Abstract
There is increasing focus on the role of the nucleus accumbens (NAc) in learning and memory, but there is little consensus as to how the core and medial shell subregions of the NAc contribute to these processes. In the current experiments, we used spontaneous object recognition to test rats with 6-hydroxydopamine lesions targeted at the core or medial shell of the NAc on a familiarity discrimination task and a location discrimination task. In the object recognition variant, control animals were able to discriminate the novel object at both 24-hr and 5-min delay. However, in the lesion groups, performance was systematically related to dopamine (DA) levels in the core but not the shell. In the location recognition task, sham-operated animals readily detected the object displacement at test. In the lesion groups, performance impairment was systematically related to DA levels in the shell but not the core. These results suggest that dopamine function within distinct subregions of the NAc plays dissociable roles in the modulation of memory for objects and place.
Keywords: nucleus accumbens, dopamine, rat, object memory, place memory
There is emerging evidence that the nucleus accumbens (NAc), a major component of the ventral striatum, plays a key role in learning and memory (Pennartz et al., 2009). The NAc receives dense afferent projections from brain structures implicated in dissociable learning and memory processes such as the hippocampal formation, prefrontal cortex, and basolateral amygdala, and in turn the NAc projects to motor output areas including the ventral pallidum. Thus, the NAc is well-placed to serve as a functional interface and integrate information from different memory systems to allow adaptive behavioral responses (Brog, Salyapongse, Deutch, & Zahm, 1993; Groenewegen, Wright, Beijer, & Voorn, 1991; Mogenson, Jones, & Yim, 1980). The NAc is also richly innervated by dopamine (DA) fibers originating in the ventral tegmental area and substantia nigra pars compacta (Brog et al., 1993; Voorn, Jorritsma-Byham, Van Dijk, & Buijs, 1986). It appears that DA may modulate learning and memory processes by regulating information flow from the different limbic inputs into the NAc (Floresco, 2007; Goto & Grace, 2008).
Consistent with this position, there is good evidence of NAc involvement in the processing of hippocampal-dependent spatial information (e.g., Annett, McGregor, & Robbins, 1989; Ferretti et al., 2005; Seamans & Phillips, 1994). However, the NAc contains at least two morphologically, hodologically, and neurochemically distinct subregions, a core and a shell, which are likely to be differentially involved in these processes (Brog et al., 1993; Zahm & Brog, 1992). Studies that have addressed the anatomical heterogeneity of the NAc have produced mixed results, with some showing deficits in the processing of spatial information following neural manipulations to the shell (e.g., Ito, Robbins, Pennartz, & Everitt, 2008; Setlow & McGaugh, 1999), whereas others have highlighted a role for the core in these processes (Maldonado-Irizarry & Kelley, 1994; Smith-Roe, Sadeghian, & Kelley, 1999). Furthermore, there is some evidence of NAc involvement in tasks that tax nonspatial memory (e.g., Sargolini, Roullet, Oliverio, & Mele, 2003). In terms of wider theories of NAc function, its role in novelty processing (e.g., Burns, Annett, Kelley, Everitt, & Robbins, 1996), and moreover the demonstrable role of DA within NAc in the behavioral response to novelty (Bardo, Donohew, & Harrington, 1996; De Leonibus, Oliverio, & Mele, 2005; De Leonibus, Verheij, Mele, & Cools, 2006; Goto & Grace, 2005, 2008; Hooks, Jones, Smith, Neill, & Justice, 1991; Rebec, Christensen, Guerra, & Bardo, 1997; Redgrave & Gurney, 2006), suggests a likely role in memory tasks that rely on familiarity discrimination.
In the current study we investigated the relative roles of DA activity within core and medial shell subregions of the NAc in the processing of spatial and nonspatial information. Behavioral testing used spontaneous object recognition (SOR), which exploits animals' natural tendency to explore novel objects or locations and has been widely used to examine the neurobiological underpinnings of mammalian memory (for reviews, see Dere, Huston, & De Souza Silva, 2007; Winters, Saksida, & Bussey, 2008). SOR tests do not require rule learning, extensive training, or reinforcement and hence avoid the effects of such confounds on performance. We used two tests, one requiring object (identity) familiarity discrimination after long and short retention intervals (Experiment 1) and the other, object location discrimination (Experiment 2). In both experiments, we tested animals with focal 6-hydroxydopamine (6-OHDA) lesions to allow an assessment of the relative involvement of DA terminals within accumbal subdivisions in memory for object identity and location.
Method
Subjects
Adult male Wistar rats (Charles River, Margate, United Kingdom) were caged in pairs on a 12:12 hr light/dark cycle with food and water ad libitum. Rats were handled for approximately 10 min per day for 1 week prior to any procedure.
In Experiment 1, 54 rats underwent surgery, while of mean weight 220 g and in the range 200–242 g. Eighteen rats were randomly allocated to each of the core and shell laterality groups, and 18 rats were allocated to the sham condition (9 were sham operated at the core coordinates, and 9 were sham operated at the shell coordinates). One core-lesioned rat died postoperatively.
In Experiment 2, 72 rats, at mean weight 234 g (range 198–270 g) underwent surgery. Twenty-four rats were randomly allocated to each of the core and shell injection groups, and a total of 24 rats were allocated to the sham condition (12 rats were sham operated at the core coordinates, and 12 rats were sham operated at the shell coordinates). One sham-operated rat died postoperatively.
All procedures were carried out in accordance with the United Kingdom (U.K.) Animals Scientific Procedures Act, 1986 (Project License No. PPL 40/3163). The U.K. Act ensures full compliance with the “Principles of Laboratory Animal Care” (National Institutes of Health Publication No. 86–23, revised 1985).
Stereotaxic Infusion of 6-OHDA
Animals received subcutaneous administration of the noradrenalin (NA) reuptake inhibitor desipramine (20 mg/kg) 40 min prior to surgery. Anesthesia was induced by isoflurane (4%) in a N2O/O2 (1:2, vol/vol) mixture and maintained thereafter with isoflurane (1–2%). Stereotaxic surgery was conducted with the incisor bar set at −3.3 mm below the intra-aural line. A craniotomy was performed with a 1-mm hand drill (to make a hole of approximate diameter 1 mm), and the dura was cut to expose the cortex. Rats received bilateral infusions of 6-OHDA or vehicle into either NAc core or medial shell at the following stereotaxic coordinates core: AP +1.6 mm; ML ±1.8 mm; DV −6.8 mm; medial shell AP +1.3 mm; ML ±0.8 mm; DV −6.4 mm; and 7.0 mm (1 infusion at each DV coordinate; Paxinos & Watson, 2005). DV coordinates were taken from dura. Infusions were made via a 31-gauge stainless steel injector attached by polythene tubing to a 1-μL Hamilton syringe. The 6-OHDA hydrobromide (24 mg/mL as salt dissolved in vehicle; Sigma, Surrey, United Kingdom) or vehicle (0.9% saline/ascorbic acid 0.01% weight/volume; wt/vol) was infused manually over 2 min on each side in a volume of 0.5 μL (core) or as 2 infusions of 0.25 μL (medial shell). The injectors were left in situ for 5 min to allow absorption of the bolus and to minimize spread of the toxin. Rimadyl (0.03 mL of 50 mg/mL solution s.c.) provided postoperative analgesia. Animals were allowed a minimum of 7 days recovery before the commencement of behavioral testing.
