Abstract
In three experiments, rats were required to find a submerged platform by referring to the boundaries of a circular swimming pool. In the first experiment, rats with lesions of the hippocampus were impaired at finding the hidden platform, lending support for the proposal that learning to find a goal that is a certain direction and distance from a boundary is dependent upon the hippocampus. Experiments 2 and 3 offered preliminary tests to see if such boundary learning occurred incidentally, irrespective of the presence of a reliable landmark. In contrast to this proposal, a landmark hanging above the platform successfully restricted learning about the location of the platform with respect to the boundary of the arena. The discussion explores the capacity of the hippocampus to encode boundary information, as well as interprets the behavioral results on the basis of an associative learning framework.
Keywords: hippocampus, boundaries, landmarks, overshadowing
Evidence is accumulating to suggest that information about the boundaries of an environment is important for spatial behavior. One rather indirect source of evidence for this claim is provided by the results from electrophysiology studies that show that the firing of cells in different regions of the brain is controlled by an animal's position relative to a boundary. Solstad, Boccara, Kropff, Moser, and Moser (2008) identified such cells, which they refer to as boundary cells, in the entorhinal cortex. O'Keefe and Burgess (1996) demonstrated that the locations at which place cells in CA1 and CA3 of the hippocampus tend to fire are specified by the various distances to environmental boundaries in different allocentric directions around that location. Finally, Lever, Burton, Jeewajee, O'Keefe, and Burgess (2009) revealed the existence of what they called boundary vector cells in the dorsal subiculum, where the firing of these cells was controlled by the distance and allocentric direction of the rat from a particular boundary.
More direct evidence that boundaries play an important role in spatial behavior can be found in a study by Doeller and Burgess (2008, see also Doeller, King, & Burgess, 2008). Human participants were required to find hidden objects in a virtual environment displayed on a computer monitor. The environment was a circular arena surrounded by a boundary wall, with landmarks serving as orientation cues lying beyond the wall. Participants in what we shall refer to as the boundary condition were required to move through the circular environment and collect a variety of hidden objects before being tested by being asked to replace the objects in their original locations. Since there were no landmarks within the circular environment, the position of the objects was defined relative to the boundary wall. Participants were able to replace the objects with a considerable degree of accuracy, which led Doeller and Burgess to conclude that the boundary was important for identifying where the objects were located.
Additional findings by Doeller and Burgess (2008) and Doeller et al. (2008) prompted them to make two claims about spatial learning based on boundaries. First, they argued that learning about boundaries progresses incidentally, which means that it is not affected by the presence of other cues that also indicate where the goal can be found. The justification for this claim was based on the results from a second condition in the experiment—the boundary + landmark condition—which was the same as the condition just described, except that landmarks within the arena could also be used to indicate where the goal was located. Subsequent tests without the landmarks revealed that the boundary cues in this condition acquired as much control over searching for the hidden object as for the boundary condition. In other words, there was no evidence that the landmarks overshadowed learning based on the boundary cues.
In order to demonstrate that such incidental learning is restricted to boundary cues, the experiment included a third, landmark condition in which the hidden objects could be found only by reference to landmarks. Subsequent test trials revealed that the landmarks gained less control over searching for the platform in the boundary + landmark condition than the landmark condition. In these circumstances, therefore, there was evidence that the boundary cues overshadowed learning about the landmarks, when both types of cue could be used to find the goal. On the basis of these findings, Doeller and Burgess argued that learning about the significance of landmarks for finding a hidden goal is different to that for boundary cues because it is not incidental but governed instead by an error-correction principle (e.g., Rescorla & Wagner, 1972).
The second claim by Doeller and Burgess (2008) and Doeller et al. (2008) is perhaps not surprising in light of some of the electrophysiological results mentioned above. They suggested that learning about the significance of a boundary for finding a goal depends upon activity in the hippocampus. The justification for this claim was based on the results of an experiment by Doeller et al. (2008). Participants were required to find objects in a virtual environment by reference either to the boundary of the environment or to landmarks within the environment. Using functional MRI, it was discovered that activation of the right posterior hippocampus accompanied searching for an object when reference was made to the boundary cues. Of less direct relevance to the present article, it was also found that using the landmarks to find an object enhanced activity in the right dorsal striatum.
To our knowledge, the idea that spatial learning based on boundary cues is both incidental and dependent upon the hippocampus is original. It is also of some theoretical importance if a complete understanding of the conditions under which spatial learning takes place is to be achieved and if the neural mechanisms of such learning are to be understood. In view of the importance of the proposals of Doeller and Burgess (2008) and Doeller et al. (2008), the overall purpose of the reported article is to determine whether their conclusions, which were derived from research with humans, also apply to animals. Using rats, Experiment 1 examined whether learning about the position of a goal relative to boundary cues involves the hippocampus; Experiments 2 and 3 were preliminary investigations to determine whether such learning is incidental.
Experiment 1
At least two different sets of findings suggest that the hippocampus might be important for spatial learning based on boundaries in animals, but neither of them provides unequivocal support for this claim. One set of experiments has involved training rats to find a submerged platform located in one corner of a rectangular swimming pool. By surrounding the pool with curtains and rotating its principal axis randomly from trial to trial, it is possible to ensure that only cues provided by the walls of the rectangle are used to find the platform. When normal rats are trained in this apparatus, then after a number of sessions they typically head directly for the corner containing the platform or for the diagonally opposite corner. Since both corners share the same geometric properties, it is impossible to distinguish between them, and they are referred to as the correct corners. This preference for the correct over the other, incorrect corners demonstrates that the animals can use information provided by the boundary of the environment to find the submerged platform (e.g., Pearce, Good, Jones, & McGregor, 2004; Wall, Botley, Black, & Shettleworth, 2004).
