Abstract
Background
Paraoxonase 1 (PON1) detoxifies oxon derivatives of some organophosphate (OP) pesticides, and its genetic polymorphisms influence enzyme activity and quantity. We previously reported that maternal urinary concentrations of dialkyl phosphate (DAP) metabolites, a marker of OP pesticide exposure, were related to poorer mental development and maternally reported symptoms consistent with pervasive developmental disorder (PDD) in 2-year-olds participating in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study.
Objective
We determined whether PON1 genotypes and enzyme measurements were associated with child neurobehavioral development and whether PON1 modified the association of in utero exposure to OPs (as assessed by maternal DAPs) and neurobehavior.
Methods
We measured DAP concentrations in maternal urine during pregnancy, PON1192 and PON1−108 genotypes in mothers and children, and arylesterase (ARYase) and paraoxonase (POase) in maternal, cord, and 2-year-olds’ blood. We assessed 353 2-year-olds on the Mental Development Index (MDI) and Psychomotor Development Index (PDI) of the Bayley Scales of Infant Development and queried their mothers on the Child Behavior Checklist to obtain a score for PDD.
Results
Children with the PON1−108T allele had poorer MDI scores and somewhat poorer PDI scores. Children were less likely to display PDD when they or their mothers had higher ARYase activity and when their mothers had higher POase activity. The association between DAPs and MDI scores was strongest in children with PON1−108T allele, but this and other interactions between DAPs and PON1 polymorphisms or enzymes were not significant.
Conclusion
PON1 was associated with child neurobehavioral development, but additional research is needed to confirm whether it modifies the relation with in utero OP exposure.
Keywords: biomarkers, Child Behavior Checklist, children, DAPs, farmworker, genetic susceptibility, in utero exposure, mental development, Mexican Americans, neurodevelopment, organophosphates, paraoxonase, pervasive developmental disorder, pesticides, PON1
The paraoxonase 1 (PON1) enzyme detoxifies organophosphate (OP) pesticides, which are known neurotoxicants at high doses (Costa et al. 2005). PON1 also inhibits low-density lipoprotein oxidation, a marker of oxidative stress (Li et al. 2003). Several common polymorphisms in the coding (e.g., PON1192) and promoter (e.g., PON1−108) regions of the PON1 gene influence both the quantity and catalytic efficiency of the PON1 enzyme (Brophy et al. 2001; Li et al. 2000), measured against specific substrates, such as arylesterase (ARYase) and paraoxonase (POase) activity.
PON1 polymorphisms and/or enzyme measurements have been associated with various diseases of the nervous system, including Alzheimer’s disease (Erlich et al. 2006; Leduc and Poirier 2008; Paragh et al. 2002), brain tumors (Kafadar et al. 2006), vascular dementia (Paragh et al. 2002), amyotrophic lateral sclerosis (Saeed et al. 2006; Slowik et al. 2006), ischemic stroke (Voetsch et al. 2002, 2004), and Parkinson disease (Zintzaras and Hadjigeorgiou 2004). Gulf War syndrome, which some have hypothesized may be attributable to exposure to an OP agent (Golomb 2008), has also been associated with low PON1 ARYase (Haley et al. 1999) and POase (Mackness et al. 2000) measurements. PON1 polymorphisms or enzyme activity may also play a role in psychiatric disease such as schizophrenia (Kucukali et al. 2008), depression (Lawlor et al. 2007), and anxiety (Sklan et al. 2004). In addition, PON1192 genotype and PON1 enzyme measurements have been associated with childhood autism (Pasca et al. 2006, 2010), at least in certain populations (D’Amelio et al. 2005).
An individual’s susceptibility to the effects of specific OP pesticide exposure may be determined by their PON1 genotypes and expression (Li et al. 2000). PON1 enzyme measurements vary widely in humans (Cole et al. 2003; Costa et al. 2005; Holland et al. 2006), and measurements in fetuses and children up to at least 7 years of age are much lower than those in adults (Chen et al. 2003; Furlong et al. 2006; Huen et al. 2010), thus presenting a potential period of greater vulnerability to OP pesticide toxicity and oxidative stress. Transgenic newborn mice expressing PON1192Q showed greater inhibition of brain acetylcholinesterase after chlorpyrifos oxon exposure than did those with PON1192R (Cole et al. 2003). In a birth cohort study from New York, an association between abnormal neonatal reflexes and maternal dimethyl OP pesticide exposure (as measured by urinary metabolites) was found only in children with lower levels of POase expression, although an association between abnormal reflexes and urinary diethyl phosphate (DE) metabolites was observed regardless of POase expression (Engel et al. 2007).
In our longitudinal birth cohort, the Center for Health Assessment of Mothers and Children of Salinas (CHAMACOS) study, we previously reported high exposure to OP pesticides, as measured by urinary dialkyl phosphate (DAP) metabolite levels, among pregnant women living in the agricultural Salinas Valley of California, relative to levels in women of reproductive age (18–40 years) participating in the National Health and Nutrition Examination Survey (NHANES) (Bradman et al. 2005). We also observed associations between maternal DAP levels during pregnancy, particularly dimethyl phosphates (DMs; resulting from exposure to DM pesticides), and CHAMACOS 2-year-olds’ mental development as assessed on the Bayley Scales of Infant Development (Bayley 1993). Maternal report of symptoms of pervasive development disorder (PDD) in the clinical range on the Child Behavior Checklist (CBCL) were also associated with maternal DAP levels (Eskenazi et al. 2007).
In the present study, we estimated associations between neurodevelopmental outcomes and PON1 genotypes and with PON1 enzyme activity measured in children at birth and at 2 years of age and in mothers at delivery. We also examined whether PON1 modified the associations we previously observed between the mothers’ DAP concentrations during pregnancy and their children’s neurobehavioral development at 2 years of age (Eskenazi et al. 2007).
Materials and Methods
Study subjects
The CHAMACOS study is a longitudinal birth cohort study of the effects of exposures to pesticides and other environmental chemicals on neurodevelopment, growth, and respiratory disease in children from primarily Latino farmworker families in the Salinas Valley, California (Eskenazi et al. 2003). Located in Monterey County, the Salinas Valley is an intensive agricultural area where > 235,000 kg of OP pesticides are applied annually (California Department of Pesticide Regulation 2005). A total of 601 pregnant women were enrolled in the CHAMACOS study, and 528 delivered newborns. Mothers in the CHAMACOS cohort were primarily low-income, Mexican-born, Spanish-speaking women who were farmworkers themselves or lived with farmworkers. In this analysis, we include those mothers who had measured levels of urinary DAP metabolites during pregnancy and whose children were followed up to 2 years of age (n = 371). Most women (n = 351) and children (n = 369) provided a blood specimen that was genotyped for PON1192 and PON1−108, and a smaller portion of these mothers and children had adequate blood samples for PON1 enzyme measurements (n = 304, 266, and 250 for mothers, umbilical cord, and 2-year-olds, respectively) (Huen et al. 2009a, 2010). Study protocols were approved by the University of California, Berkeley, Committee for the Protection of Human Subjects. Written informed consent was obtained from all mothers for themselves and their children.