Quantification of 6-OHDA Lesion
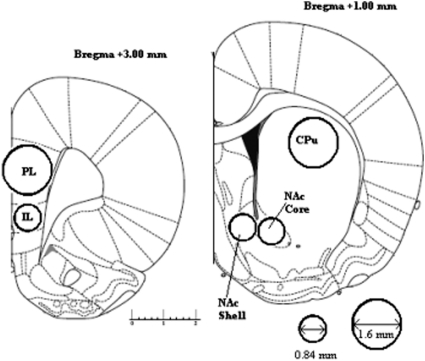
After the completion of behavioral testing, the rats were humanely killed by dislocation of the neck and decapitated. The dissection and micropunch technique used was as described by Peleg-Raibstein et al. (2004; see Figure 1). The brains were removed rapidly and were placed ventral side up in an ice-chilled rat brain matrix (Harvard Apparatus, Kent, United Kingdom). Using ice-chilled razor blades, two 2-mm coronal brain sections were cut. The posterior side of the slices corresponded to approximately +3 mm and +1 mm from Bregma according to the atlas of Paxinos and Watson (2005). The brain samples were then immediately frozen on dry ice and stored at −80 °C. Subsequently, the two 2-mm coronal sections were placed posterior side up onto an ice-chilled plate. From the first section (+3 mm bregma) a 1.6-mm diameter stainless steel micropunch was used to remove samples of tissue from the prelimbic cortex (PL) and a 0.84-mm diameter stainless steel micropunch was used to remove tissue from the infralimbic cortex (IL). From the second section (+1 mm bregma), samples of tissue from core NAc and medial shell NAc and a 1.6-mm diameter stainless steel micropunch was used to remove sample tissue from the caudate-putamen (CPu). In each case, one punch was used per brain hemisphere. Tissue punch samples were stored in 1.5-mL Eppendorf tubes and frozen at −80 °C.
Figure 1. Forebrain regions dissected for postmortem neurochemical analysis. Regions of interest were dissected by pushing micropunch needles of 0.84 or 1.6 mm diameter into the posterior face of the coronal slices, as is indicated. Adapted from “The Rat Brain in Stereotaxic Coordinates, 5e,” by George Paxinos and Charles Watson, Copyright 2005. Reprinted with permission of Elsevier. Numbers indicate distance from bregma in millimeters.
Neurotransmitter levels in the samples were determined by high-pressure liquid chromatography (HPLC) with electrochemical detection. The tissue samples were homogenized in 0.1 M PCA solution by sonication and centrifuged at 17,400 g for 20 min at 4 °C. The supernatant was injected onto the HPLC system. The mobile phase consisted of 50 mM citric acid, 0.1 mM EDTA, 8 mM KCl, 50 mM phosphoric acid, 100 mg/L octanesulfonic acid, and 6% methanol, pH adjusted to 3.85 by the addition of sodium hydroxide. The mobile phase was pumped at a flow rate of 0.2 mL/min by an Alexys LC100 pump connected via an Alexys AS100 autosampler to an Antec Leyden reverse phase analytical column (ALF-215 150 mm × 2.1 mm i.d.) maintained at 35 °C. Neurotransmitter levels were detected using a glassy carbon flow cell (VT-03 Antec) with an Ag/AgCl reference electrode. An external standard consisting of DA, NA, and metabolites in concentrations of 10−7, 0.5 × 10−7, and 10−8 M was injected at a volume of 4 μL for calibration. Samples were injected onto the column at 4-μL volumes, except for PL and IL samples, which were injected at 8 μL.
Results were analyzed using Alexys software data system. Bradford assay was used to adjust for protein content using the pellet remaining after sample centrifugation.
Behavioral Apparatus
All testing was conducted in a rectangular arena that was made of opaque plastic and measured 38 cm × 40 cm. The walls were 54 cm high. An overhead camera was used to record animals' behavior for subsequent analysis.
The stimuli consisted of duplicate copies of objects made of glass, metal, or plastic that varied in shape, color, and size and were too heavy to be displaced by the animal. Pairs of objects were placed in opposite corners of the arena. Objects used included bottles, flasks, and cans. The objects differed markedly and did not appear to share common features. The test box and objects were cleaned with an alcohol-based solution (20% wt/vol) before each trial to remove odor cues. The particular set of objects was counterbalanced across tests, and the placement (left or right of arena) of the novel object (Experiment 1) or displaced object (Experiment 2) was counterbalanced between animals. The familiar object at test was always an identical copy of the object seen at sampling. Time spent exploring each object was defined as directing the nose at the object at a distance of less than 1 cm and actively exploring it (i.e., sniffing and or interacting with the object). Object exploration was not scored if the animal was in contact with but not facing the object or if it sat on the object or used it as a prop to look around or above the object (Dix & Aggleton, 1999; Ennaceur & Delacour, 1988).
Behavioral Testing
Pretraining
Prior to the start of testing, animals received one habituation session. The rats were placed individually into the arena for 10 min.
Experiment 1: Novel object recognition
Animals were taken into the experimental room and placed in the center of the test arena. During the sample phase, animals were allowed to explore two identical copies of the sample object for a period of 10 min. This length of sample phase was selected as control animals' ability to recognize novel objects after a 24-hr retention interval has been shown to be improved by increasing the duration of the sample phase (Albasser, Davies, Futter, & Aggleton, 2009). The total time spent exploring the two identical objects was recorded. A different pair of identical objects was used for each sample phase. After a delay of 24 hr (Experiment 1A) or 5 min (Experiment 1B), which was spent in the home cage with its cage mate, each rat was returned to the arena, which now contained a novel object and an identical copy of the object previously seen during the sampling phase. Each rat was tested once for 3 min. Time spent exploring the familiar and novel object was recorded. For each rat there was a 3-day gap after the test session and the commencement of Experiment 1B.