Of particular relevance to the present discussion is the finding that rats with hippocampal lesions are severely impaired on this task and find it difficult to discriminate between the correct and the incorrect corners (Jones, Pearce, Davies, Good, & McGregor, 2007; McGregor, Hayward, Pearce, & Good, 2004; Pearce et al., 2004). Since the walls of the rectangular pool create the boundary of the environment, it might be argued that the hippocampal lesions were effective by making it difficult for subjects to navigate with reference to boundary cues. There is, however, at least one other possible explanation for the effect of the lesions. Pearce et al. (2004) suggested that the failure to discriminate between the correct and incorrect corners occurred because the lesions made it difficult to tell the difference between the long and short walls of the training environment (see also Pearce, 2009). A measure of support for this proposal can be found in an experiment by Jones et al. (2007) in which rats were required to find a submerged platform positioned in the acute-angled corner of a kite-shape pool. Here, rats do not need to find the platform by making a judgment based on the relative lengths of the sides of the pool, all that is necessary is to search for the only acute-angled corner in the environment. If the hippocampus allows rats to discriminate between long and short walls, then lesions to this region should not disrupt performance on this problem, which was the outcome that was observed. Thus, although the effects of the lesions of the hippocampus reported by Pearce et al. (2004) are consistent with the proposal that this region is important for navigation based on boundaries, there is an alternative explanation for the outcome of this study, and there is at least one result showing that such lesions do not necessarily disrupt navigation based on cues provided by the boundary of an environment.
The second set of findings that suggests the hippocampus is important for learning about boundaries comes from the finding that hippocampal lesions make it difficult to locate a submerged platform in a Morris pool (e.g., Morris, Garrud, Rawlins, & O'Keefe, 1982). It is possible that to locate the platform in these experiments, rats learn to search at a certain distance from the boundary, in a direction and orientation that is determined by the landmarks outside the pool (Hamilton, Akers, Weisend, & Sutherland, 2007). If this is correct, then it could be argued that the hippocampal lesions were effective by making it difficult for subjects to use the boundary to indentify where the platform was located. However, the strategy just described is not the only means by which the platform could be located. An alternative would be to identify the position of the platform with reference to the compass bearing of two or more landmarks outside the pool, as perceived from the goal, and make no use of the boundary at all (e.g., Cartwright & Collett, 1983). In view of this possibility, it would be unwise to regard the demonstrations of impaired performance in a Morris pool as evidence that the hippocampus is important for learning about boundaries. This region may, instead, play a role in the calculation of compass bearings.
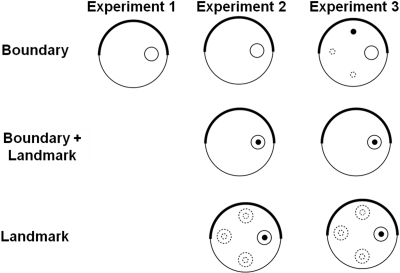
A plan of the apparatus for Experiment 1 is shown in Figure 1. Rats were placed in a circular pool and required to find a submerged platform that was located at a fixed distance from the boundary. In order to provide orientation cues, which could be used to identify precisely the location of the platform, the pool was enclosed by two curtains, one of which was black and surrounded half of the pool and one of which was white and surrounded the other half of the pool. The platform was located on the diameter created by the points where the two curtains met but nearer to one of these points than the other. It was thus necessary to identify the position of the platform by referring to its distance from the boundary together with its direction with respect to the cues created by the joins between the black and white curtains.
Figure 1. Schematic representations of the training arenas used for the groups in Experiments 1–3. The thick black line encompassing half of the large circle represents the black curtain, while the thin black line represents the white curtain. The small white circle represents the submerged platform and the small black circle represents the landmark. Dotted lines indicate possible positions of the platform and/or landmark across the four trials per session.
There were two groups in the experiment: a hippocampal group with bilateral lesions of the hippocampus and a control group with sham lesions. If lesions of the hippocampus are effective because they make it difficult to discriminate between long and short walls, then it is hard to see how they would disrupt performance on the present task. Likewise, if these lesions are effective because they affect the ability of an animal to calculate compass bearings, then damage to the hippocampus should again not have an impact on the proposed task. On the other hand, if the lesions make it difficult to identify locations with reference to the boundary, then the hippocampal group should experience more difficulty than the control group with finding the platform.
Method
Subjects
Twenty-four, male, hooded, Lister rats (Rattus norvegicus), obtained from Harlan Olac (Bicester, Oxfordshire, England) and weighing between 300–350 g at the start of the experiment, were used. All rats were previously used in an appetitive conditioning experiment but had no experience in a swimming pool.
Apparatus
A white, circular pool measuring 2 m in diameter and 0.6 m deep was used. The pool was mounted on a platform 0.6 m from the floor in the middle of the room (4 m × 4 m × 2.3 m). The pool was filled with water to a depth of 27 cm and was maintained at a temperature of 25 °C (±2 °C). To make the water opaque, 0.5 L of white opacifer E308 (Roehm and Haas, U.K., Ltd., Dewsbury) was used. The water was changed daily.