Maternal interviews and neurobehavioral assessments
Information on different covariates was collected from maternal interviews and medical record review. Women were interviewed twice during pregnancy (mean = 13.4 and 25.8 weeks’ gestation), shortly after delivery, and when children were 6 months and 1 and 2 years of age. Interviews were conducted in Spanish or English by bilingual, bicultural interviewers. Mothers were administered the Peabody Picture Vocabulary Test (PPVT) (Dunn and Dunn 1981) at the 6-month visit to assess their scholastic/cognitive abilities, and the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977) at the 1-year visit. The Infant-Toddler Home Observation for Measurement of the Environment (HOME) instrument (Caldwell and Bradley 1984), a measure of the home and social environment, was completed when the children were 6 months and 1 year of age, and 32 of 45 items were completed at 2 years. Prenatal and delivery medical records were abstracted by a registered nurse.
Children were assessed at 2 years of age on the Bayley Scales of Infant Development, 2nd edition (Bayley 1993), a test of developmental functioning of infants and young children comprising two subscales: the Mental Development Index (MDI), which characterizes a variety of cognitive abilities, and the Psychomotor Development Index (PDI), which characterizes large-muscle and fine-motor coordination. Both scales were administered in Spanish and/or English by psychometricians blind to exposure. Psychometricians were trained using standardized protocols and were supervised for quality assurance by a clinical neuropsychologist. Assessments were performed in a private room at the CHAMACOS research office or in a recreational vehicle (RV) modified to be a mobile testing facility. Children were assessed on average (mean ± SD) at 24.6 ± 1.1 months. Each scale is standardized by age to mean ± SD = 100 ± 15.
At the time of the child’s 2-year assessment, the mother was administered the CBCL for ages 1.5 to 5 years, a 99-item tool to assess children’s emotional/behavioral problems and competencies (Achenbach and Rescorla 2000). The CBCL has been widely used in cross-cultural research and collects data on a range of behavior problems, yielding scores for several syndrome scales and five scales designed to be consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) diagnoses (American Psychiatric Association 2000). For this study, we focus on the results of the DSM-IV–oriented pervasive developmental disorder (PDD) scale, which includes such items as “avoids eye contact,” “rocks head, body,” and “unresponsive to affection,” which are considered consistent with Asperger’s disorder and autistic disorder (Achenbach and Rescorla 2000). A score considered of “clinical” significance is > 97th percentile of the national normative sample.
OP pesticide exposure: DAP metabolites
Urine specimens were collected from the mother twice during pregnancy. Urine was aliquoted and stored at −80°C until shipment on dry ice to the Centers for Disease Control and Prevention (Atlanta, GA), where specimens were analyzed using gas chromatography/tandem mass spectrometry and quantified using isotope dilution calibration (Bravo et al. 2002) for six DAP metabolites: three DMs (dimethylphosphate, dimethylthiophosphate, dimethyldithiophosphate) and three DEs (diethylphosphate, diethylthiophosphate, and diethyldithiophosphate) (Bradman et al. 2005). These six metabolites represent the by-products of approximately 80% of OP pesticides used in the Salinas Valley. The most commonly used DM pesticides in the Salinas Valley are malathion and oxydemeton-methyl, and the most commonly used DE pesticides are diazinon and chlorpyrifos. Values below the limit of detection (LOD) were assigned a value of 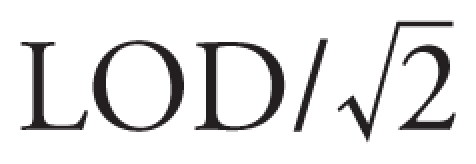 .
.
PON1 genotype and enzyme measurements
Maternal blood and fetal umbilical cord blood were collected at the time of delivery, and child blood samples were collected at 2 years of age using BD Vacutainers (Becton, Dickinson and Company, Franklin Lakes, NJ) as previously described (Holland et al. 2006). Heparinized plasma was used to assess PON1 substrate-specific activities at each time point, and maternal and child genotyping was conducted using DNA isolated from blood clots.
The coding polymorphism PON1192 was genotyped using the Taqman real-time polymerase chain reaction (PCR) method as previously described (Holland et al. 2006) using probes custom-designed by Applied Biosystems, Inc. (Foster City, CA). The promoter single-nucleotide polymorphism (SNP) PON1−108 assay required a two-part nested PCR strategy, where the region surrounding the SNP was preamplified using nonallelic flanking primers. The forward primer sequence was 5′-ATAGACAAAGGGATC GATGGGCG-3′, and the reverse primer sequence was 5′-TTTGGCTGAAAGT GCTGAGCTCCTG-3′. The amplicon was then diluted and used as the template for the Amplifluor assay (Flowgen Biosciences Ltd., Nottingham, UK). Quality assurance procedures for genotyping PON1 SNPs included assessment of randomly distributed blank samples and duplicates with independently isolated DNA from the same subjects. Repeated analysis (4% of samples) in several runs showed a high degree (> 99%) of concordance. All discrepancies were resolved with additional genotyping.
PON1 enzyme activities toward paraoxon (POase) and phenyl acetate (ARYase) were determined using spectrophotometric methods as described by Huen et al. (2009b). The ARYase assay is a quantitative measure of PON1 enzyme level (Connelly et al. 2008; Kujiraoka et al. 2000), which is mostly affected by the promoter polymorphism PON1−108. In contrast, the POase substrate-specific assay reflects both quantity and catalytic efficiency of the PON1 enzyme and is affected primarily by the coding polymorphism PON1192. We refer to enzyme quantity, reflected by ARYase, as PON1 enzyme levels and to POase as PON1 enzyme activity. When we refer to both, we use PON1 measurements. All assays were performed in triplicate. Quality assurance included assessment of repeat samples and internal controls. The average coefficient of variation (CV) for repeated samples ranged from 6–9%, and interassay variability, as measured by the average CV for internal control samples, was between 7% and 9% [for more details on validation of these assays for longitudinal studies, see Huen et al. (2009b)].
Statistical analysis
PON1 genotypes were examined categorically with two indicator variables per model: For PON1−108, the CC genotype was the reference, and for PON1192, the RR genotype was the reference. Supplemental analyses were performed classifying genotypes as ordinal variables (none, one, or two T alleles of PON1−108 or Q alleles of PON1192). ARYase and POase activity in cord, maternal pregnancy, and blood from 2-year-olds was examined categorically in tertiles and continuously, with values normalized by dividing each value by the SD of the range of values.