Experiment 2: Novel location recognition
Four identical objects (glass bottles) were used. During the sample phase, the animals explored two identical objects for 5 min. The total time spent exploring the two identical objects was recorded. After a delay of 10 min, spent in the home cage with respective cage mates, the animals were returned to the arena and allowed to explore two identical copies of the objects sampled earlier. One object was in the identical spatial location as in the sample phase, and the other was now placed in a novel location (adjacent corner to other object rather than opposite corner). These parameters have previously been shown to produce robust object-in-place memory in shams that is sensitive to hippocampal damage (Mumby, Gaskin, Glenn, Schramek, & Lehmann, 2002). Total time spent exploring the object in the familiar and novel locations was recorded.
Interrater Reliability
An independent experimenter unaware of the lesion group and object contingencies rescored 20% of all test phases from the original video footage. The rescored results significantly correlated with the original scores (r = .91, p < .001), indicating robust interrater reliability.
Design and Analysis
The discrimination ratio—the total time spent exploring the novel object divided by the time exploring both objects—was calculated. The behavioral data for each test were analyzed in separate analyses of variance (ANOVAs) with lesion as the between-subjects factor. The alpha level was set as 0.05. Where appropriate, differences between groups were explored with two-tailed independent t tests. In order to establish whether animals' performance at test was above chance, one-sample t tests were performed (with the test value set at 0.5 indicating equivalent exploration of the two objects). In Experiment 2, 6 animals failed to explore the objects at test, and consequently these animals' scores are not included in the analyses. Partial correlations were used to explore the relationship between NAc neurotransmitter (DA and NA) levels in lesioned animals and recognition performance at test. Because any deficit seen at test could potentially be explained by neurotransmitter loss that is selective to one accumbal subregion or alternatively by interactive effects of neurotransmitter depletion in both subregions, we used two-tailed partial correlations. The use of partial correlations tests the relationship between neurotransmitter levels in one accumbal subregion and discrimination performance while controlling for any variability in this relationship attributable to neurotransmitter levels in the adjacent accumbal subregion. This approach has previously been used to dissociate the potential contribution of separate neural systems to object recognition performance (Albasser et al., 2009).
Results
Neurochemical
Quantification of the 6-OHDA lesions by HPLC revealed that 6 animals in Experiment 1 and 5 animals in Experiment 2 showed little evidence of NAc DA denervation (<30% depletion); consequently, these animals were not considered for further behavioral or neurochemical analysis.
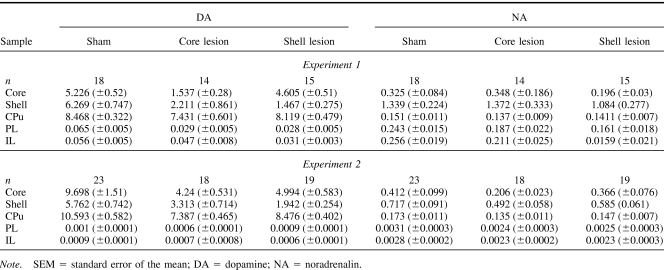
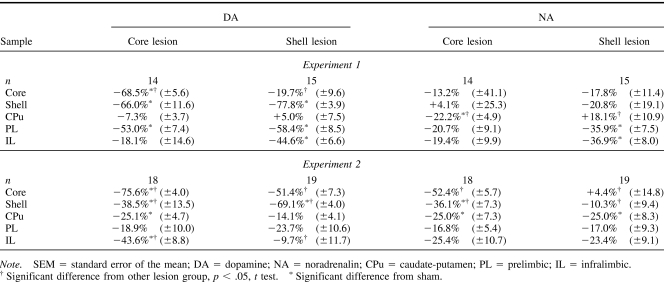
Table 1 displays the levels (pmoles/μg protein) of DA and NA in the five brain regions from which samples were taken as absolute levels, and Table 2 displays the levels of DA and NA as the percentage depletion in relation to sham levels. In both experiments, infusion of 6-OHDA into the core produced significant depletions (68.5% and 75.6%) in DA in relation to core controls but also in the adjacent shell subregion (66% and 38.5%). The shell infusions produced comparable depletions in the target structure (77.8% and 69.1%), but DA levels in the adjacent core were not significantly different from shell control levels. Some changes in DA levels were also found in the three control regions that were selected on the basis of their location (CPu dorsal) and known interconnectivity (IL and PL). There are some inconsistencies in the pattern of catecholaminergic depletion obtained across the two experiments. Compensatory changes may underlie these differences because analysis by HPLC was performed 3 weeks later in Experiment 2 (e.g., Robinson, Mocsary, Camp, & Whishaw, 1994). Critically, however, the animals in the two experiments were tested behaviorally at the same time point postoperatively. Desipramine pretreatment did not fully protect NA because there were effects of the 6-OHDA infusions on NA in the various brain regions sampled. However, the pattern of NA depletion did not match that of the DA depletion.
Table 1. Mean Absolute Levels (±SEM) of DA and NA (pmoles/μg Protein) of Sham (Pooled), Core, and Shell-Lesioned Animals in Core, Shell, Caudate-Putamen (CPu), Prelimbic (PL), and Infralimbic (IL) Cortices.
Table 2. Mean Percentage Difference (±SEM) in DA and NA Levels of Core- and Shell-Lesioned Animals in Comparison With Core and Shell Vehicle-Infused Sham Animals in the Five Brain Regions Assayed.
Behavioral
6-OHDA lesions and novel object recognition with 24 hour delay (Experiment 1A)
Sample
There was no overall effect of lesion on total exploration during the sample phase (F <1); mean total exploration time, in seconds (plus or minus standard error of the mean, SEM): sham = 85.5 (±5.9); core injected = 82.9 (±4.0); and shell injected = 85.9 (±7.2).