A white circular ceiling, measuring 2 m in diameter, was suspended 1.75 m above the floor of the pool. In the center of the ceiling was a hole measuring 30 cm in diameter in which a video camera with a wide-angled lens was situated. The lens of the camera was 25 cm above the hole and was connected to a video monitor and computer equipment in an adjacent room. During tests, the rats' movements were analyzed using Watermaze software (Morris & Spooner, 1990). The pool was illuminated by eight 45-W lights that were located in the circular ceiling above the pool. The lights were 22.5 cm in diameter and were equidistant from each other in a 1.6 m diameter circle whose center was coincident with the center of the circular ceiling. A platform measuring 10 cm in diameter and mounted on a column was used during all training trials. The surface of the platform had a series of concentric ridges. For all trials, the base of the column rested on the bottom of the pool and the platform surface was 2 cm below the surface of the water. Two different colored curtains surrounded the pool during training trials. A white curtain was drawn around the whole pool for all training trials. A black curtain could be attached just inside the white curtain via Velcro and surrounded half of the pool at any given time. Each curtain was attached to the edge of the circular ceiling, was 1.5 m high, and fell 25 cm below the edge of the pool. The training room was additionally lit by two 1.53-m strip lights connected end to end on each of the East and West walls. These lights ran parallel with the floor and were situated 75 cm above the floor. There was a door (1.75 m × 2 m) in the center of the South wall.
A beacon could be attached to the platform 2.5 cm from its edge. The beacon consisted of a plastic rod painted with alternating black and white hoops. A white disk, 3 cm in diameter and 0.5 cm thick, was attached to the top of the beacon.
Surgery
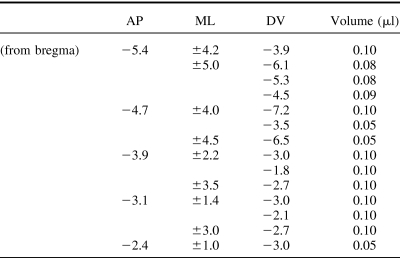
There were two groups of lesioned rats in Experiment 1: Sham (n = 12) and Hippocampal (n = 12). Following anesthesia using an isoflurane-oxygen mix, all rats were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) and the bone above the region to be lesioned was removed. Rats in the hippocampal group were infused with ibotenic acid (Biosearch Technologies, San Rafael, CA; dissolved in phosphate-buffered saline (pH 7.4) to provide a solution with a concentration of 63 mM). The neurotoxin was administered through a 2-μl Hamilton syringe held with a microinjector (Kopf Instruments, Model 5000). Table 1 depicts the coordinates and volume of infusions for rats in the hippocampal group. For this group, a total of 14 infusions per hemisphere was made with an infusion rate of 0.10 μl/min and diffusion time of 2 min. Rats in the sham group received identical treatment with the exception that the dura was perforated with a 25-gauge Microlance3 needle (Becton Dickinson, Drogheda, Ireland), but no fluid was infused into the brain. A minimum of 14 days postoperative recovery was allowed before testing began.
Table 1. Stereotaxic Coordinates and Volume of Ibotenic Acid for Lesions of the Hippocampus.
Procedure
The training consisted of two stages. For the three sessions of Stage 1, all rats were required to swim to the submerged platform with the beacon attached to it. Each session contained four trials. Rats were brought into a room adjacent to the test room in groups of six in a light-tight box. They remained in this box between trials. Each rat was carried from the box to the pool and was released facing the wall of the pool at one of four randomly determined release sites (NE, NW, SW, and SE) with the stipulation that each release point was used once during a session. During a trial, the rat was required to swim to the submerged platform. Each trial lasted a maximum of 60 s. If the rat did not find the platform within 60 s, the experimenter guided it to the platform. After climbing on the platform, the rat remained there for 20 s before being lifted from the pool, dried, and returned to its holding container. The intertrial interval for each rat was approximately 5 min. Between each trial, the experimenter rotated the black curtain 90°, 180°, or 270° clockwise. Four possible orientations of the black curtain were used. Two where the black curtain was drawn from North to South, and two where the curtain was drawn from East to West. Each orientation of the black curtain was used once during a session. The center of the platform was always situated 50 cm from the edge of the pool, on a notional line between the two points where the black and white curtains met. For six rats, the platform was 50 cm from the line created by the white curtain being to the left of the black curtain, as viewed from the pool, and for the remaining rats, the platform was 50 cm from the line created by the white curtain being to the right of the black curtain.
For the 12 sessions of the second stage, the beacon was removed from the platform, but the remaining details of the training were the same as for Stage 1. A record was also taken of the path followed by the rat from the point of release to the platform. The fourth trial of the final session of the experiment was a test trial with the platform removed from the pool but with the black and white curtains in place. Rats were released from the center of the pool and allowed to swim for 60 s.
Throughout the experiment, except for the test trial, a record was taken of the latencies to find the platform for each rat. For the purpose of analyzing the results from the test trials, two circular search zones with a diameter of 30 cm were used. The center of the correct zone was coincident with the center of where the platform had previously been located. The center of the other, incorrect zone was also on the line between the two joins between the curtains, but 1 m away from the correct zone and thus 50 cm from the far boundary of the pool. The amount of time spent in the correct and incorrect zones was recorded.
Histology
Following behavioral testing, all rats received a lethal overdose of sodium pentobarbitone (Euthatal) and were transcardially perfused, first with 0.9% saline and then with 10.0% formal-saline. Their brains were extracted, postfixed for 24 hrs and then transferred to phosphate-buffered (0.1M) 30.0% sucrose solution in which they remained for a further 24hrs. Subsequently all brains were frozen in a −20 °C cryostat and sectioned coronally. The 40 μm sections were collected on gelatin-coated slides, left to dry at room temperature over 24 hrs, and then stained with cresyl violet. The sections were examined under a microscope and histological borders and nomenclature for the hippocampal lesions were verified with reference to the boundaries defined by Paxinos and Watson (1996).