DAP metabolite concentrations (nanomoles per liter) were summed and transformed to the log10 scale. We created “pregnancy” values for DE, DM, and total DAP concentrations by averaging the two log-transformed pregnancy measures. The two total DAP measurements during pregnancy were correlated (r = 0.15, p = 0.007) and did not differ significantly (paired t-test = −0.38, p = 0.71). For 22 women, only a single DAP measurement was available.
Statistical methods and base models were similar to those described previously (Eskenazi et al. 2007). To examine the relationship between PON1 and neurobehavioral development at 2 years of age, we constructed separate multiple regression models for PON1 genotype (PON1−108 and PON1192) and enzyme measurements (ARYase and POase) and each outcome, adjusting for DAP metabolite concentrations and the covariates included in our previous analysis (Eskenazi et al. 2007). Specifically, all models were adjusted for log10-transformed total DAP, DE, or DM concentrations and exact age at assessment, sex, parity, breast-feeding duration (months), HOME score (continuous), maternal PPVT (continuous), and household income in relation to the federal poverty threshold (U.S. Census Bureau 2000). Models with enzyme measurements were also adjusted for temperature during the assay. Multiple linear regression models for Bayley MDI and PDI scores also included psychometrician (n = 4) and testing location (office or RV), whereas multiple logistic regression models for clinical-range PDD also included maternal depression (assessed at 12 months postpartum). Covariates in final models were categorized as noted in Table 1 unless otherwise specified above. To preserve the size of the analytic population, each missing covariate value was imputed by randomly selecting a value from participants with nonmissing values. In models of genotype, tests for trend were performed by substituting 0, 1, or 2 to represent the number of PON1−108 T or PON1192 Q alleles. Studies suggest that PON1 status, which takes into account both enzyme level and PON1192 genotype, is useful for investigating the relationship between PON1 and health outcomes in epidemiologic studies (Li et al. 2003; Richter et al. 2010). Therefore, to incorporate a measure of PON1 status, we included variables for both ARYase (as a measure of PON1 enzyme levels) and PON1192 genotype (as a proxy measure of PON1 enzyme activity) within the same statistical models and also considered their interaction.
Table 1.
Demographic characteristics of CHAMACOS mothers with children followed to 2 years of age: Salinas Valley, California, 2000–2001 (n = 371).
| Characteristic | n (%) |
|---|---|
| Child sex | |
| Female | 189 (50.9) |
| Male | 182 (49.1) |
| Maternal age at delivery (years) | |
| 18–24 | 153 (41.2) |
| 25–29 | 123 (33.2) |
| 30–34 | 63 (17.0) |
| ≥ 35 | 32 (8.6) |
| Marital status during pregnancy | |
| Married/living as married | 307 (82.8) |
| Single | 64 (17.3) |
| Parity | |
| 0 | 117 (31.5) |
| ≥ 1 | 254 (68.5) |
| Maternal education during pregnancy | |
| < 6th grade | 163 (43.9) |
| 7th–12th grade | 135 (36.4) |
| Completed high school | 73 (19.7) |
| Maternal country of birth | |
| Mexico | 323 (87.1) |
| USA | 44 (11.9) |
| Other | 4 (1.1) |
| Alcohol use during pregnancy | |
| Yes | 4 (1.1) |
| No | 354 (98.9) |
| Smoking during pregnancy | |
| Smoker | 17 (4.6) |
| Nonsmoker living with smoker | 28 (7.6) |
| Nonsmoker | 326 (87.9) |
| Maternal depressive symptoms (CES-D ≥ 16) at 1 year | |
| Yes | 181 (50.8) |
| No | 175 (49.2) |
| Breast-feeding at 2 years | |
| Yes | 30 (8.1) |
| No | 341 (91.9) |
| Household incomea | |
| ≤ Federal poverty threshold | 218 (58.8) |
| > Federal poverty threshold | 153 (41.2) |
| Worked in agriculture during pregnancy | |
| Yes | 163 (44.2) |
| No | 206 (55.8) |
| Lived with agricultural worker(s) during pregnancy | |
| Yes | 302 (82.1) |
| No | 66 (17.9) |
| Lived within 200 feet of agricultural field | |
| Yes | 85 (23.0) |
| No | 284 (77.0) |
Percentages represent those of known values. Values are limited to participants who had a Bayley assessment or maternal CBCL report performed at 24 months.
Poverty level compares federal poverty thresholds with household income divided by the number of people supported (U.S. Census Bureau 2000).
Potential interactions between maternal urinary DAP concentrations (log-transformed continuous variables) and PON1 genotypes were explored by adding interaction terms. We also examined interaction by number of variant alleles (0, 1, 2). Models for DAP concentrations and neurobehavioral outcomes stratified by PON1 genotype are presented. Similarly, interactions between DAP concentrations and PON1 enzyme levels and activity were examined using interaction terms for DAP concentrations and continuous PON1 enzyme measurements; models stratified by tertile of enzyme quantity or activity are presented.
Statistical significance for main effects terms was based on a p-value of 0.05 and for interaction terms, on a p-value of 0.15. All analyses were performed in STATA 10.0 (StataCorp LP, College Station, TX).
Results
Most study mothers were young (mean ± SD = 26.2 ± 5.2 years of age at delivery), born in Mexico, and married or living as married (Table 1). Nearly 60% were living at or below the federal poverty threshold. About 44% of the women worked in agriculture during pregnancy, 82% had at least one household member working in agriculture, and 23% lived near a field during pregnancy. Nearly all women breast-fed; median duration was 6 months. The women’s PPVT scores were below the expected standardized average of 100 (mean ± SD = 86 ± 21).
The geometric mean (GM) of the average total DAP levels for the women during pregnancy was 110 nmol/L [95% confidence interval (CI), 101–120] with a larger proportion composed of DM DAP metabolites (GM = 77; 95% CI, 70–85) than of DE DAP metabolites (GM = 18; 95% CI, 16–19). The children’s mean ± SD scores were 85 ± 12 on the Bayley MDI and 98 ± 11 on the Bayley PDI. Based on maternal report on the CBCL, 51 (14%) exceeded the clinical cutoff criteria for PDD (χ2 test, p < 0.001 comparing 14% observed with 3% expected).
As we previously reported, about half of the children were heterozygous for PON1192 and PON1−108 (Huen et al. 2009a, 2009b), with allelic frequencies for both of approximately 0.5 [see Supplemental Material, Table 1 (doi:10.1289/ehp.1002234)]. PON1−108TT and PON1192QQ genotype were associated with mostly lower enzyme measures, suggesting that individuals with these genotypes might be more susceptible to effects of OPs. Neonates with PON1−108TT genotype (20.3%) had the lowest measures of ARYase and POase among PON1−108 genotypes. Neonates with PON1192QQ (22.9%) showed lower measures of POase than did neonates with other PON1192 genotypes but no significant difference in ARYase levels (Holland et al. 2006; Huen et al. 2009a). At 2 years of age, average POase activities and ARYase levels were two to three times higher than in cord blood. Two-year-olds showed similar POase enzyme patterns in relation to their genotype as when they were neonates. ARYase levels were somewhat higher in 2-year-olds with PON1192QQ than in those with other PON1192 genotypes. We observed similar associations between mother’s PON1 genotype and enzyme measurements [Supplemental Material, Table 2 (doi:10.1289/ehp.1002234)].