Test
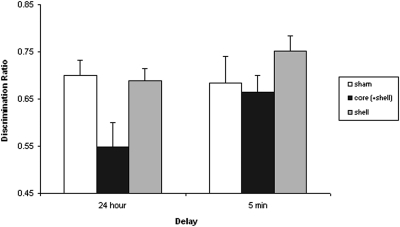
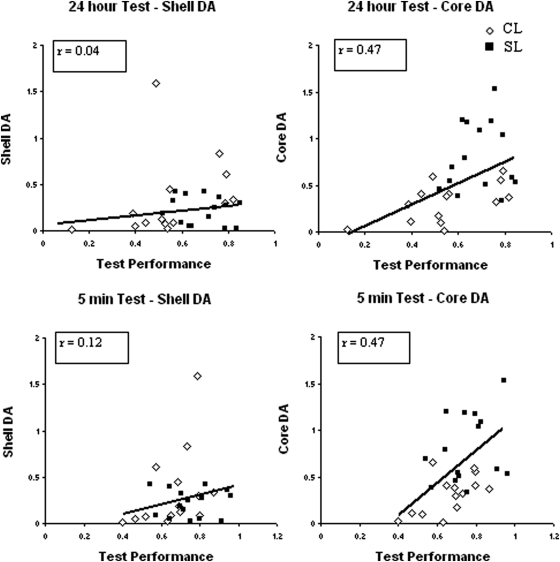
Analysis of object exploration at test also showed no difference in total time spent interacting with the two objects (F < 1): mean total exploration time (in seconds, ±SEM): sham = 46.6 (±3.9); core injected = 41.3 (±4.5); and shell injected = 44.3 (±3.4). However, as is clear from the discrimination ratios displayed in Figure 2, the groups differed markedly in the proportion of time spent exploring the novel and familiar objects. Both shams and shell-injected animals showed a clear preference for the novel object over the familiar object. However, the core-injected animals failed to detect the novel object and explored the familiar and novel object equally. This description of the data was confirmed by an ANOVA, which yielded an effect of lesion, F(2, 44) = 4.86, p < .05. This effect arose because both the shams, t(30) = 2.6, p < .05, and shell-injected animals, t(25) = 2.44, p < .05, had higher discrimination ratios than did the core-injected group. Critically, one-sample t tests confirmed that both sham, t(17) = 6.26, p < .001, and shell-injected groups, t(14) = 6.9, p < .001, readily solved the discrimination at test (scores above 0.5), but performance in the core-injected group did not differ statistically from chance (t < 1). Figure 3 (top half) displays the relationship between DA levels in each accumbal subregion and task performance.
Figure 2. Effect of 6-hydroxydopamine (6-OHDA) lesions to the core and medial shell nucleus accumbens (NAc) on recognition memory for objects after a 24-hr (Experiment 1A) or 5-min (Experiment 1B) retention interval. Mean discrimination ratios (plus standard error of the mean) by lesion group are shown.
Figure 3. Correlation between dopamine (DA) levels in core and medial shell of nucleus accumbens (NAc) and performance of core (plus shell) lesioned (CL) and shell-lesioned (SL) animals on a test of object recognition memory after delays of 24 hr (top half) and 5 min (bottom half). DA levels are presented as a proportion of sham levels. Test performance is the discrimination ratio. The best fit slopes correspond to the Pearson correlations. The partial correlations (controlling for DA levels in the adjacent accumbal region) are presented in the boxes.
Analysis with partial correlations revealed that there was no significant relationship between levels of DA in the shell and the discrimination ratio (r = .04, p = .85). However, as is clear from Figure 3, test performance correlated significantly with DA levels in the core NAc (r = .47, p < .05). There was no relationship between NA levels in either accumbal territory and the ability to recognize the novel object (maximum r = .05, p = .8).
6-OHDA lesions and novel object recognition with 5-min delay (Experiment 1B)
Sample
Total exploration times did not differ by lesion group in the sample phase (F < 1): mean total exploration time (in seconds, ±SEM): sham = 82.5 (±7.0); core injected = 73.0 (±7.8); and shell injected = 73.5 (±6.4).
Test
Similarly, there was no effect of lesion on total amount time spent exploring the objects at test (F < 1): mean total exploration time (in seconds, ±SEM): sham = 38.0 (±4.7); core injected = 36.6 (±6.1); and shell injected = 37.9 (±5.0). In contrast to the 24 delay condition (see Figure 2), there was no statistical evidence of any differences between the lesion groups in task performance after a 5-min delay, F(2, 41) = 1.1, p = .34. The performance of all three lesion groups was statistically different from chance level, minimum t(16) = 3.2, p < .005, indicating that all the animals were able to discriminate the novel from the familiar object with a 5-min delay between sample and test.
Despite the lack of overall impairment on this task, it is clear from the correlational analysis presented in the bottom half of Figure 3 that, as in Experiment 1A, lesioned animals' ability to detect the novel object was significantly related to DA levels in the core NAc. Partial correlations confirmed a significant relationship between DA in core NAc and discrimination performance in the 5-min delay condition (r = .47, p < .05) but not shell NAc (r = .12 p = .54). NA levels in neither the core (r = −0.16, p = .42) nor the shell (r = .11, p = .57) correlated with discrimination performance at test.
6-OHDA lesions and novel location recognition (Experiment 2)
Sample
There were no differences by lesion group in the time spent exploring the two objects in the sample phase (F < 1): mean total exploration time (in seconds, ±SEM): sham = 33.0 (±3.8); core injected = 28.0 (±3.1); and shell injected = 28.8 (±4.2).
Test
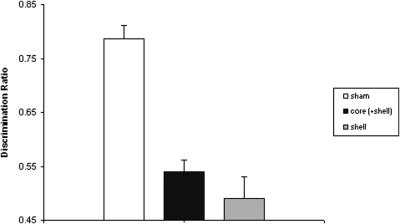
Lesion did not affect total time spent exploring the objects at test (F < 1): mean total exploration time (in seconds, ±SEM): sham = 22.6 (±3.3); core = 19.2 (±1.8); and shell = 19.7 (±2.3). However, as is clear from Figure 4, the groups explored the displaced and nondisplaced objects differentially. An ANOVA revealed an effect of lesion, F(2, 57) = 29.02, p < .001, as both the core-injected group, t(39) = 7.12, p < .001, and the shell-injected group, t(40) = 6.38, p < .001, had lower discrimination ratios than did shams but did not differ from each other, t(35) = 1.04, p = .31. One-sample t tests confirmed that shams readily detected the change in the spatial array of the objects, t(22) = 11.48, p < .001, but performance on the task in the shell-injected animals (t < 1) and core-injected animals, t(17) = 1.74, p = .1, was not different from chance.
Figure 4. Effect of 6-hydroxydopamine (6-OHDA) lesions to the core and medial shell nucleus accumbens (NAc) on novel location recognition memory (Experiment 2). Mean discrimination ratios (plus standard error of the mean) by lesion group are shown.
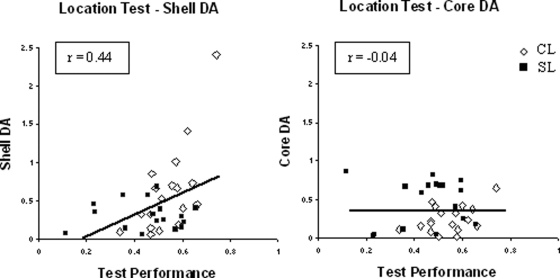
Inspection of Figure 5 shows subregionally specific correlations between performance on the location task and DA levels. Analysis with partial correlations showed that DA levels in the shell subregion correlated with test performance (r = .44, p < .01) but there was no statistical evidence that DA levels in the core subregion were related to the ability of lesioned animals to recognize the displaced object (r = −0.04, p = .803). NA levels in core and shell were unrelated to performance on the location task (maximum r = −0.29, p = .09).