Results
Histology results
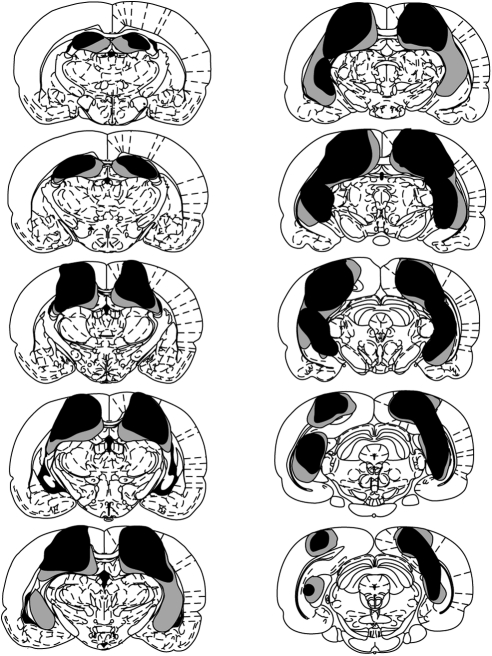
Figure 2 depicts a series of coronal sections (adapted from Paxinos & Watson, 1998) showing the maximum and minimum extent of hippocampal damage for Experiment 1. The nomenclature used to describe the extent of hippocampal cell loss was taken from Swanson (1992). Histological analyses revealed that in two rats from the hippocampal group over 80% of the hippocampus was spared including most of CA1-3 and the dentate gyrus. Only parts of the dorsal subiculum and the cortex overlaying the hippocampus showed damage. Therefore, those rats were excluded from the behavioral analyses. In the remaining 10 rats, damage to the hippocampus was more extensive in the dorsal than the ventral parts. CA1-3 and the lateral parts of the dentate gyrus, including the granular, polymorph, and molecular layers, were damaged in all rats. In nine rats, the medial blade of the dentate gyrus was spared. The fimbria was damaged in all rats. In more ventral parts of the hippocampus, all rats showed sparing of the granular, polymorph, and molecular layers of the lateral blade of the dentate gyrus, as well as the most ventral part of the CA1 and CA3, including the stratum oriens, the deep and superficial pyramidal layers, and the stratum radium. This ventral sparing reached the ventral part of the CA2. In eight rats, the ventral CA2 was spared and in the remaining two rats the posterior parts of the CA2 were damaged. The ventral subiculum was spared in eight rats.
Figure 2. Coronal sections taken throughout the dorsoventral extent of the brain depicting the extent of the damage in the rats with hippocampal lesions (Experiment 1). The largest and smallest lesions are depicted in gray and black, respectively. The sections are posterior to and at specific distances (in mm) from Bregma (top to bottom, left then right: 2.12, 2.80, 3.30, 3.80, 4.30, 4.80, 5.30, 5.80, 6.30, 6.80). Adapted from “The Rat Brain in Stereotaxic Coordinates.
At anterior sections, no damage extended ventrally to the dorsal hippocampus, leaving the mediodorsal and laterodorsal thalamic nuclei intact. All rats sustained some limited damage dorsal to the hippocampus, including the deep layers of the primary somatosensory area, the parietal region of the posterior association area, and at posterior sections parts of the primary and rostrolateral visual areas were damaged. Very limited damage to the dysgranular retrosplenial cortex was seen in seven rats. It is important to note that the damage seen to these cortical areas adjacent to the hippocampus was not correlated with the behavioral effects of interest. In fact, the two rats excluded from the analyses showed performance comparable to that of the shams, yet they suffered damage to the cortex overlaying the hippocampus at least to the same extent if not more than the hippocampally lesioned rats.
Behavioral results
A Type I error rate of 0.05 was adopted for the statistical tests in this article. Figure 3 shows the group mean latencies to find the platform for each of the 18 sessions of the experiment. During the first three sessions, when a landmark was attached to the platform, the performance of both groups improved considerably as training progressed. The latencies for the hippocampal group were marginally longer than those for the sham control group. The removal of the landmark for the remaining 12 sessions of the experiment disrupted the performance of both groups and, throughout this stage, the escape latencies for the hippocampal group were considerably longer than for the sham group. These observations were supported by the results of a two-way ANOVA based on individual mean escape latencies for the first three sessions combined and for the remaining 12 sessions combined. There was a significant effect of stage, F(1, 20) = 43.98, of lesion, F(1, 20) = 26.21, and a significant Stage × Lesion interaction, F(1, 20) = 6.20. The interaction is important because it indicates that the removal of the landmark from the platform, thus forcing subjects to rely solely on the boundary cues to find the platform, resulted in a more severe disruption in performance for the hippocampal than the sham group.
Figure 3. Mean escape latencies for the hippocampal and sham groups of Experiment 1. On Sessions 1–3, all rats received a beacon attached to a submerged platform. For the remaining sessions, the beacon was removed.
The implication of the foregoing analysis, that the hippocampal group found it harder to navigate with reference to the boundary cues than the sham group, was supported by the results from the test trial conducted on Session 18. Figure 4 shows for both groups the mean proportion of time spent in the correct and incorrect zones throughout the 60-s trial. Both groups spent a greater proportion of time in the correct rather than incorrect zone; however, the hippocampal group spent less time in the correct zone than the sham group. A 2 × 2 (Group X Zone) ANOVA was conducted and revealed a significant main effect of zone, F(1, 20) = 35.62, and a significant Group X Zone interaction, F(1, 20) = 5.41. The main effect of group was not significant, F(1, 20) = 2.37. A simple effects analysis was conducted on the interaction and revealed that both groups spent significantly more time in the correct rather than incorrect zone, Fs (1, 20) > 6.63. It is more important to note that the percentage of time spent in the correct zone was significantly less in the hippocampal group than the sham group, F(1, 40) = 7.65. Time spent in the incorrect zone did not differ between the groups, F < 1.