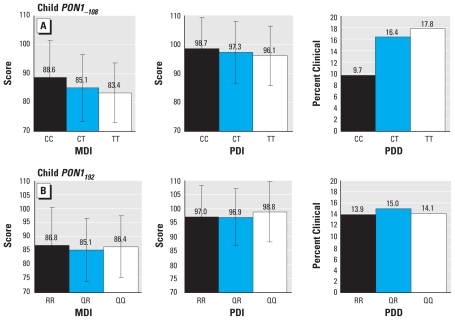
Children with the PON1−108T allele had lower average MDI and PDI scores and were more likely to be reported by their mothers as having symptoms characteristic of PDD (Figure 1). We saw no clear pattern of associations with outcomes with PON1192 genotype. The PON1−108T allele remained associated with poorer performance on the Bayley MDI and PDI (Table 2) after adjustment for covariates. Specifically, compared with children with PON1−108CC genotype, children with PON1−108CT and PON1−108TT genotypes performed, respectively, 3.9 points (p = 0.004) and 5.7 points (p = 0.001) lower on the Bayley MDI (p-value for trend = 0.01) and 1.4 (p = 0.27) and 2.8 points (p = 0.07) lower on the Bayley PDI (p-value for trend = 0.07). We noted similar patterns, albeit muted, for maternal genotype for PON1−108 and MDI scores [see Supplemental Material, Table 3 (doi:10.1289/ehp.1002234)]. Children with the PON1−108T allele were slightly but not significantly more likely to be reported by their mothers as having symptoms consistent with PDD, after controlling for potential confounders. We found no clear relationship between the child’s or mother’s PON1192 genotype and Bayley scores or maternal report of PDD symptoms, except for somewhat better PDI scores among children with PON1192QQ (p-value for trend = 0.10).
Figure 1.
Unadjusted relationship (mean ± SD) of child PON1−108 (A) and PON1192 (B) genotypes and MDI and PDI scores and maternal report of PDD on the CBCL.
Table 2.
Adjusteda association of child PON1−108 and PON1192 genotypes and neurobehavioral development at 2 years of age: CHAMACOS study, Salinas Valley, California, 2000–2003.
| Genotype | nb | Bayley MDI β (95% CI) | p-Value trend | Bayley PDI β (95% CI) | p-Value trend | CBCL PDD OR (95% CI) | p-Value trend |
|---|---|---|---|---|---|---|---|
| PON1−108 | |||||||
| CC | 111 | Reference | < 0.01 | Reference | 0.07 | Reference | 0.14 |
| CT | 179 | −3.9 (−6.6 to −1.2)# | −1.4 (−3.8 to 1.0) | 1.5 (0.7 to 3.3) | |||
| TT | 74 | −5.7 (−9.0 to −2.5)# | −2.8 (−5.7 to 0.2)* | 2.0 (0.8 to 5.1) | |||
| PON1192 | |||||||
| RR | 94 | Reference | 0.65 | Reference | 0.10 | Reference | 0.94 |
| QR | 188 | −0.5 (−3.4 to 2.4) | 0.3 (−2.2 to 2.9) | 1.0 | (0.4 to 2.2) | ||
| 86 | 0.7 (−2.6 to 4.0) | 2.4 (−0.5 to 5.4) | 1.0 | (0.4 to 2.4) | |||
Adjusted for prenatal DAPs and age at assessment, sex, parity, breast-feeding duration, HOME score, maternal PPVT, and household poverty status. Bayley MDI and PDI models also included psychometrician and testing location; PDD models also included maternal depression.
Numbers may vary slightly depending on the model.
p < 0.10.
p < 0.01.
Continuous measures of ARYase and POase in maternal, cord, or child blood were not related to neurobehavioral performance at 2 years of age on the Bayley MDI or PDI (Table 3). Cord PON1 measures also were not associated with maternal report of PDD, although 2-year-old ARYase levels [odds ratio (OR) = 0.6; 95% CI, 0.4–0.9] and maternal POase activity (OR = 0.6; 95% CI, 0.4–1.0) were associated with decreased odds of PDD at 2 years of age. Results were similar when we categorized ARYase and POase into tertiles, although those in the highest tertiles of ARYase had the highest MDI scores (ptrend = 0.04; data not shown). Associations between the outcomes and ARYase were comparable when we included PON1192 genotype in the model, and we found no evidence of statistical interaction (although children with PON1192QQ and in the highest tertile of ARYase did have the highest adjusted MDI and PDI scores).
Table 3.
Adjusteda association of PON1 enzyme measurements (per SD increase) in cord and child blood and neurobehavioral development at 2 years of age: CHAMACOS study, Salinas Valley, California, 2000–2003.
| Enzyme | Mean ± SD | Bayley MDI [β (95% CI)] | Bayley PDI [β (95% CI)] | CBCL PDD [OR (95% CI)] |
|---|---|---|---|---|
| Fetal enzymes (n = 265) | ||||
| ARYase | 34.0 ± 16.8 | 0.8 (−0.6 to 2.1) | −0.6 (−1.8 to 0.7) | 0.8 (0.6 to 1.2) |
| POase | 254.3 ± 165.0 | 0.1 (−1.3 to 1.5) | −1.0 (−2.2 to 0.2) | 1.0 (0.7 to 1.4) |
| 2-year-old enzymes (n = 249) | ||||
| ARYase | 87.0 ± 25.1 | 0.4 (−1.0 to 1.8) | 0.1 (−1.2 to 1.3) | 0.6 (0.4 to 0.9)** |
| POase | 665.2 ± 385.8 | −0.2 (−1.6 to 1.3) | −0.5 (−1.7 to 0.8) | 0.8 (0.6 to 1.3) |
| Maternal enzymes during pregnancy (n = 302) | ||||
| ARYase | 133.3 ± 40.9 | 0.7 (−0.7 to 2.1) | 0.4 (−0.8 to 1.7) | 0.7 (0.5 to 1.0)* |
| POase | 958.6 ± 599.3 | −0.1 (−1.4 to 1.2) | 0.0 (−1.2 to 1.2) | 0.6 (0.4 to 1.0)** |
Adjusted for prenatal DAPs and age at assessment, sex, parity, breast-feeding duration, HOME score, maternal PPVT, household poverty status, and assay temperature. Bayley MDI and PDI models also included psychometrician and testing location; PDD models also included maternal depression.
p < 0.10.
p < 0.05.
