 0.336 | |
| 4/19 | 13/135 | 12/133 | 11/160 | 21/129 | ||||
| Referent | 0.2 (0.1–0.7) | 0.3 (0.1–0.9) | 0.2 (0.0–0.8) | 0.6 (0.2–2.0) | ||||
| PCB-151 (ng/g) | 80.2 | 0.19 | 0.30 | 0.41 | 0.79 | 0.030 | 0.068 | |
| 11/115 | 14/113 | 8/112 | 10/116 | 19/121 | ||||
| Referent | 1.1 (0.3–3.6) | 0.8 (0.3–2.1) | 1.0 (0.4–2.3) | 2.6 (1.2–5.8) | ||||
| PCB-153 (ng/g) | 100.0 | 5.59 | 15.18 | 35.01 | 75.55 | 0.006 | 0.023 | |
| 17/145 | 13/147 | 14/153 | 19/136 | |||||
| Referent | 1.5 (0.6–3.6) | 2.3 (0.7–7.4) | 7.2 (1.7–29.9) | |||||
| PCB-170 (ng/g) | 99.2 | 1.40 | 4.56 | 11.00 | 22.70 | 0.015 | 0.042 | |
| 0/3 | 15/147 | 18/141 | 17/151 | 13/137 | ||||
| Referent | 2.1 (1.0–4.3) | 3.1 (1.1–8.7) | 4.4 (1.3–14.4) | |||||
| PCB-172 (ng/g) | 77.1 | 0.42 | 1.10 | 1.99 | 3.73 | 0.007 | 0.023 | |
| 13/131 | 15/120 | 12/107 | 13/119 | 10/100 | ||||
| Referent | 1.4 (0.7–3.1) | 2.1 (0.8–5.4) | 2.7 (0.9–8.1) | 3.4 (1.2–9.7) | ||||
| PCB-177 (ng/g) | 89.3 | 0.53 | 1.30 | 2.70 | 6.00 | < 0.001 | < 0.001 | |
| 7/60 | 9/123 | 16/144 | 15/124 | 16/128 | ||||
| Referent | 0.7 (0.4–1.3) | 2.0 (1.0–3.9) | 4.2 (1.7–10.4) | 6.5 (2.8–15.3) | ||||
| PCB-178 (ng/g) | 85.9 | 0.40 | 1.20 | 2.44 | 4.86 | 0.014 | 0.042 | |
| 7/70 | 16/130 | 13/133 | 17/127 | 10/118 | ||||
| Referent | 1.7 (0.8–3.9) | 2.1 (1.0–4.6) | 4.6 (1.4–15.3) | 4.8 (1.3–17.4) | ||||
| PCB-180 (ng/g) | 99.8 | 3.40 | 11.90 | 29.40 | 66.51 | 0.206 | 0.336 | |
| 16/145 | 17/147 | 19/151 | 11/137 | |||||
| Referent | 1.5 (0.7–3.2) | 2.5 (0.9–7.3) | 2.4 (0.6–10.4) | |||||
| PCB-183 (ng/g) | 93.6 | 0.60 | 1.40 | 2.99 | 6.37 | 0.017 | 0.042 | |
| 2/33 | 16/131 | 17/150 | 11/133 | 17/132 | ||||
| Referent | 2.4 (0.4–15.4) | 4.0 (0.6–26.8) | 3.1 (0.4–23.2) | 7.8 (0.9–63.9) | ||||
| PCB-187 (ng/g) | 99.2 | 1.10 | 3.40 | 8.33 | 18.40 | < 0.001 | 0.002 | |
| 0/4 | 13/136 | 19/158 | 14/142 | 17/138 | ||||
| Referent | 2.8 (1.6–5.0) | 4.6 (1.6–13.3) | 10.5 (3.2–34.6) | |||||
| PCB-194 (ng/g) | 87.8 | 1.00 | 3.57 | 7.87 | 16.57 | 0.881 | 0.958 | |
| 9/68 | 17/125 | 13/123 | 15/137 | 9/114 | ||||
| Referent | 1.2 (0.6–2.5) | 1.0 (0.4–2.4) | 1.1 (0.4–2.7) | 1.2 (0.3–4.6) | ||||
| PCB-195 (ng/g) | 65.5 | 0.60 | 1.40 | 2.33 | 4.23 | 0.862 | 0.958 | |
| 26/198 | 9/97 | 13/93 | 6/79 | 9/93 | ||||