Figure 5. Correlation between dopamine (DA) levels in core and medial shell of nucleus accumbens (NAc) and performance of core (plus shell) lesioned (CL) and shell-lesioned (SL) animals on a test of location recognition memory. DA levels are presented as a proportion of sham levels. Test performance is the discrimination ratio. The best fit slopes correspond to the Pearson correlations. The partial correlations (controlling for DA levels in the adjacent accumbal region) are presented in the boxes.
Discussion
In both experiments, injection at core coordinates depleted DA in shell and core subregions of NAc. The shell lesion was more selective in Experiment 1. However, in Experiment 2, DA in the core was also 50% depleted by the shell lesion. This lack of selectivity (consistent with other reports; see below) means that the lesion group effect on novel object recognition of 6-OHDA injected at core coordinates could in principle rather indicate that this behavior is sensitive to bigger (core plus shell) lesions. Similarly, the equivalent effect of both lesions on the object location variant in Experiment 2 could indicate a role for the shell or reflect an equivalent role for the shell and core in mediating the psychological processes necessary to performance on this task variant.
However, the partial correlational analyses clearly enable us to identify a differential role of core (in object recognition) and shell (in the location recognition variant). The systematic relationship between core DA levels and performance in the object recognition task clearly points to the importance of core DA in mediating the underlying processes necessary to performance on this task. Although in principle the lack of a correlation between shell DA levels and test performance could result from the limited variability in shell DA values as both lesions depleted shell DA, a role for shell per se would seem to be precluded by the absence of any overall effect on object recognition in the shell lesion group. A selective role for core DA in these processes is further supported by the use of partial correlations that explicitly control for any variability due to shell DA levels. Similarly, the systematic relationship revealed by partial correlations between shell but not core DA levels and performance in the object location variant suggests that the underlying processes are mediated by DA in shell rather than core.
Neuroanatomical and Neurochemical Selectivity of the Lesion
Consistent with previous reports, the anatomical selectivity of the 6-OHDA lesions to the core and medial shell was not complete (Boye, Grant, & Clarke, 2001; Sellings & Clarke, 2003; Sellings, McQuade, & Clarke, 2006). Analysis by HPLC revealed differential depletion by infusion site, but the 6-OHDA infusions into the core did significantly deplete DA in the adjacent shell sample. The recent evidence of widespread intra-accumbal connections, with particularly strong projections from the core to the shell, may underlie the pattern of DA denervation that we and others have observed with this kind of lesion (van Dongen et al., 2005). Despite this, core and shell DA levels were only marginally related (Pearsons r = .38), and there was sufficient intersubject variability to allow partial correlational analysis. The pattern of depletion did vary across the two experiments, and this could potentially undermine the validity of direct comparisons between the two experiments. However, the presence of subregion-specific partial correlations between DA levels and performance allows dissociation of the relative contribution of DA function in accumbal subregions to the behavioral tasks under investigation, irrespective of the lesion extent in each experiment. In line with previous reports (e.g., Parkinson et al., 2002; Robbins, Giardini, Jones, Reading, & Sahakian, 1990), secondary changes in DA levels were also observed in the mPFC and CPu. However, the deficits we observed are more likely to be mediated by disrupted NAc DA function. The PL, where significant depletions were seen, is not involved in object recognition (Akirav & Maroun, 2006; Bachevalier & Mishkin, 1986). Similarly, the mPFC has been implicated in spatial temporal order memory but not spatial–object recognition (Hannesson, Vacca, Howland, & Phillips, 2004). There is some evidence of dorsal striatum involvement in spatial memory (De Leonibus et al., 2005), but the level of depletion (maximum 25%) seen in the CPu was unlikely to be sufficient to disrupt task performance in the current experiments. DMI pretreament did afford a degree of neurochemical selectivity, but this protection was not uniform because there were some significant changes in NA. However, these changes did not follow the pattern of DA depletions (see Tables 1 and 2) and are therefore unlikely to account for the behavioral results. Indeed, the correlational analyses confirmed that there was no relationship between NA levels in either accumbal subregion and behavioral performance.
Nonspecific Lesion Effects
The deficits observed are not simply the result of disrupted locomotor activity or exploratory behavior; as in line with previous studies (e.g., Winn & Robbins, 1985), there was no effect of accumbal 6-OHDA lesions on these parameters. The lesioned animals did not differ from shams in the total time spent exploring the objects at either the sample or test phase. There is good evidence that the NAc and mesolimbic DA plays a role in the behavioral response to novelty (Bardo et al., 1996; De Leonibus et al., 2006; Goto & Grace, 2005, 2008; Hooks et al., 1991; Redgrave & Gurney, 2006). For example, entering a novel environment is associated with increased DA efflux in the NAc shell (Rebec et al., 1997). As the familiarity discriminations fundamental to performance on novel object test procedures could be argued to rely to some extent on novelty detection, the deficits seen here could potentially be explained by effects of reduced NAc DA transmission on the processing of novel stimuli rather than recognition memory per se. However, the present study demonstrates effects more specific than those attributable to novelty processing in that there was no effect of DA depletion in either subregion on initial exploratory approach responses to the objects in the sample phases (when all the objects were novel). Moreover, the demonstration that the shell-lesioned animals were unimpaired on the object-familiarity discrimination in Experiment 1 and preferentially explored the novel object at test indicates a role beyond the general processing of novelty. Similarly, because the deficits produced by DA depletion in the core were delay dependent, it is unlikely that these effects are simply due to the behavioral impact of novelty.
Alternatively, it could be argued that the observed deficits result from impaired behavioral flexibility. The NAc has been shown to be important in modulating behavioral flexibility, and lesions to the NAc can produce perseverative responding, impair reversal learning, and reduce behavioral switching (e.g., Haluk & Floresco, 2009). Indeed, in the current study, animals with the largest depletions tended to show a preference for the familiar object over the novel object, which could be indicative of perseverative responding. Although this cannot be completely ruled out as a possible explanation of the results, it is unlikely to be the sole underlying deficit, because a tendency toward perseveration would presumably disrupt performance on all variants of the task, in stark contrast to the actual results obtained.