Figure 4. Mean (+SEM) percentage of time spent in the correct and incorrect zones for the hippocampal and sham groups during the boundary test of Experiment 1.
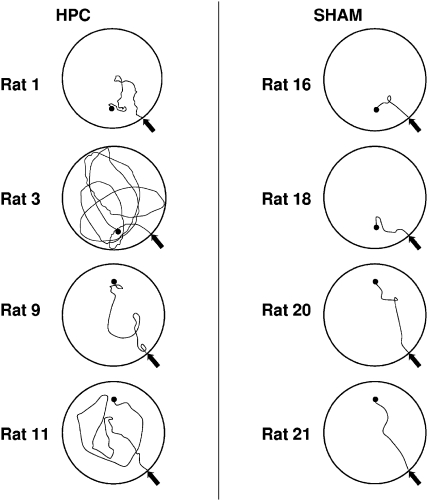
The impairment of the hippocampal group that was found during the test mirrors that of the paths taken to find the platform during training. Figure 5 shows the swim paths for selected rats from the hippocampal and sham groups, taken from Trial 3 of Session 15. As can be seen, the sham group headed directly for the platform while the paths taken by the hippocampal group were considerably more circuitous. A t test conducted on the path lengths for this trial revealed that the hippocampal group ( = 591.20 cm, SEM = 150.39 cm) took significantly longer paths to find the platform than the shams ( = 212.95 cm, SEM = 15.03 cm), t(20) = 2.75.
Figure 5. Representative paths taken by rats in the hippocampal and sham groups during the last trial of Session 15. The filled black circle represents the location of the platform, and the arrow represents the animals' release point.
Discussion
The sustained impairment in the performance of the hippocampal group throughout the 12 sessions of training once the beacon was removed and during the test trial indicates that the lesions made it difficult to find the platform by referring to cues that were some distance from it. Since the only cues that could be used for finding the platform were provided by the boundary of the pool, the results demonstrate the importance of the hippocampus for navigation with reference to boundary cues. The outcome of the experiment is thus in keeping with the proposals of Doeller and Burgess (2008). The results also have implications for the suggestion by Pearce et al. (2004) that hippocampal lesions impair performance in a rectangular pool because they make it difficult to tell the difference between the long and the short walls. Although this conclusion may still be correct, the possibility must be considered that the lesions were effective in the study by Pearce et al. (2004) because they had a more general effect of making it difficult to refer to a boundary for the purposes of navigation. It is also possible that the disruptive effect of hippocampal lesions on navigation in a Morris pool occurs because they make it difficult for animals to use the boundary as an important cue for finding the submerged platform.
Experiment 2
The results from Experiment 1 imply that learning about the position of a hidden goal relative to a boundary depends upon activity within the hippocampus. The purpose of Experiments 2 and 3 is to determine if this learning takes place incidentally, as maintained by Doeller and Burgess (2008), or if it takes place according to well established error-correction rules of learning (e.g., Rescorla & Wagner, 1972). The experiments were based on the design of Experiment 1, but a landmark was suspended from the ceiling directly above the platform for some of the groups. If the proposals of Doeller and Burgess (2008) are correct, then the boundary cues will overshadow learning about the landmark, but the landmark will not overshadow learning about the boundary cues.
There were three groups in the experiment. A boundary group was trained in the same manner as rats in Experiment 1, except that the preliminary training with a beacon attached to the platform was omitted. This group, therefore, had to rely on the boundary to find the platform. A boundary + landmark group was trained in the same way, but a landmark was suspended over the platform on every trial. This group could use both the boundary and the landmark to find the platform. Finally, the landmark group was trained in a similar manner to the other two groups, except that the position of the platform was changed from trial to trial and a landmark was always suspended above the platform. This group could use only the landmark to find the platform.
Toward the end of training, two test trials were conducted in the absence of the platform. One test took place in the circular pool in the presence of the black and white curtain. The rats were released from the center of the pool, and the time spent searching in the place where the platform had previously been located was recorded. If landmarks do not overshadow boundary cues, then both the boundary and the boundary + landmark groups should spend a similar amount of time searching in the correct region of the pool. Moreover the amount of time spent by these groups in this region of the pool should be greater than for the landmark group.
The second test was conducted in the circular pool surrounded entirely by a white curtain and with the landmark suspended from the ceiling. On this occasion, the proposals of Doeller and Burgess (2008) lead to the prediction that the landmark group will spend more time searching for the platform beneath the landmark than the boundary + landmark group. This prediction follows because the boundary cues were expected to overshadow learning about the landmark in the latter group. Finally, as the test trial will be the first occasion that the boundary group is exposed to the landmark, it would not be expected to spend much time searching beneath it for the platform.
Method
Subjects and apparatus
The 36 rats were from the same stock of approximately the same age as the rats for Experiment 1. They were experimentally naïve prior to the start of the experiment. The apparatus was the same as for Experiment 1, but with the addition of a landmark. The landmark was a Premierlight® LED, waterproof, camping lantern (white, 60 Lumens) that was inverted for the purpose of the experiment. The lantern, which was always switched on, was 18 cm high (including base) and was cylindrical with a diameter of 14 cm. The landmark was suspended from various points above the pool by a wire attached to the base of the lantern. The lowest point of the landmark was 33 cm above the surface of the water at a distance of 50 cm from the edge of the pool.