| Referent | 0.6 (0.2–1.6) | 1.3 (0.5–3.4) | 0.6 (0.2–1.8) | 1.2 (0.5–2.9) | ||||
| PCB-196 and PCB-203 (ng/g) | 93.6 | 0.80 | 2.60 | 5.92 | 12.50 | < 0.001 | 0.002 | |
| 2/38 | 18/133 | 13/139 | 16/138 | 14/130 | ||||
| Referent | 3.6 (0.9–13.8) | 4.1 (1.1–16.0) | 8.2 (1.7–39.3) | 14.7 (3.3–65.3) | ||||
| PCB-199 (ng/g) | 92.8 | 0.81 | 3.00 | 7.34 | 16.79 | 0.093 | 0.194 | |
| 4/40 | 19/127 | 12/149 | 17/129 | 11/128 | ||||
| Referent | 1.9 (0.9–4.2) | 1.2 (0.7–2.1) | 2.5 (1.2–5.3) | 3.3 (0.9–12.4) | ||||
| PCB-206 (ng/g) | 96.9 | 0.50 | 1.60 | 4.40 | 10.90 | 0.971 | 0.982 | |
| 2/15 | 20/148 | 13/141 | 20/140 | 8/131 | ||||
| Referent | 1.0 (0.4–2.6) | 0.8 (0.3–2.3) | 1.3 (0.6–3.2) | 0.9 (0.3–2.2) | ||||
| PCB-209 (ng/g) | 96.4 | 0.40 | 0.93 | 2.73 | 8.79 | 0.982 | 0.982 | |
| 0/18 | 23/147 | 15/142 | 14/134 | 10/131 | ||||
| Referent | 0.9 (0.4–2.0) | 0.8 (0.4–1.8) | 1.1 (0.3–3.9) | |||||
Non–dioxin-like PCBs were measured in serum and are reported as lipid-adjusted values.
ORs were adjusted for age, sex, race/ethnicity, PIR, HOMA-IR, and BMI.
Additionally adjusted for multiple comparisons.
Discussion
The prevalence of ALT elevation unexplained by viral hepatitis, hemochromatosis, or alcoholism (i.e., suspected NAFLD) was 10.6% in NHANES 2003–2004, which was nearly double the prevalence (5.4%) reported by a study of NHANES 1988–1994 adult participants that used similar exclusion criteria and a similar ALT reference range (Clark et al. 2003). As in our study, Clark et al. (2003) also noted that ALT elevation was associated with BMI, Hispanic ethnicity, and middle age. The observed increase in the prevalence of ALT elevation from NHANES 1988–1994 to NHANES 2003–2004 is consistent with the growing burden of obesity and NAFLD.
Because liver biopsy was not performed in NHANES, we used unexplained ALT elevation as a proxy measure of liver disease and NAFLD and identified several ubiquitous environmental pollutants that were dose-dependently associated with suspected NAFLD, including lead, mercury, and PCBs. Although levels of many pollutants are decreasing in the environment, PCB, lead, and mercury exposures remain problematic. For example, even though PCBs were banned in 1977, 100% of subjects in this study had detectable PCB levels.