NAc Core and Object Memory
One-trial object memory was related to DA function in the core, but on average, task performance was only disrupted in the 24-hr delay condition. Because DA function was manipulated prior to sampling and testing, it is possible that several processes were affected: encoding, consolidation, and retrieval. Indeed, the similar relationship between DA levels in core NAc and performance at 5 min and 24 hr might suggest that we cannot draw any definitive conclusions about the role of core DA function in the encoding and consolidation/retrieval of object recognition memory. However, the demonstration that core-lesioned animals only showed a deficit in discriminating a familiar from a novel object after a 24-hr delay but not a 5-min delay is more consistent with a specific role for core DA in consolidation and retrieval processes rather than the encoding of object memory because these animals clearly had on a representation of the previously sampled objects after a 5-min delay. Such a time-dependent lesion effect provides arguably the best evidence that DA depletion in the core does not affect encoding but instead has effects specifically on consolidation and long-term storage or retrieval processes. The equivalent correlation between core DA and performance in the 5-min condition clearly indicates that DA in the core plays a role in the regulation of these processes but the length of the delay between sample and test phases was not sufficient to disrupt performance on average. Future experiments using selective manipulation before and after individual stages of the object recognition procedure will be required to provide a definite answer as to the role of core DA in the modulation of the different memory processes that underpin object recognition memory.
Nevertheless, the current findings are in line with reports that DA and in particular D1 receptors in the NAc core mediate memory consolidation in a variety of behavioral tasks (Dalley et al., 2005; Di Ciano, Cardinal, Cowell, Little, & Everitt, 2001; Managò, Castellano, Oliverio, Mele, & De Leonibus, 2008; Smith-Roe & Kelly, 2000). The demonstration that activation of DA can potentiate or attenuate the responsiveness of NAc neurons to HPC and basolateral amygdala (BLA) evoked firing may underlie these effects (Charara & Grace, 2003; Floresco, Blaha, Yang, & Phillips, 2001a, 2001b). However, with respect to the behavior under investigation here, there is no evidence that the BLA is involved in object recognition, and the literature on the role of the HPC is far from unequivocal (for a review, see Winters et al., 2008). Rather, there is overwhelming evidence that the perirhinal cortex is necessary for object recognition memory (e.g., Brown & Aggleton, 2001; Bussey, Muir, & Aggleton, 1999). Critically, the NAc core and to a lesser extent the lateral shell are preferentially innervated by the perirhinal cortex (Brog et al., 1993; Phillipson & Griffiths, 1985). The current finding of profound deficits in novel object recognition following DA depletion in the core suggests that interactions between the perirhinal cortex and NAc contribute to consolidation of memory processes necessary to support novel object recognition. DA transmission within the core may facilitate this interaction to allow adaptive responding.
NAc Shell and Location Recognition
The finding here that performance in the location recognition task was systematically related to DA levels in NAc shell is consistent with an emerging literature implicating plasticity within the NAc in the processing of spatial information (e.g., Annett et al., 1989; Seamans & Phillips, 1994). Recent work has demonstrated that DA and glutamate receptor activation within NAc is required for the consolidation of spatial memory, suggesting a modulatory role of these neurotransmitter systems in the intracellular mechanisms posited to be involved in long-term plasticity (e.g., Ferretti et al., 2005; Ferretti, Sargolini, Oliverio, Mele, & Roullet, 2007; Mele et al., 2004). The current data suggest that DA activity within the shell may be critical to these processes. Neuroanatomical evidence suggests that the medial shell receives input from the ventral hippocampus (HPC), whereas the core and rostrolateral shell are more innervated by the dorsal HPC (Groenewegen et al., 1999). Although the HPC has been characterized by a functional gradient along the dorsoventral axis, with the dorsal HPC being more important for spatial memory (e.g., Moser, Moser, & Andersen, 1993; Pothuizen, Zhang, Jongen-Relo, Feldon, & Yee, 2004), recent evidence has highlighted a critical role for the intermediate HPC in rapid place learning and its translation into behavioral performance, perhaps via its connections with the medial shell and prefrontal cortex (Bast, 2007; Bast, Wilson, Witter, & Morris, 2009). There is good evidence that performance on spatial tasks depends on functional interaction between the HPC and the NAc (e.g., Floresco & Phillips, 1999; Ito et al., 2008). Consistent with this suggestion, stimulation of hippocampal inputs to the NAc has been shown to increase DA efflux in the NAc (e.g., Floresco et al., 2001b; Legault, Rompre, & Wise, 2000) and in particular within the NAc shell (Peleg-Raibstein & Feldon, 2006). The effects of DA on synaptic activity of medium spiny output neurons are complex and can be either inhibitory or excitatory (e.g., Pennartz, Dolleman-vender Weel, Kitai, & Lopes Da Silva, 1991). However, DA release induced by HPC stimulation has been shown to augment subsequent hippocampal-evoked neural activity of NAc neurons (Floresco, 2007; Floresco et al., 2001b). Consistent with this role of DA in regulating hippocampal inputs into NAc, amphetamine exposure has been shown to selectively enhance hippocampal-dependent place conditioning (Ito & Canseliet, 2010). It follows that attenuated DA activity should disrupt performance on tasks mediated by hippocampal-NAc circuits, and this was the finding in the current experiments.
Conclusion
The current data provide the first evidence that core and shell subregions of the NAc and their DA innervation are involved in different forms of recognition memory. Indeed, the demonstration that DA depletion in the NAc core disrupted performance on the object identity recognition task, whereas DA denervation in the shell selectively impaired performance on the location recognition task, highlights a role for NAc DA beyond the general processing of novelty. This apparent dissociable contribution of DA within core and shell NAc to these two forms of recognition memory may be related to the differential connectivity of these NAc subregions to the HPC and perirhinal cortex. Converging evidence, such as would be provided by the infusion of selective DA agents, will ultimately be necessary for a more definitive conclusion as to the role of DA terminals within these subregions (and if so, which receptor subtypes) in different aspects of novel object processing. Similarly, to our knowledge, the effects of excitotoxic lesions to shell versus core NAc on tests of novel object and novel location recognition memory have yet to be examined. In other behavioral tests, depletion of the DA input to the NAc has been shown to produce very different results to those of excitotoxic lesions (e.g., Joseph et al., 2000; Tai, Cassaday, Feldon, & Rawlins, 1995), and thus anatomically selective excitotoxic lesions will be required to test the generality of the current findings.
Nevertheless, these findings are consistent with the suggestion that the NAc serves as a site of integration of information flow from its divergent corticolimbic inputs and highlight a critical role of DA transmission in gating this afferent input to facilitate adaptive behavioral responses by influencing the pattern of information transmission to motor output areas (Floresco, 2007; Goto & Grace, 2005, 2008). Changes in the balance of these systems may contribute to the memory impairments and cognitive dysfunction seen in schizophrenia and other human psychopathologies (Young, Powell, Risbrough, Marston, & Geyer, 2009).
Acknowledgments
This work was supported by the Wellcome Trust (Ref. No. 082940). We thank Julia Dudley for assistance with behavioral testing.