Procedure
At the start of the experiment, the rats were assigned at random to each of the three groups in equal numbers. The method of training was the same as for Experiment 1, with the following exceptions. There were 14 sessions of training. For the boundary and the boundary + landmark group, the platform was located 50 cm away from the point at which the two curtains met, with the black curtain to the left of the white curtain. The platform for the landmark group was located in the position just described for one trial of each session. For the remaining trials, it was again located 50 cm from the boundary wall, but it was rotated by 90, 180, and 270o from the position just described, with reference to the center of the pool. The sequence in which these positions were occupied varied randomly from session to session. The landmark was suspended directly above the platform for every training trial with the landmark and the landmark + boundary group.
The fourth trial of Session 12 was a boundary test with the platform removed from the pool, but with the black and white curtains surrounding the pool in the normal manner. The fourth trial of Session 14 was a landmark test. The platform was again removed from the pool, but this time the curtain surrounding the pool was entirely white, and the landmark was suspended above the water 50 cm from the edge of the pool. Rats were released from the center of the pool for both tests and allowed to swim for 60 s. The manner of recording the results from the training trials and from the boundary test was the same as for Experiment 1. For the landmark test, the time spent swimming in a 30-cm diameter search zone, with its center directly below the center of the landmark, was recorded.
Results and Discussion
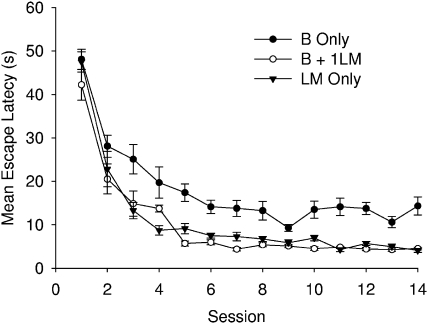
Figure 6 shows the mean escape latencies to reach the platform across the 14 sessions of the experiment for the three groups. All groups took less time to find the platform as training progressed, but the mean escape latencies were consistently longer for the boundary group than the other two groups. To examine the asymptotic performance of the groups, a one-way ANOVA was conducted with individual mean escape latencies combined across the last three sessions before the first test trial (i.e., Sessions 10, 11, and 12). This analysis revealed a significant difference among the groups, F(2, 33) = 39.20. Newman–Keuls' tests revealed that latencies for the boundary group were significantly longer than for either of the other two groups, which did not differ. The results from the boundary group suggest that the absence of the landmark made it difficult to pin point the exact location of the platform by reference solely to the boundary cues.
Figure 6. Mean escape latencies for the B only, B + 1LM, and LM only groups of Experiment 2.
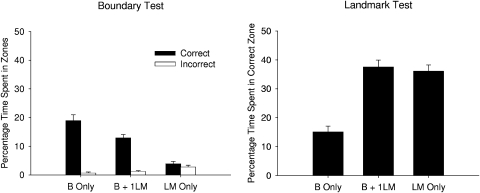
The left-hand panel of Figure 7 shows the percentage of time spent in the correct and incorrect zones for the Session-12 boundary test trial. The boundary and the boundary + landmark groups, but not the landmark group, spent more time in the correct rather than incorrect zone. In addition, the boundary group spent more time in the correct zone than the boundary + landmark group. A 3 × 2 (Group X Zone) ANOVA revealed significant main effects of group, F(2, 33) = 23.21, zone, F(1, 33) = 130.94, and a significant Group X Zone interaction, F(2, 33) = 30.26. A simple main effects analysis of the interaction revealed that the boundary and the boundary + landmark groups spent a greater proportion of time in the correct rather than incorrect zone, Fs (1, 33) > 55.89, while the landmark group did not express a preference for either zone, F < 1. There was also a significant difference among the groups in the proportion of time spent in the correct zone, F(2, 66) = 53.42, but not in the incorrect zone, F(2, 66) = 1.08. Newman–Keuls tests revealed that the boundary group spent more time in the correct zone than the boundary + landmark group, which spent more time in the correct zone than the landmark group.
Figure 7. Mean (+SEM) percentage of time spent in the correct and incorrect zones during the boundary test (left-hand panel) and landmark test (right-hand panel) for the three groups of Experiment 2.
The right-hand panel of Figure 7 shows the time spent in the correct zone for the landmark test trial conducted in Session 14 for each group. The boundary group spent the least amount of time in the correct zone, compared the other two groups, which spent approximately the same proportion of time in the correct zone. A one-way ANOVA was conducted and revealed a significant difference among the groups, F(2, 33) = 32.15. Newman–Keuls' tests revealed that the boundary group differed from the remaining groups, which did not differ from each other.
The results from both tests failed to confirm the predictions derived from the proposals of Doeller and Burgess (2008). On the one hand, it was anticipated that the landmark would not overshadow learning about the boundary, which led to the prediction that the boundary + landmark group and the boundary group should have performed similarly during the boundary test. In fact this test revealed evidence of overshadowing because the boundary + landmark group spent significantly less time in the correct search zone than the boundary group. On the other hand, it was anticipated that the boundary would overshadow learning about the landmark, which leads to the prediction that the boundary + landmark group will spend less time than the landmark group searching beneath the landmark during the test with this cue. On this occasion, however, there was no difference between the two groups.
A possible explanation for the outcome of the boundary test can be based on generalization decrement. The boundary test for both the boundary and the boundary + landmark groups took place in the absence of the landmark. However, this landmark had been present throughout the training trials for the boundary + landmark group but not the boundary group. It is thus conceivable that both groups learned to the same degree about the position of the platform relative to the boundary cues, but the generalization decrement consequent upon the removal of the landmark weakened responding during the test trial in the boundary + landmark group. The purpose of the next experiment was, in part, to test this explanation for the results of Experiment 2 and, in part, to confirm their reliability.