Table 4 shows the relation of maternal DAP levels and neurobehavioral outcomes stratified by child genotype. As we previously reported (Eskenazi et al. 2007), maternal DAP concentrations were negatively associated with child MDI scores at 2 years of age, and this relationship was primarily with DM DAPs. The interaction between genotype and DAP concentrations was not statistically significant (interaction p = 0.98); however, the inverse association between DAP concentrations and MDI scores was progressively stronger among children with PON1−108CT and PON1−108TT genotypes relative to PON1−108CC. This relationship was most apparent for DM DAP concentrations: For each 10-fold increase in maternal DM DAP levels, we observed a drop in child MDI scores of −2.2 points (p = 0.45) for children with PON1−108CC, −3.4 points (p = 0.09) for children with PON1−108CT, and −5.9 points (p = 0.03) for children with PON1−108TT (interaction p = 0.91). We observed a similar trend (albeit nonsignificant) for DE DAP concentrations and MDI scores by genotype. The relation between DAP concentrations and MDI scores did not differ progressively by PON1192 genotype.
Table 4.
Adjusteda associations of prenatal DAPs and neurobehavioral development at 2 years of age, stratified by child PON1−108 and PON1192 genotype: CHAMACOS study, Salinas Valley, California, 2000–2003.
| DAP | Bayley MDI β (95% CI) | p-Value interactionb | Bayley PDI β (95% CI) | p-Value interactionb | CBCL PDD OR (95% CI) | p-Value interactionb |
|---|---|---|---|---|---|---|
| Total DAPs | ||||||
| PON1−108 | ||||||
| CC | −3.2 (−9.8 to 3.5) | 0.98 | −2.3 (−7.8 to 3.3) | 0.89 | 4.2 (0.5−36.8) | 0.91 |
| CT | −3.7 (−8.0 to 0.6)* | −0.8 (−4.8 to 3.3) | 2.0 (0.6–6.0) | |||
| TT | −5.5 (−11.1 to 0.1)* | −1.0 (−7.1 to 5.1) | 1.9 (0.3–10.4) | |||
| PON1192 | ||||||
| RR | −6.5 (−15.6 to 2.6) | 0.33 | −1.7 (−8.7 to 5.4) | 0.53 | 5.4 (0.7–44.0) | 0.29 |
| QR | −1.2 (−5.2 to 2.9) | 0.1 (−3.5 to 3.8) | 1.2 (0.4–3.6) | |||
| −6.9 (−12.8 to −0.9)** | −5.1 (−11.1 to 1.0)* | 5.2 (0.8–35.1)* | ||||
| DM DAPs | ||||||
| PON1−108 | ||||||
| CC | −2.2 (−8.0 to 3.6) | 0.91 | −1.6 (−6.4 to 3.3) | 0.87 | 3.3 (0.5–21.3) | 0.94 |
| CT | −3.4 (−7.4 to 0.6)* | −0.3 (−4.0 to 3.4) | 2.2 (0.8–5.9) | |||
| TT | −5.9 (−11.1 to −0.6)** | −1.2 (−6.9 to 4.4) | 1.9 (0.4–9.8) | |||
| PON1192 | ||||||
| RR | −4.4 (−12.4 to 3.6) | 0.38 | −2.1 (−8.3 to 4.0) | 0.36 | 4.8 (0.8–31.1)* | 0.20 |
| QR | −1.3 (−4.9 to 2.4) | 0.7 (−2.6 to 4.0) | 1.2 (0.5–3.3) | |||
| −7.4 (−13.0 to −1.9)** | −4.7 (−10.4 to 1.0) | 6.1 (1.0–39.3)* | ||||
| DE DAPs | ||||||
| PON1−108 | ||||||
| CC | −0.3 (−7.2 to 6.7) | 0.84 | 0.9 (−4.9 to 6.8) | 0.66 | 7.4 (0.6–93.9) | 0.44 |
| CT | −1.7 (−6.3 to 3.0) | −2.2 (−6.5 to 2.1) | 0.8 (0.2–2.8) | |||
| TT | −3.4 (−8.8 to 2.1) | −1.5 (−7.3 to 4.2) | 0.8 (0.1–4.3) | |||
| PON1192 | ||||||
| RR | 1.4 (−8.4 to 11.1) | 0.47 | 4.5 (−2.9 to 11.9) | 0.14 | 1.0 (0.1–8.2) | 0.97 |
| QR | −1.1 (−5.2 to 3.0) | −1.9 (−5.6 to 1.8) | 0.8 (0.2–2.6) | |||
| −2.5 (−8.7 to 3.6) | −3.8 (−9.9 to 2.3) | 1.2 (0.2–7.7) | ||||
Adjusted for age at assessment, sex, parity, breast-feeding duration, HOME score, maternal PPVT, and household poverty status. Bayley MDI and PDI models also included psychometrician and testing location; PDD models also included maternal depression.
Interaction p-value was calculated using a postestimation combined F-test (or chi-square test) for the two interaction variables between genotype and DAPs (e.g., 192QR × DAP and 192QQ × DAP). Conclusions regarding interaction were similar if a single interaction term with the number of C or Q alleles (0, 1, 2) was used instead.
p < 0.10.
p < 0.05.
We previously found no relation between maternal DAP concentrations and child PDI scores at 2 years of age, and we also observed no clear relation when we stratified by child PON1−108 (Table 4). However, we found an inverse association between DAP concentrations and PDI scores among 2-year-olds with PON1192QQ (p = 0.10) and an interaction of DE DAP concentrations and PDI scores by PON1192 genotype (interaction p = 0.14).
Although we observed a strong relation of maternal DAP concentrations and maternal report of PDD overall, we found no clear difference in the odds across strata of PON1−108 and only a suggestion of a higher odds in children with PON1192QQ or PON1192RR (for DMs). However, numbers within each stratum are too small to provide reliable ORs, and none of the interactions between DAP concentrations and genotype was statistically significant. We observed similar, albeit weaker, associations between DAP concentrations and neurobehavioral outcomes when we stratified results by maternal genotype rather than child genotype [Supplemental Material, Table 4 (doi:10.1289/ehp.1002234)].
We did not observe statistically significant interaction between DAPs and enzyme measurements (as a continuous measure) in relation to any of the neurobehavioral end points. We present the relation of maternal DAP concentrations and neurobehavioral outcomes within tertiles of cord and maternal ARYase and POase in Supplemental Material, Tables 5 and 6 (doi:10.1289/ehp.1002234). (We do not present the results stratified by 2-year-old PON1 measurements because the biological mechanism of in utero exposure being modified by child’s enzyme levels 2 years later would be uncertain.) Consistent with results by child PON1 genotype, the relation of DAP concentrations and child MDI scores was strongest within the group of children with the lowest tertiles of cord ARYase and POase, although the pattern was not as clear. We observed slightly stronger relationships of PDI scores and maternal DAP concentrations, particularly for DE DAPs, among children with cord blood in the lowest tertile of ARYase relative to children with higher cord blood PON1 activity. Maternal DAP concentrations were consistently positively associated, although not significantly, with reports of PDD across all tertiles of cord ARYase activity. However, the relationship of DAP concentrations and PDD was significant only in the group with the lowest tertile of POase. Again, relatively small numbers in each tertile made these estimates unreliable. Results for DAP concentrations and MDI scores, PDI scores, and PDD assessment across maternal enzyme levels and activities [Supplemental Material, Table 5 (doi:10.1289/ehp.1002234)] were similar to those for cord blood enzyme levels.