Diet-induced obesity probably plays the primary role in the pathogenesis of most cases of NAFLD (Cave et al. 2007), but nutrient–toxicant interactions and genetic susceptibility to environmental pollution may be important cofactors, which we did not address in this study. Data from our group and others suggest that diet-induced obesity and fatty liver decrease antioxidant defenses and impair xenobiotic metabolism and disposition, which could sensitize the liver to chemical injury (Fisher et al. 2009a, 2009b; Kirpich et al. in press). Further complicating this issue, lead, mercury, and coplanar PCBs concentrate within the liver, whereas non–dioxin-like PCBs concentrate in adipose tissue and possibly in steatotic (fatty) livers [Klein et al. 1972; Mudipalli 2007; National Toxicology Program (NTP) 2006a]. Therefore, tissue levels may not always correlate with serum levels. However, it is important to recognize that multiple animal studies demonstrate that PCBs and methylmercury (MeHg) exposures induce fatty liver, even in the absence of diet-induced obesity (Chang and Yamaguchi 1974; Desnoyers and Chang 1975b; Lin et al. 1996; NTP 2006a, 2006b, 2006c). Although lead has been associated with hepatic hyperplasia and not NAFLD, to our knowledge lead and diet-induced obesity coexposures have not been performed in animal models (Mudipalli 2007). The results of these aforementioned studies lend biologic plausibility to the hypothesis that lead, mercury, and PCBs may play a previously unsuspected role in the pathogenesis of some cases of suspected NAFLD.
PCBs are polyhalogenated aromatic hydrocarbons that consist of up to 10 chlorine atoms attached to a biphenyl group. About 130 of the 209 theoretical PCB congeners were manufactured between 1929 and 1977 as mixtures and were sold as a function of chlorine content. For example, Monsanto marketed Aroclors 1221, 1231, and 1242 up to 1268, which contain, respectively, 21%, 31%, and 42% to 68% chlorine by weight. Aroclors were used in multiple industrial applications and were components in dielectric insulating fluids for transformers and capacitors, hydraulic fluids, plastics, and paints. An estimated 1.3 million tons of PCBs were manufactured almost exclusively (97%) in the northern hemisphere (Breivik et al. 2002). Although PCBs have been banned in the United States for > 30 years, their high thermodynamic stability makes them resistant to biodegradation and thus persistent organic pollutants. More highly chlorinated PCBs tend to be metabolized and eliminated more slowly, and the PCBs identified in biologic samples in this study were indeed the more highly chlorinated varieties.
From a mechanistic standpoint, a PCB’s structure determines its ability to interact with nuclear receptors. Like PCDDs and PCDFs, coplanar PCBs are aryl hydrocarbon receptor (AhR) agonists, and PCB-126 accounts for 52% of the toxic equivalency of dioxin-like PCBs in human tissues (NTP 2006b; Safe 1993). In comparison, some non–dioxin-like PCBs such as PCB-153 do not activate AhR but may be constitutive androstane receptor agonists (Dean et al. 2002). Animal studies demonstrate that non–dioxin-like PCBs such as PCB-153 are concentrated most heavily within the adipose tissue because of their high lipid solubility (NTP 2006b). Coplanar PCBs, such as PCB 126, despite high lipid solubility, paradoxically concentrate primarily within the liver (NTP 2006b). In our study, both types of PCBs, including PCB-126 and PCB-153, were dose-dependently associated with ALT elevation.
Extensive animal studies conducted by the NTP and others have defined a role for PCBs in liver disease. The NTP has performed 2-year toxicity studies on PCB-126 and PCB-153 in female Harlan Sprague-Dawley rats (NTP 2006a, 2006b, 2006c). These studies demonstrated that the liver was the principal target organ for these compounds. Both benign (toxic hepatopathy, including steatosis) and malignant (hepatocellular carcinoma and cholangiocarcinoma) liver lesions were observed at high frequencies in a dose-dependent fashion, particularly in animals treated with PCB-126 alone or combined with PCB-153. Importantly, both of these PCBs were associated with human ALT elevation in our study. Hennig et al. (2005) demonstrated that PCB-77 exacerbated high-fat-diet (corn oil)–induced hepatic steatosis in mice and increased hepatic gene expression of genes involved in apoptosis, inflammation, and oxidative stress. However, this particular coplanar PCB was not measured in NHANES 2003–2004. In contrast to animal studies, human data on PCBs in liver disease are lacking. However, in Taiwan, 13 years after the “Yucheng” incident where cooking oil was contaminated by PCBs, the mortality rate due to cirrhosis was 2.7 times higher than expected (Yu et al. 1997).