References
- Akirav I., & Maroun M. (2006). Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cerebral Cortex, 16, 1759–1765. [DOI] [PubMed] [Google Scholar]
- Albasser M. M., Davies M., Futter J. E., & Aggleton J. P. (2009). Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behavioral Neuroscience, 123, 115–124. [DOI] [PubMed] [Google Scholar]
- Annett L. E., McGregor A., & Robbins T. W. (1989). The effects of ibotenic acid lesions of the nucleus accumbens on spatial learning and extinction in the rat. Behavioural Brain Research, 31, 231–242. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., & Mishkin M. (1986). Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behavioural Brain Research, 20, 249–261. [DOI] [PubMed] [Google Scholar]
- Bardo M. T., Donohew R. L., & Harrington N. G. (1996). Psychobiology of novelty seeking and drug seeking behavior. Behavioural Brain Research, 77, 23–43. [DOI] [PubMed] [Google Scholar]
- Bast T. (2007). Toward an integrative perspective on hippocampal function: From the rapid encoding of experience to adaptive behavior. Reviews in Neurosciences, 18, 253–281. [DOI] [PubMed] [Google Scholar]
- Bast T., Wilson I. A., Witter M. P., & Morris R. G. (2009). From rapid place learning to behavioral performance: A key role for the intermediate hippocampus. Public Library of Science Biology, 7, 730–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S. M., Grant R. J., & Clarke P. B. (2001). Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology, 40, 792–805. [DOI] [PubMed] [Google Scholar]
- Brog J. S., Salyapongse A., Deutch A. Y., & Zahm D. S. (1993). The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. Journal of Comparative Neurology, 338, 255–278. [DOI] [PubMed] [Google Scholar]
- Brown M. W., & Aggleton J. P. (2001). Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience, 2, 51–61. [DOI] [PubMed] [Google Scholar]
- Burns L. H., Annett L., Kelley A. E., Everitt B. J., & Robbins T. W. (1996). Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: Implication for limbic–striatal interactions. Behavioral Neuroscience, 110, 60–73. [DOI] [PubMed] [Google Scholar]
- Bussey T. J., Muir J. L., & Aggleton J. P. (1999). Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. Journal of Neuroscience, 19, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A., & Grace A. A. (2003). Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology, 28, 1412–1421. [DOI] [PubMed] [Google Scholar]
- Dalley J. W., Lääne K., Theobald D. E., Armstrong H. C., Corlett P. R., Chudasama Y., & Robbins T. W. (2005). Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America, 102, 6189–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus E., Oliverio A., & Mele A. (2005). A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learning & Memory, 12, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus E., Verheij M. M., Mele A., & Cools A. (2006). Distinct kinds of novelty processing differentially increase extracellular dopamine in different brain regions. European Journal of Neuroscience, 23, 1332–1340. [DOI] [PubMed] [Google Scholar]
- Dere E., Huston J. P., & De Souza Silva M. A. (2007). The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and Biobehavioral Reviews, 31, 673–704. [DOI] [PubMed] [Google Scholar]
- Di Ciano P., Cardinal R. N., Cowell R. A., Little S. J., & Everitt B. J. (2001). Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. Journal of Neuroscience, 21, 9471–9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix S. L., & Aggleton J. P. (1999). Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behavioural Brain Research, 99, 191–200. [DOI] [PubMed] [Google Scholar]
- Ennaceur A., & Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats: 1. Behavioral data. Behavioural Brain Research, 31, 47–59. [DOI] [PubMed] [Google Scholar]
- Ferretti V., Florian C., Costantini V. J., Roullet P., Rinaldi A., De Leonibus E., . . . Mele A. (2005). Co-activation of glutamate and dopamine receptors within the nucleus accumbens is required for spatial memory consolidation in mice. Psychopharmacology, 179, 108–116. [DOI] [PubMed] [Google Scholar]
- Ferretti V., Sargolini F., Oliverio A., Mele A., & Roullet P. (2007). Effects of intra-accumbens NMDA and AMPA receptor antagonists on short-term spatial learning in the Morris water maze task. Behavioural Brain Research, 179, 43–49. [DOI] [PubMed] [Google Scholar]
- Floresco S. B. (2007). Dopaminergic regulation of limbic–striatal interplay. Journal of Psychiatry and Neuroscience, 32, 400–411. [PMC free article] [PubMed] [Google Scholar]
- Floresco S. B., Blaha C. D., Yang C. R., & Phillips A. G. (2001a). Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. Journal of Neuroscience, 21, 6370–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S. B., Blaha C. D., Yang C. R., & Phillips A. G. (2001b). Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: Cellular mechanisms of input selection. Journal of Neuroscience, 21, 2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S. B., & Phillips A. G. (1999). Dopamine and hippocampal input to the nucleus accumbens play an essential role in the search for food in an unpredictable environment. Psychobiology, 27, 277–286. [Google Scholar]
- Goto Y., & Grace A. A. (2005). Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature Neuroscience, 8, 805–812. [DOI] [PubMed] [Google Scholar]
- Goto Y., & Grace A. A. (2008). Limbic and cortical information processing in the nucleus accumbens. Trends in Neurosciences, 31, 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen H. J., Wright C. I., Beijer A. V., & Voorn P. (1999). Convergence and segregation of ventral striatal inputs and outputs. Annals of the New York Academy of Sciences, 877, 49–63. [DOI] [PubMed] [Google Scholar]
- Haluk D. M., & Floresco S. B. (2009). Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology, 34, 2041–2052. [DOI] [PubMed] [Google Scholar]
- Hannesson D. K., Vacca G., Howland J. G., & Phillips A. G. (2004). Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behavioural Brain Research, 153, 273–285. [DOI] [PubMed] [Google Scholar]
- Hooks M. S., Jones G. H., Smith A. D., Neill D. B., & Justice J. B. (1991). Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse, 9, 121–128. [DOI] [PubMed] [Google Scholar]
- Ito R., & Canseliet M. (2010). Amphetamine exposure selectively enhances hippocampus-dependent spatial learning and attenuates amygdala-dependent cue learning. Neuropsychopharmacology, 35, 1440–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R., Robbins T. W., Pennartz C. M., & Everitt B. J. (2008). Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. Journal of Neuroscience, 28, 6950–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M. H., Peters S. L., Moran P. M., Grigoryan G. A., Young A. M. J., & Gray J. A. (2000). Modulation of latent inhibition in the rat by altered dopamine transmission in the nucleus accumbens at the time of conditioning. Neuroscience, 101, 921–930. [DOI] [PubMed] [Google Scholar]