Discussion
Our previous study demonstrated that women’s levels of OP pesticide DAP metabolites in urine during pregnancy are related to their 2-year-olds’ mental development and reported symptoms of PDD (Eskenazi et al. 2007). In the present study, we examined whether these associations were modified by the mother and child’s PON1 genotypes and associated enzyme levels and activities. In analyses of PON1 and neurobehavior, we found that the PON1−108T allele in children was associated with poorer Bayley MDI scores and with somewhat poorer Bayley PDI scores. Maternal, cord, and child PON1 enzyme levels and activities, although related to genotype, were not significantly associated with these neurobehavioral outcomes except that children were less likely to display symptoms of PDD when they or their mothers had higher ARYase levels or POase activities.
Although we have observed a relationship of DAP concentrations and neurobehavioral outcomes and of PON1 and these same end points, we are less certain as to whether genotype or enzyme measurements modify the association of DAP concentrations and neurobehavior—that is, whether a subgroup of children by virtue of their genetic makeup are more susceptible to the adverse effects of maternal exposure to OP pesticides during pregnancy. There is a suggestion with MDI scores that children with PON1−108T allele show a stronger association with OP pesticide exposure in utero (as measured by maternal DAPs), but the interaction is not significant.
PON1 has been implicated in the etiology of a number of adult-onset neurologic diseases such as Parkinson and Alzheimer diseases (Erlich et al. 2006; Leduc and Poirier 2008; Paragh et al. 2002; Zintzaras and Hadjigeorgiou 2004). In addition, PON1 polymorphisms and enzyme activities have been associated with autism spectrum disorder. In a family-based linkage study, D’Amelio et al. (2005) found that Caucasian-American, but not Italian, patients diagnosed with autism were more likely to carry the PON1192R allele and more likely, although not significantly, to carry the PON1−108T allele. In line with these results, Pasca et al. (2010) reported that ARYase levels and POase activity were significantly lower in 50 Romanian autistic children compared with 30 age- and sex-matched nonautistic controls, but they observed no differences in PON1192 or PON155 allelic frequencies between groups (and they did not examine PON1−108). Another study (Serajee et al. 2004) also failed to find a relation of PON155 allelic frequency in a family-based association study of 196 trios but did not examine PON1−108 or PON1192.
In the above studies, autistic patients were diagnosed by clinicians. In the present study, we based PDD on maternal report of symptomatology associated with autism-like behavior, and these reports were not confirmed by a clinician’s assessment. Nevertheless, for the most part, our findings are in line with those reported by Pasca et al. and D’Amelio et al. (2005): We observed a nonsignificant increase in maternal report of PDD in primarily Mexican-American children with the PON1−108T allele but not with the PON1192R allele, and we found that 2-year-olds with higher ARYase levels and children whose mothers had higher ARYase or POase measures during pregnancy were less likely to be reported by their mother as having PDD.
D’Amelio et al. (2005) hypothesized that genetic vulnerability in the presence of exposure to OP pesticides may contribute to the development of childhood autism, especially in North America, where until recently OP pesticides have been more commonly used in homes than in Europe. OP pesticides can induce oxidative stress (Milatovic et al. 2006), and PON1 may protect the body from the effects of OP pesticides both through its antioxidant properties and its ability to metabolize the activated form (e.g., chlorpyrifos-oxon) of the pesticides. We previously reported that with every 10-fold increase in DAP metabolites concentrations in the urine of the women in this study during pregnancy, we found more than a doubling in odds of their reporting signs of PDD in their 2-year-olds. Nevertheless, we observed no evidence that PON1 genotype or enzyme activity modifies this association as suggested by the hypothesis of D’Amelio et al. (2005). However, given the lack of clinical diagnosis and that our sample sizes when stratified by PON1 genotype were relatively small, this hypothesis cannot be rejected.
Few studies have tested the hypothesis that PON1 genotype modifies the effects of pesticide exposure on neuropsychologic functioning. One study from Israel demonstrated increased frontal cortical brain activity and decreased temporal lobe activity in pesticide-exposed workers with PON1192R compared with either those without the R allele or unexposed adults (Browne et al. 2006). Only one previous study has examined the ability of PON1 enzyme to modify the association of OP pesticide exposure and neurobehavioral end points in children. Engel et al. (2007) found that DAP metabolite levels during pregnancy were associated with an increase in abnormal reflexes in the neonates as assessed on the Brazelton Neonatal Assessment Scale, and the association between DAP concentrations, specifically DM DAPs, and abnormal reflexes was strongest in the infants born to women with the lowest levels of ARYase.
In the present study, we extend the observations of Engel et al. (2007) to toddlers. Although we find a clear relationship of PON1−108 genotype with MDI scores and, to a lesser extent, PDI scores and a trend of increasingly stronger associations of maternal DAPs and MDI scores, we cannot provide firm evidence that the PON1 genotype or phenotype modifies the relation of exposure and mental development. Recent studies demonstrate that PON1 is a multifunctional enzyme, and it plays a significant role in protection against oxidative stress and lipid peroxidation in addition to OP detoxification. Thus, it is possible that the relationship between PON1 and mental development is more strongly influenced by the role of PON1 in protection from oxidative stress than by its OP pesticide detoxification capabilities, which may explain the absence of strong effect modification. Further, other pesticides not hydrolyzed by PON1, including malathion (devolves to DM DAPs), have been shown to induce oxidative stress (Durak et al. 2009; Franco et al. 2009). This may explain why we observed stronger associations of neurobehavior with DM DAPs than with DE DAPs (after stratification by PON1 genotype) despite the fact that DEs are derived from known PON1 substrates (chlorpyrifos-oxon and diazoxon).
Our research is limited in a number of ways. DAP metabolites are an imperfect measure of OP pesticide exposure given the short half-life of OP pesticides, the variability in exposure over time, differences in metabolism that may affect excretion (Wessels et al. 2003), and the potential for exposure to preformed DAPs in the environment (Quirós-Alcalá 2010). In fact, it is possible that because the PON1 gene is involved with the ability to metabolize OP pesticides, levels of excreted DAPs may be related both to exposure and to genetics. We are currently examining this issue by analyzing the interrelationship of blood OP pesticide measurements, genotype, and urinary DAP concentrations in this cohort. Our study results may not be generalizable given this unique cohort of primarily Mexican-American children from an agricultural area. Other populations may have a different spectrum of OP pesticide exposures and PON1 allelic distributions, which are known to vary widely among different ethnic groups (Chen et al. 2003; Rojas-Garcia et al. 2005). The relationship between genotype and enzyme activities in adults, however, appears to be comparable in different ethnic groups (Costa et al. 2005).