Whole-blood total mercury, present in 92.5% of subjects, but not urinary total (inorganic plus elemental) mercury, was dose-dependently associated with ALT elevation and suspected NAFLD. These results suggest that the organic form of mercury was associated with liver disease. MeHg is the principal form of organic mercury historically associated with organ toxicity. Since the 1950s outbreak of Minamata disease (MeHg intoxication) in a Japanese fishing village, MeHg has been recognized as one of the most hazardous environmental pollutants. Coal-fired power plants have been identified as the primary source of current mercury emissions, and atmospheric mercury may be converted into MeHg in water-body sediment and subsequently enter the aquatic food chain and bioaccumulate in fish (Charnley 2006). The primary route of human MeHg exposure is consumption of contaminated fish and shellfish, and PCB coexposure may occur (Charnley 2006). MeHg has well-characterized toxic effects on the human nervous system, developing fetus, and kidney (Charnley 2006).
Despite the fact that MeHg concentrates considerably within the liver because of enterohepatic recirculation, few animal studies have examined the potential role of MeHg in liver disease. However, acute and chronic toxicity studies conducted in rats and cats demonstrated that mercury exposure resulted in the depletion of body fat, the development of centrilobular hepatic steatosis, an increase in lipid peroxidation products, the proliferation of the endoplasmic reticulum, and floccular degeneration of the mitochondria with extrusion of diseased organelles into the sinusoidal space (Chang and Yamaguchi 1974; Desnoyers and Chang 1975a, 1975b; Klein et al. 1972; Lin et al. 1996). Many of these changes were irreversible after exposure to MeHg was discontinued. The primary mechanism of MeHg hepatotoxicity may be related to its high affinity for sulfhydryl residues and consequent poisoning of cysteine-containing proteins and glutathione depletion (Lin et al. 1996). Previous human epidemiological studies have inconsistently linked mercury contamination in Japanese fishing villages to increased liver-related mortality in villagers (Futatsuka et al. 1987, 1992, 2005).
With a detection rate of 99.6%, lead exposure was nearly universal in adult NHANES subjects. In contrast to PCBs and MeHg, lead hepatotoxicity is relatively well recognized and was recently reviewed (Mudipalli 2007). Lead exposure most commonly occurs through the respiratory or gastrointestinal system. Regardless of the route of exposure, the liver is the largest lead repository in the body (Mudipalli 2007). The pathologic liver lesion of lead exposure has been termed “lead-induced hepatic hyperplasia,” but hepatic steatosis has not been reported. Multiple molecular events have been described in association with lead-induced hepatic hyperplasia. Oxidative stress, proinflammatory cytokine production and sensitivity, and liver and serum cholesterol levels were all increased by lead (Aykin-Burns et al. 2003; Honchel et al. 1991; Kojima et al. 2004; Milosevic and Maier 2000; Sandhir and Gill 1995).
Several potential problems are inherent to the design of this study. The exact specificity of ALT for liver disease in NHANES is unknown because liver biopsies were not performed. However, ALT should be relatively specific, because the incidence of myopathy, the most important extrahepatic source of ALT, is likely low in the general population (Green and Flamm 2002). In contrast, at the reference range used in this study, the sensitivity of ALT is likely lower than its specificity. In fact, some authors have suggested using lower laboratory cutoffs to gain more sensitivity (Prati et al. 2002; Ruhl and Everhart 2009). Importantly, ALT may be normal in NAFLD, and this appears to be an even bigger problem in fatty liver and TASH due to some industrial chemicals (Brautbar and Williams 2002; Cave et al. 2010). Therefore, low-level environmental pollution may pose an even greater risk for liver disease in the general U.S. population than suggested by the results of this study. Lastly, the cross-sectional study design of NHANES cannot determine the direction of causation for the identified associations between environmental pollutant levels and elevated ALT. It is possible that these pollutant concentrations may be elevated because of the presence of liver disease or another predisposing factor for elevated ALT, rather than the risk of elevated ALT being increased because of elevated pollutant levels.