Experiment 3
The experiment included three groups that were given the same names and treated in much the same manner as the three groups from the previous experiment. The main difference between the experiments concerned the treatment for the boundary group. This group was again required to find the platform with reference to the boundary cues but, in contrast to Experiment 2, the landmark was present on every training trial, although it was never located above the platform. Moreover, for all three groups, the landmark was removed from the apparatus for the boundary test. Given that both the boundary and the boundary + landmark group experienced a similar change from the conditions of training to those of testing, it would now be difficult to attribute any difference in the performance of the two groups during the boundary test to generalization decrement.
Method
Subjects and apparatus
The 30 naïve rats were from the same stock and housed in the same manner as for Experiment 1. Ten rats were assigned at random to each of the three groups at the start of the experiment. The apparatus was identical to that of the previous experiment.
Procedure
There were 14 sessions of training. For half the rats in the boundary and the boundary + landmark groups, the platform was located in the same position as for their namesakes of Experiment 2. For the remaining rats in these groups, the platform was in the diametrically opposite position, so that it was again 50 cm from a point where the two curtains met but this time with the black curtain to the right of the white curtain. The remaining details for the three groups were the same as for the three groups of Experiment 2, except that for the boundary group the landmark was suspended from the ceiling in randomly selected locations with the constraint that it was never directly above the platform. The fourth trial of Sessions 12 and 14 was a boundary and landmark test carried out in the same manner as for Experiment 2.
Results and Discussion
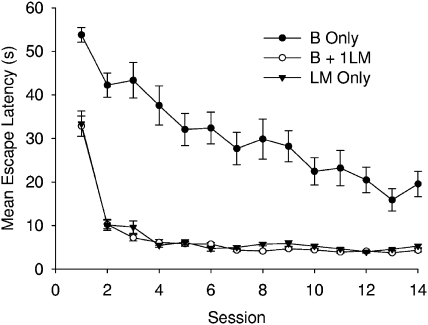
Figure 8 shows the mean escape latencies for the three groups across the 14 sessions of training. The boundary group was slower at finding the platform throughout the experiment than the other two groups, which performed similarly to each other. A two-way ANOVA was conducted on the individual mean latencies combined across the last three sessions before the first test trial (Sessions 10, 11, and 12) and revealed a significant difference among the groups, F(2, 27) = 37.92. Newman–Keuls' tests revealed that the boundary group took significantly more time to find the platform than the boundary + landmark and the landmark groups, which did not differ.
Figure 8. Mean escape latencies for the B only, B + 1LM, and LM only groups of Experiment 3.
The left-hand panel of Figure 9 shows the mean percentage of time spent in the correct and incorrect zones for the boundary test for the three groups. Both the boundary and the boundary + landmark groups spent more time in the correct zone than the incorrect zone, while the landmark group showed no preference for either zone. In addition, the boundary group spent a greater proportion of time in the correct zone compared to the boundary + landmark group. A 3 × 2 (Group X Zone) ANOVA was conducted and revealed a significant main effect of group, F(2, 27) = 5.66, zone, F(1, 27) = 27.02, and a significant Group X Zone interaction, F(2, 27) = 13.72. A simple effects analysis was carried out on the interaction and revealed that the boundary and the boundary + landmark groups preferred the correct zone over the incorrect zone, F(1, 27) > 5.66, whereas the landmark group showed no preference for either zone, F(1, 27) < 1. There was a significant difference among the groups for the amount of time spent in the correct zone, F(2, 54) = 18.32, but not the incorrect zone, F(2, 54) = 2.27. Comparison using the Newman–Keuls' procedure revealed that the boundary group spent significantly more time in the correct zone than either of the other two groups and that the boundary + landmark group spent significantly more time in this zone than the landmark group.
Figure 9. Mean (+SEM) percentage of time spent in the correct and incorrect zones during the boundary test (left-hand panel) and landmark test (right-hand panel) for the three groups of Experiment 3.
The right-hand panel of Figure 9 shows the time spent in the correct zone, situated directly under the landmark, during the landmark test on Session 14. Both the landmark and the boundary + landmark groups exhibited a strong tendency to search in the correct zone compared to the boundary group, which spent little time in the correct zone. A one-way ANOVA revealed a significant difference among the groups, F(2, 27) = 65.38. Subsequent comparisons, using the Newman–Keuls' procedure, revealed that the boundary group spent significantly less time in the test zone than either of the other two groups, which themselves did not differ.
Despite the difference in procedure, the results from three groups replicate the findings from their counterparts in Experiment 2. The landmark again overshadowed learning about the boundary cue in the boundary + landmark group and, on this occasion, it is hard to explain the effect in terms differences in generalization decrement between this group and the boundary group. There was also, again, no indication that the presence of the boundary cues affected learning about the significance of the landmark in the boundary + landmark group.
General Discussion
The experiments were conducted in order to test the proposal by Doeller and Burgess (2008, see also Doeller et al., 2008) that learning about the significance of a boundary for finding a goal depends upon the hippocampus and takes place incidentally. The results from Experiment 1 supported the first of these proposals by showing that the ability of rats to search at a certain distance from a boundary and in a certain direction is disrupted by lesions to the hippocampus. The results from the remaining experiments, however, failed to support the second of these proposals by showing that a landmark near a goal can overshadow learning about the position of the goal relative to the boundary. The following discussion will explore, in turn, the implications of each of these findings.