Another limitation is that we have constructed a fairly simple model for neurobehavioral development. Although we have examined and/or controlled for a number of key potential confounders, neurobehavioral development is complex and multifactorial, and numerous genes and environmental agents, social, biological, physical, and chemical, are likely involved. In addition, when data are stratified by genotype, the fairly large numbers of this study become relatively small in each stratum, making it difficult to find significant statistical interactions.
We have not examined other genes that have been implicated in the hypothesized gene–environment interaction such as RELN or AChE or other PON1 polymorphisms (Moy and Nadler 2008). We have recently shown that other PON1 polymorphisms are also associated with ARYase and POase measurements in mothers and children of the CHAMACOS cohort; however, most of them were in strong linkage disequilibrium with those examined in this report (Huen et al. 2010) and are unlikely to affect the associations noted here. Haplotype analysis is sometimes proposed as a more informative approach for analysis of genetic effects, but we did not see an improvement of association with neurodevelopment using haplotypes in this study (data not shown). Additionally, we did not find a significant difference in functional effects of PON1 haplotypes compared with multiple polymorphisms in predicting PON1 enzyme measurements (Holland et al. 2006; Huen et al. 2010).
The strengths of this study are that we have focused on an agricultural population with relatively high levels of OP pesticide exposures, that we have measured this exposure during pregnancy in this longitudinal birth cohort study, and that we have genotyped and measured enzyme activity in mothers and children at birth and during infancy.
In summary, we have found that PON1−108T allele is related to mental development and, to a lesser extent, psychomotor development in toddlers. This study adds to the growing evidence that the PON1 gene is associated with an array of neurologic end points in adults and in children. Although we did not observe a statistical interaction between prenatal OP pesticide exposure as measured by pesticide metabolites and PON1, we did observe a consistent trend, and our results need replication in other populations and perhaps pooling with similar studies to provide adequate sample sizes.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002234 via http://dx.doi.org/).
We acknowledge the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) staff, students, community partners, and participants and families, without whom this study would not be possible. We specifically thank the CHAMACOS field office staff.
This publication was made possible by research supported by grant RD 83171001 from the U.S. Environmental Protection Agency (EPA), P01 ES009605 from the National Institute for Environmental Health Sciences (NIEHS), R01 ES012503-03 from NIEHS, and U.S. EPA Science to Achieve Results award RD-83273401.
The contents are solely the authors’ responsibility and do not necessarily represent the official views of the NIEHS, National Institutes of Health, or U.S. EPA.
Since this paper was accepted, B.E. worked as an expert witness in a pesticide poisoning case. The authors declare they have no actual or potential competing financial interests.
References
- Achenbach T, Rescorla L. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association, 943; 2000. Text Revision. [Google Scholar]
- Bayley M. The Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Bradman A, Eskenazi B, Barr D, Bravo R, Castorina R, Chevrier J, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Driskell WJ, Whitehead RD, Jr, Needham LL, Barr DB. Quantitation of dialkyl phosphate metabolites of organophosphate pesticides in human urine using GC-MS-MS with isotopic internal standards. J Anal Toxicol. 2002;26((5)):245–252. doi: 10.1093/jat/26.5.245. [DOI] [PubMed] [Google Scholar]
- Brophy VH, Jampsa RL, Clendenning JB, McKinstry LA, Jarvik GP, Furlong CE. Effects of 5′ regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. Am J Hum Genet. 2001;68((6)):1428–1436. doi: 10.1086/320600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne RO, Moyal-Segal LB, Zumsteg D, David Y, Kofman O, Berger A, et al. Coding region paraoxonase polymorphisms dictate accentuated neuronal reactions in chronic, sub-threshold pesticide exposure. FASEB J. 2006;20((10)):1733–1735. doi: 10.1096/fj.05-5576fje. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas; 1984. [Google Scholar]
- California Department of Pesticide Regulation. Pesticide Use Report, Annual 2005. Sacramento, CA: California Environmental Protection Agency; 2005. [Google Scholar]
- Chen J, Kumar M, Chan W, Berkowitz G, Wetmur JG. Increased influence of genetic variation on PON1 activity in neonates. Environ Health Perspect. 2003;111:1403–1409. doi: 10.1289/ehp.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM, et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13((6)):357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- Connelly PW, Maguire GF, Picardo CM, Teiber JF, Draganov D. Development of an immunoblot assay with infrared fluorescence to quantify paraoxonase 1 in serum and plasma. J Lipid Res. 2008;49((1)):245–250. doi: 10.1194/jlr.D700022-JLR200. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta. 2005;352((1–2)):37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- D’Amelio M, Ricci I, Sacco R, Liu X, D’Agruma L, Muscarella LA, et al. Paraoxonase gene variants are associated with autism in North America, but not in Italy: possible regional specificity in gene-environment interactions. Mol Psychiatry. 2005;10((11)):1006–1016. doi: 10.1038/sj.mp.4001714. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test, Revised. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- Durak D, Uzun FG, Kalender S, Ogutcu A, Uzunhisarcikli M, Kalender Y. Malathion-induced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ Toxicol. 2009;24((3)):235–242. doi: 10.1002/tox.20423. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165((12)):1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Erlich PM, Lunetta KL, Cupples LA, Huyck M, Green RC, Baldwin CT, et al. Polymorphisms in the PON gene cluster are associated with Alzheimer disease. Hum Mol Genet. 2006;15((1)):77–85. doi: 10.1093/hmg/ddi428. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland NT. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J Childrens Health. 2003;1((1)):3–27. [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JL, Posser T, Mattos JJ, Trevisan R, Brocardo PS, Rodrigues AL, et al. Zinc reverses malathion-induced impairment in antioxidant defenses. Toxicol Lett. 2009;187((3)):137–143. doi: 10.1016/j.toxlet.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet Genomics. 2006;16((3)):183–190. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci USA. 2008;105((11)):4295–4300. doi: 10.1073/pnas.0711986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley RW, Billecke S, La Du BN. Association of low PON1 type Q (type A) arylesterase activity with neurologic symptom complexes in Gulf War veterans. Toxicol Appl Pharmacol. 1999;157((3)):227–233. doi: 10.1006/taap.1999.8703. [DOI] [PubMed] [Google Scholar]