The pollutant subclassifications created by NHANES, although generally reasonable, may not always have the most biologic relevance. For example, heavy metals were grouped differently according to the method of measurement (blood or urine). Given the large number of measured pollutants, looking at all possible groupings of pollutants and mixtures of subgroups was not practical. However, we created new PCB subclasses for coplanar and total PCBs because these molecules were consistently associated with ALT elevation.
Regarding PCBs, NHANES reported levels for only a quarter of the 130 manufactured PCB congeners, so it must be acknowledged that this study did not actually model the effects of total lipid-adjusted serum PCB burden. However, because PCBs were sold in mixtures, it is likely that subjects high in the measured PCBs would also be high in the others. As with all other subclasses, members of the tetrachlorodibenzo-p-dioxin, PCDDs and coplanar PCB subclasses were ranked by serum concentration, which did not account for their toxic equivalency factors. This method allowed us to combine the coplanar PCB and non–dioxin-like PCB subclasses to form the total PCB subclass. However, AhR-dependent hepatotoxicities could be examined by alternate models. Also, although ranking individuals on the basis of exposure levels rather than modeling serum pollutant levels directly allowed us to compare results between individual pollutants and pollutant subclasses, this approach limits comparisons with other study populations.
Conclusion
PCBs, lead, and mercury are present in nearly all U.S. adults. These common pollutants are associated with significant dose-dependent increased ORs for ALT elevation in subjects whose ALT elevations were not explained by viral hepatitis, hemochromatosis, or alcohol abuse. These results suggest a possible association between low-level environmental pollution and the development of liver disease and suspected NAFLD. Future studies should be performed to confirm the potential role of these environmental pollutants in NAFLD.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002720 via http://dx.doi.org/).
We acknowledge the assistance of the Bioinformatics, Biostatistics, and Computational Biology Core of the University of Louisville Center for Environmental Genomics and Integrative Biology.
This research was supported in part by the National Institute of Environmental Health Sciences (P30ES014443-01A1), National Center for Research Resources (5P20RR024489-02), National Institute on Alcohol Abuse and Alcoholism (1P01AA017103-01, K23AA18399-01A, RC2AA019385), Department of Veterans Affairs, the Sheila Sherlock Clinical and Translational Research Award in Liver Diseases from the American Association for the Study of Liver Diseases, and the National Institutes of Health Loan Repayment Program.