- Legault M., Rompre P. P., & Wise R. A. (2000). Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. Journal of Neuroscience, 20, 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry C. S., & Kelley A. E. (1994). Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions. Psychopharmacology, 116, 65–72. [DOI] [PubMed] [Google Scholar]
- Managò F., Castellano C., Oliverio A., Mele A., & De Leonibus E. (2008). Role of dopamine receptors subtypes, D1-like and D2-like, within the nucleus accumbens subregions, core and shell, on memory consolidation in the one-trial inhibitory avoidance task. Learning & Memory, 16, 46–52. [DOI] [PubMed] [Google Scholar]
- Mele A., Avena M., Roullet P., De Leonibus E., Mandillo S., Sargolini F., . . . Oliverio A. (2004). Nucleus accumbens dopamine receptors in the consolidation of spatial memory. Behavioural Pharmacology, 15, 423–431. [DOI] [PubMed] [Google Scholar]
- Mogenson G. J., Jones D. L., & Yim C. Y. (1980). From motivation to action: Functional interface between the limbic system and the motor system. Progress in Neurobiology, 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Moser E., Moser M. B., & Andersen P. (1993). Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience, 13, 3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby D. G., Gaskin S., Glenn M. J., Schramek T. E., & Lehmann H. (2002). Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learning & Memory, 9, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. A., Dalley J. W., Cardinal R. N., Bamford A., Fehnert B., Lachenal G., . . . Everitt B. J. (2002). Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behavior: Implications for mesoaccumbens dopamine function. Behavioural Brain Research, 137, 149–163. [DOI] [PubMed] [Google Scholar]
- Paxinos G., & Watson C. (2005). The rat brain in stereotaxic coordinates (5th ed.). San Diego, CA: Academic Press. [Google Scholar]
- Peleg-Raibstein D., & Feldon J. (2006). Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology, 51, 947–957. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D., Pezze M. A., Ferger B., Zhang W. N., Murphy C. A., Feldon J., & Bast T. (2004). Activation of dopaminergic neurotransmission in the medial prefrontal cortex by N-methyl-d-aspartate stimulation of the ventral hippocampus in rats. Neuroscience, 132, 219–232. [DOI] [PubMed] [Google Scholar]
- Pennartz C. M., Berke J. D., Graybiel A. M., Ito R., Lansink C. S., van der Meer M., . . . Voorn P. (2009). Corticostriatal interactions during learning, memory processing, and decision making. Journal of Neuroscience, 29, 12,831–12,838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz C. M., Dolleman-vender Weel M. J., Kitai S. T., & Lopes Da Silva F. H. (1991). Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory inputs to the shell region of the rat nucleus accumbens studied in vitro. Journal of Neurophysiology, 67, 1325–1333. [DOI] [PubMed] [Google Scholar]
- Phillipson O. T., & Griffiths A. C. (1985). The topographic order of inputs to nucleus accumbens in the rat. Neuroscience, 16, 275–296. [DOI] [PubMed] [Google Scholar]
- Pothuizen H. H., Zhang W. N., Jongen-Relo A. L., Feldon J., & Yee B. K. (2004). Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: A within-subject, within-task comparison of reference and working spatial memory. European Journal of Neuroscience, 19, 705–712. [DOI] [PubMed] [Google Scholar]
- Rebec G. V., Christensen J. R., Guerra C., & Bardo M. T. (1997). Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Research, 776, 61–67. [DOI] [PubMed] [Google Scholar]
- Redgrave P., & Gurney K. (2006). The short-latency dopamine signal: A role in discovering novel actions? Nature Reviews Neuroscience, 7, 967–975. [DOI] [PubMed] [Google Scholar]
- Robbins T. W., Giardini V., Jones G. H., Reading P., & Sahakian B. J. (1990). Effects of dopamine depletion from the caudate-putamen and nucleus accumbens septi on the acquisition and performance of a conditional discrimination task. Behavioural Brain Research, 38, 243–261. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Mocsary Z., Camp D. M., & Whishaw I. Q. (1994). Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. Journal of Neuroscience, 14, 2687–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F., Roullet P., Oliverio A., & Mele A. (2003). Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behavioural Brain Research, 138, 153–163. [DOI] [PubMed] [Google Scholar]
- Seamans J. K., & Phillips A. G. (1994). Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behavioral Neuroscience, 108, 456–468. [DOI] [PubMed] [Google Scholar]
- Sellings L. H. L., & Clarke P. B. S. (2003). Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. Journal of Neuroscience, 23, 6295–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings L. H., McQuade L. E., & Clarke P. B. (2006). Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. Journal of Pharmacology and Experimental Therapeutics, 317, 1178–1187. [DOI] [PubMed] [Google Scholar]
- Setlow B., & McGaugh J. L. (1999). Differential effects of immediate posttraining sulpride microinfusions into the nucleus accumbens shell and core on Morris waze retention. Psychobiology, 27, 248–255. [Google Scholar]
- Smith-Roe S. L., & Kelley A. E. (2000). Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. Journal of Neuroscience, 20, 7737–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Roe S. L., Sadeghian K., & Kelley A. E. (1999). Spatial learning and performance in the radial arm maze is impaired after N-methyl-D-aspartate (NMDA) receptor blockade in striatal subregions. Behavioral Neuroscience, 113, 703–717. [DOI] [PubMed] [Google Scholar]
- Tai C.-T., Cassaday H. J., Feldon J., & Rawlins J. N. P. (1995). Both electrolytic and excitotoxic lesions of nucleus accumbens disrupt latent inhibition of learning in rats. Neurobiology of Learning and Memory, 64, 36–48. [DOI] [PubMed] [Google Scholar]
- van Dongen Y. C., Deniau J.-M., Pennartz C. M. A., Galis-de Graaf Y., Voorn P., Thierry A.-M., & Groenewegen H. J. (2005). Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience, 136, 1049–1071. [DOI] [PubMed] [Google Scholar]
- Voorn P., Jorritsma-Byham B., Van Dijk C., & Buijs R. M. (1986). The dopaminergic innervation of the ventral striatum in the rat: A light- and electron-microscopical study with antibodies against dopamine. Journal of Comparative Neurology, 251, 84–99. [DOI] [PubMed] [Google Scholar]
- Winn P., & Robbins T. W. (1985). Comparative effects of infusions of 6-hydroxydopamine into nucleus accumbens and anterolateral hypothalamus induced by 6-hydroxydopamine on the response to dopamine agonists, body weight, locomotor activity and measures of exploration in the rat. Neuropharmacology, 24, 25–31. [DOI] [PubMed] [Google Scholar]
- Winters B. D., Saksida L. M., & Bussey T. J. (2008). Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience and Biobehavioral Reviews, 32, 1055–1070. [DOI] [PubMed] [Google Scholar]
- Young J. W., Powell S. B., Risbrough V., Marston H. M., & Geyer M. A. (2009). Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacology & Therapeutics, 122, 150–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm D. S., & Brog J. S. (1992). On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience, 50, 751–767. [DOI] [PubMed] [Google Scholar]