- Holland N, Furlong C, Bastaki M, Richter R, Bradman A, Huen K, et al. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ Health Perspect. 2006;114:985–991. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Brooks J, Hubbard A, Bradman A, Eskenazi B, et al. Developmental changes in PON1 enzyme activity in young children and effects of PON1 polymorphisms. Environ Health Perspect. 2009a;117:1632–1638. doi: 10.1289/ehp.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Richter R, Furlong C, Eskenazi B, Holland N. Validation of PON1 enzyme activity assays for longitudinal studies. Clin Chim Acta. 2009b;402((1–2)):67–74. doi: 10.1016/j.cca.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Bradman A, Eskenazi B, Holland N. Longitudinal changes in PON1 enzymatic activities in Mexican-American mothers and children with different genotypes and haplotypes. Toxicol Appl Pharmacol. 2010;244((2)):181–189. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafadar AM, Ergen A, Zeybek U, Agachan B, Kuday C, Isbir T. Paraoxonase 192 gene polymorphism and serum paraoxonase activity in high grade gliomas and meningiomas. Cell Biochem Funct. 2006;24((5)):455–460. doi: 10.1002/cbf.1284. [DOI] [PubMed] [Google Scholar]
- Kucukali CI, Aydin M, Ozkok E, Orhan N, Cakir U, Kilic G, et al. Paraoxonase-1 55/192 genotypes in schizophrenic patients and their relatives in Turkish population. Psychiatr Genet. 2008;18((6)):289–294. doi: 10.1097/YPG.0b013e3283060f94. [DOI] [PubMed] [Google Scholar]
- Kujiraoka T, Oka T, Ishihara M, Egashira T, Fujioka T, Saito E, et al. A sandwich enzyme-linked immunosorbent assay for human serum paraoxonase concentration. J Lipid Res. 2000;41((8)):1358–1363. [PubMed] [Google Scholar]
- Lawlor DA, Day IN, Gaunt TR, Hinks LJ, Timpson N, Ebrahim S, et al. The association of the paraoxonase (PON1) Q192R polymorphism with depression in older women: findings from the British Women’s Heart and Health Study. J Epidemiol Community Health. 2007;61((1)):85–87. doi: 10.1136/jech.2006.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc V, Poirier J. Polymorphisms at the paraoxonase 1 L55M and Q192R loci affect the pathophysiology of Alzheimer’s disease: emphasis on the cholinergic system and beta-amyloid levels. Neurodegener Dis. 2008;5((3–4)):225–227. doi: 10.1159/000113709. [DOI] [PubMed] [Google Scholar]
- Li HL, Liu DP, Liang CC. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J Mol Med. 2003;81((12)):766–779. doi: 10.1007/s00109-003-0481-4. [DOI] [PubMed] [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, et al. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10((9)):767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Mackness B, Durrington PN, Mackness MI. Low paraoxonase in Persian Gulf War Veterans self-reporting Gulf War syndrome. Biochem Biophys Res Commun. 2000;276((2)):729–733. doi: 10.1006/bbrc.2000.3526. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Gupta RC, Aschner M. Anticholinesterase toxicity and oxidative stress. Sci World J. 2006;6:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13((1)):4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- Paragh G, Balla P, Katona E, Seres I, Egerhazi A, Degrell I. Serum paraoxonase activity changes in patients with Alzheimer’s disease and vascular dementia. Eur Arch Psychiatry Clin Neurosci. 2002;252((2)):63–67. doi: 10.1007/s004060200013. [DOI] [PubMed] [Google Scholar]
- Pasca SP, Dronca E, Nemes B, Kaucsar T, Endreffy E, Iftene F, et al. Paraoxonase 1 activities and polymorphisms in autism spectrum disorders. J Cell Mol Med. 2010;14((3)):600–607. doi: 10.1111/j.1582-4934.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca SP, Nemes B, Vlase L, Gagyi CE, Dronca E, Miu AC, et al. High levels of homocysteine and low serum paraoxonase 1 arylesterase activity in children with autism. Life Sci. 2006;78((19)):2244–2248. doi: 10.1016/j.lfs.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Quirós-Alcalá L. PhD dissertation. Berkeley, CA: University of California, Berkeley; 2010. Children’s Residential Exposures to Flame Retardants, Pesticides and Pesticide Degradation Products, and the Relationship of Pesticides with Autonomic Nervous System Functioning. [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1((3)):385–401. [Google Scholar]
- Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 status as a risk factor for disease or exposure. In: Reddy S, editor. Paraoxonases in Inflammation, Infection, and Toxicology. New York: Humana Press; 2010. pp. 29–35. Advances in Experimental Medicine and Biology 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Garcia AE, Solis-Heredia MJ, Pina-Guzman B, Vega L, Lopez-Carrillo L, Quintanilla-Vega B. Genetic polymorphisms and activity of PON1 in a Mexican population. Toxicol Appl Pharmacol. 2005;205((3)):282–289. doi: 10.1016/j.taap.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Saeed M, Siddique N, Hung WY, Usacheva E, Liu E, Sufit RL, et al. Paraoxonase cluster polymorphisms are associated with sporadic ALS. Neurology. 2006;67((5)):771–776. doi: 10.1212/01.wnl.0000227187.52002.88. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Nabi R, Zhong H, Huq M. Polymorphisms in xenobiotic metabolism genes and autism. J Child Neurol. 2004;19((6)):413–417. doi: 10.1177/088307380401900603. [DOI] [PubMed] [Google Scholar]
- Sklan EH, Lowenthal A, Korner M, Ritov Y, Landers DM, Rankinen T, et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in health, risk factors, exercise training, and genetics study. Proc Natl Acad Sci USA. 2004;101((15)):5512–5517. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowik A, Tomik B, Wolkow PP, Partyka D, Turaj W, Malecki MT, et al. Paraoxonase gene polymorphisms and sporadic ALS. Neurology. 2006;67((5)):766–770. doi: 10.1212/01.wnl.0000219565.32247.11. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Poverty Thresholds 2000, Current Population Survey. Washington, DC: U.S. Census Bureau; 2000. [Google Scholar]
- Voetsch B, Benke KS, Damasceno BP, Siqueira LH, Loscalzo J. Paraoxonase 192 Gln→Arg polymorphism: an independent risk factor for nonfatal arterial ischemic stroke among young adults. Stroke. 2002;33((6)):1459–1464. doi: 10.1161/01.str.0000016928.60995.bd. [DOI] [PubMed] [Google Scholar]
- Voetsch B, Benke KS, Panhuysen CI, Damasceno BP, Loscalzo J. The combined effect of paraoxonase promoter and coding region polymorphisms on the risk of arterial ischemic stroke among young adults. Arch Neurol. 2004;61((3)):351–356. doi: 10.1001/archneur.61.3.351. [DOI] [PubMed] [Google Scholar]
- Wessels D, Barr DB, Mendola P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children’s environmental health. Environ Health Perspect. 2003;111:1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zintzaras E, Hadjigeorgiou GM. Association of paraoxonase 1 gene polymorphisms with risk of Parkinson’s disease: a meta-analysis. J Hum Genet. 2004;49((9)):474–481. doi: 10.1007/s10038-004-0176-x. [DOI] [PubMed] [Google Scholar]