References
- Aykin-Burns N, Laegeler A, Kellogg G, Ercal N. Oxidative effects of lead in young and adult Fisher 344 rats. Arch Environ Contam Toxicol. 2003;44((3)):417–420. doi: 10.1007/s00244-002-2023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57((1)):289–300. [Google Scholar]
- Brautbar N, Williams J., 2nd Industrial solvents and liver toxicity: risk assessment, risk factors, and mechanisms. Int J Hyg Environ Health. 2002;205((6)):479–491. doi: 10.1078/1438-4639-00175. [DOI] [PubMed] [Google Scholar]
- Breivik K, Sweetman A, Pacyna JM, Jones KC. Towards a global historical emission inventory for selected PCB congeners—a mass balance approach. 1. Global production and consumption. Sci Total Environ. 2002;290((1–3)):181–198. doi: 10.1016/s0048-9697(01)01075-0. [DOI] [PubMed] [Google Scholar]
- Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, et al. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18((3)):184–195. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R, et al. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010;51((2)):474–481. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Yamaguchi S. Ultrastructural changes of the liver after long-term diet of mercury-contaminated tuna. Environ Res. 1974;7((2)):133–148. [Google Scholar]
- Charnley G. Assessing and managing methylmercury risks associated with power plant mercury emissions in the United States. [[accessed 2 November 2010]];MedGenMed. 2006 8(1):64. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1681952/?tool=pubmed. [PMC free article] [PubMed] [Google Scholar]
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40((suppl 1)):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98((5)):960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- Cotrim HP, Andrade ZA, Parana R, Portugal M, Lyra LG, Freitas LA. Nonalcoholic steatohepatitis: a toxic liver disease in industrial workers. Liver. 1999;19((4)):299–304. doi: 10.1111/j.1478-3231.1999.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Cotrim HP, De Freitas LA, Freitas C, Braga L, Sousa R, Carvalho F, et al. Clinical and histopathological features of NASH in workers exposed to chemicals with or without associated metabolic conditions. Liver Int. 2004;24((2)):131–135. doi: 10.1111/j.1478-3231.2004.0897.x. [DOI] [PubMed] [Google Scholar]
- Dean CE, Jr, Benjamin SA, Chubb LS, Tessari JD, Keefe TJ. Nonadditive hepatic tumor promoting effects by a mixture of two structurally different polychlorinated biphenyls in female rat livers. Toxicol Sci. 2002;66((1)):54–61. doi: 10.1093/toxsci/66.1.54. [DOI] [PubMed] [Google Scholar]
- Desnoyers PA, Chang LW. Ultrastructural changes in rat hepatocytes following acute methyl mercury intoxication. Environ Res. 1975a;9((3)):224–239. doi: 10.1016/0013-9351(75)90003-1. [DOI] [PubMed] [Google Scholar]
- Desnoyers PA, Chang LW. Ultrastructural changes in the liver after chronic exposure to methylmercury. Environ Res. 1975b;10((1)):59–75. doi: 10.1016/0013-9351(75)90074-2. [DOI] [PubMed] [Google Scholar]
- Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103((9)):2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Oude Elferink RP, Besselsen DG, Erickson RP, et al. Experimental nonalcoholic fatty liver disease results in decreased hepatic uptake transporter expression and function in rats. Eur J Pharmacol. 2009a;613((1–3)):119–127. doi: 10.1016/j.ejphar.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009b;37((10)):2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsuka M, Kitano T, Nagano M, Inaoka T, Arimatsu Y, Ueno T, et al. An epidemiological study with risk analysis of liver diseases in the general population living in a methyl mercury polluted area. J Epidemiol Community Health. 1992;46((3)):237–240. doi: 10.1136/jech.46.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsuka M, Kitano T, Shono M, Nagano M, Wakamiya J, Miyamoto K, et al. Long-term follow-up study of health status in population living in methylmercury-polluted area. Environ Sci. 2005;12((5)):239–282. [PubMed] [Google Scholar]
- Futatsuka M, Shibata Y, Kinjo Y. Cause specific standard mortality ratio for Minamata disease patients. Kumanoto Med J. 1987;40:119–128. [Google Scholar]
- Graubard BI, Korn EL. Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst. 1999;91((12)):1005–1016. doi: 10.1093/jnci/91.12.1005. [DOI] [PubMed] [Google Scholar]
- Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123((4)):1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, et al. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect. 2005;113:83–87. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honchel R, Marsano L, Cohen D, Shedlofsky S, McClain CJ. Lead enhances lipopolysaccharide and tumor necrosis factor liver injury. J Lab Clin Med. 1991;117((3)):202–208. [PubMed] [Google Scholar]
- Kirpich I, Gobejishvili L, Bon Homme M, Waigel S, Cave M, Arteel G, et al. Integrated hepatic transcriptome and proteome analysis of mice with high-fat diet-induced nonalcoholic fatty liver disease. J Nutr Biochem. doi: 10.1016/j.jnutbio.2009.11.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Herman SP, Brubaker P, Lucier GW. A model of acute methyl mercury intoxication in rats. Arch Path. 1972;93((5)):408–418. [PubMed] [Google Scholar]
- Kojima M, Masui T, Nemoto K, Degawa M. Lead nitrate-induced development of hypercholesterolemia in rats: sterol-independent gene regulation of hepatic enzymes responsible for cholesterol homeostasis. Toxicol Lett. 2004;154((1–2)):35–44. doi: 10.1016/j.toxlet.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81((9)):1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007a;30((3)):622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007b;50((9)):1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S, Chalasani N. Relationship between unexplained elevations in alanine aminotransferase and serum leptin in U.S. adults: results from the Third National Health and Nutrition Examination Survey (NHANES III) J Clin Gastroenterol. 2004;38((10)):891–897. doi: 10.1097/00004836-200411000-00012. [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III) Am J Med Sci. 2005;329((3)):111–116. doi: 10.1097/00000441-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Lin TH, Huang YL, Huang SF. Lipid peroxidation in liver of rats administrated with methyl mercuric chloride. Biol Trace Elem Res. 1996;54((1)):33–41. doi: 10.1007/BF02785318. [DOI] [PubMed] [Google Scholar]
- Milosevic N, Maier P. Lead stimulates intercellular signalling between hepatocytes and Kupffer cells. Eur J Pharmacol. 2000;401((3)):317–328. doi: 10.1016/s0014-2999(00)00473-8. [DOI] [PubMed] [Google Scholar]
- Mudipalli A. Lead hepatotoxicity and potential health effects. Indian J Med Res. 2007;126((6)):518–527. [PubMed] [Google Scholar]
- National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6((suppl 2)):51S–209S. [PubMed] [Google Scholar]
- NCHS (National Center for Health Statistics) NHANES 2003–2004 Public General Release File Documentation. 2005. [[accessed 2 November 2010]]. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf.
- NCHS (National Center for Health Statistics) NHANES 2003–2004 Laboratory Procedure Manual: Alanine Amino Transferase (ALT) in Refrigerated Serum. 2003–2004. [[accessed 17 February 2010]]. Available: http://origin.cdc.gov/nchs/data/nhanes/nhanes_03_04/l40_c_met_alanine_amino_transferase.pdf.
- NTP (National Toxicology Program) NTP technical report on the toxicology and carcinogenesis studies of 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) (CAS no. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies) Natl Toxicol Program Tech Rep Ser. 2006a;(529):4–168. [PubMed] [Google Scholar]
- NTP (National Toxicology Program) NTP toxicology and carcinogenesis studies of a binary mixture of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) (CAS no. 57465-28-8) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153) (CAS no. 35065-27-1) in female Harlan Sprague-Dawley rats (gavage studies) Natl Toxicol Program Tech Rep Ser. 2006b;(530):1–258. [PubMed] [Google Scholar]
- NTP (National Toxicology Program) NTP toxicology and carcinogenesis studies of a binary mixture of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) (CAS no. 57465-28-8) and 2,3’,4,4’,5-pentachlorobiphenyl (PCB 118) (CAS no. 31508-00-6) in female Harlan Sprague-Dawley rats (gavage studies) Natl Toxicol Program Tech Rep Ser. 2006c;(531):1–218. [PubMed] [Google Scholar]
- Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137((1)):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 1971;17:275–284. [PubMed] [Google Scholar]
- Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136((2)):477–485. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- Safe SH. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ Health Perspect. 1993;100:259–268. doi: 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Gill KD. Effect of lead on lipid peroxidation in liver of rats. Biol Trace Elem Res. 1995;48((1)):91–97. doi: 10.1007/BF02789081. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26((2)):468–470. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- Yu ML, Guo YL, Hsu CC, Rogan WJ. Increased mortality from chronic liver disease and cirrhosis 13 years after the Taiwan “yucheng” (“oil disease”) incident. Am J Ind Med. 1997;31((2)):172–175. doi: 10.1002/(sici)1097-0274(199702)31:2<172::aid-ajim6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]