In Experiment 1, rats were required to find a platform that was 50 cm from the boundary wall, at a point that lay on the line between the diagonally opposite vertical edges of the black curtain. Although it seems reasonable to conclude that the hippocampal lesions were effective by disrupting learning about the boundaries, a number of questions are raised by this conclusion. Did the lesions, for example, make it difficult to judge the distance of the platform from the boundary, or did they make it difficult to judge the direction in which to search, relative to the boundary? Alternatively, do the lesions impair any type of navigation based on the information provided by the boundary? Doeller and Burgess (2008) do not offer any hints concerning the answers to these questions, and it is not possible to address them by referring to the results from Experiment 1. Further experiments are therefore needed if the role that the hippocampus plays in navigation with respect to boundaries is to be fully understood. It is worth noting that Pearce, Roberts, and Good (1998) found that hippocampal lesions did not impair the ability of rats to find a submerged platform that was at a fixed direction and distance from a landmark within a Morris pool, even when the landmark moved from trial to trial. It thus seems unlikely that the effect reported in Experiment 1 was a consequence of rats being unable to make judgments about direction and distance. Instead, it may well have been the fact that the position of the platform was defined relative to the boundary that was responsible for the outcome of the experiment.
The results from Experiments 2 and 3 demonstrate that when the platform could be found by reference to both the boundary and a landmark, then the latter overshadowed the former. On the basis of the results by Doeller and Burgess (2008), this outcome is surprising because they concluded that, at least for humans, learning about boundary cues progresses incidentally. Perhaps, therefore, the conclusions of Doeller and Burgess (2008) do not extend to nonhuman animals like rats. Having said that, it must be acknowledged that overshadowing need not necessarily be a consequence of cues competing for a limited pool of associative strength, as dictated by error-correction theories of learning (Horne & Pearce, 2009). To return to Experiments 2 and 3, the boundary + landmark group may have devoted so much attention to the landmark during the search for the platform that it failed to notice its position with reference to the boundary, and it was for this reason that learning about the boundary was impaired. In view of this possibility, it would be unreasonable to conclude too forcefully that learning about boundary cues does not take place incidentally in animals.
In order to reduce the likelihood that a landmark would disrupt learning about a boundary by distracting attention away from it, Doeller and Burgess (2008) placed the landmark some distance from their hidden goal. However, pilot experiments have revealed that in the sort of environment in which the present experiments were conducted landmarks located away from the submerged platform fail to gain control over behavior. It was for this reason that we decided to locate the landmark directly above the goal. Despite this aspect of the design, it is noteworthy that Experiments 2 and 3 both revealed that the boundary cues gained a measure of control over searching for the platform. These cues were thus not ignored completely, and if learning takes place about them incidentally, with sufficient training, overshadowing should not have been observed.
One result that may not appear to fit comfortably with our proposal that learning about landmarks and boundaries is governed by the same error-correction principle is the failure of the boundary to overshadow the landmark in Experiments 2 and 3. One possibility is that the failure of the boundary to restrict learning about the landmark may indicate that the boundary acquired zero associative strength. If this is the case, then the control by the boundary in the boundary + landmark group must have resulted from a form of incidental learning that does not compete with the landmark. A more likely explanation for this outcome can be based on the assumption that the salience of the boundary was substantially less than of the landmark. Error-correction theories of learning would then predict that the overshadowing of the weak cue on the strong cue will be negligible (e.g., Mackintosh, 1976). The poor performance of the boundary group during training and testing in the last two experiments lends support to the assumption that the salience of the boundary was low.
The present experiments constitute the first attempt to assess whether the claims made by Doeller and Burgess (2008, see also Doeller et al., 2008) concerning spatial learning about boundaries in humans apply to animals—in particular, rats. We have provided support for their proposal that learning about boundaries depends upon the hippocampus. We have not provided support for their claim that this learning progresses incidentally. It remains to be seen whether this lack of support occurred because the landmark distracted attention away from the boundary cues or whether it disrupted learning about these cues for reasons envisaged by error-correction theories of learning.
References
- Cartwright B. A., & Collett T. S. (1983). Landmark learning in bees. Journal of Comparative Physiology A, 151, 521–543. [DOI] [PubMed] [Google Scholar]
- Doeller C. F., & Burgess N. (2008). Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proceedings of the National Academy of Sciences, USA, 105, 5909–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller C. F., King J. A., & Burgess N. (2008). Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences, USA, 105, 5915–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D. A., Akers K. G., Weisend M. P., & Sutherland R. J. (2007). How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. Journal of Experimental Psychology: Animal Behavior Processes, 33, 100–114. [DOI] [PubMed] [Google Scholar]
- Horne M. R., & Pearce J. M. (2009). A landmark blocks searching for a hidden platform in an environment with a distinctive shape after extended pretraining. Learning and Behavior, 37, 167–178. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Pearce J. M., Davies V. J., Good M. A., & McGregor A. (2007). Impaired processing of local geometric features during navigation in a water maze following hippocampal lesions in rats. Behavioral Neuroscience, 121, 1258–1271. [DOI] [PubMed] [Google Scholar]
- Lever C., Burton S., Jeewajee A., O'Keefe J., & Burgess N. (2009). Boundary vector cells in the subiculum of the hippocampal formation. Journal of Neuroscience, 29, 9771–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh N. J. (1976). Overshadowing and stimulus intensity. Animal Learning & Behavior, 4, 186–192. [DOI] [PubMed] [Google Scholar]
- McGregor A., Hayward A. J., Pearce J. M., & Good M. A. (2004). Hippocampal lesions disrupt navigation based on the shape of the environment. Behavioral Neuroscience, 118, 1011–1021. [DOI] [PubMed] [Google Scholar]
- Morris R. G. M., Garrud P., Rawlins J. N. P., & O'Keefe J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683. [DOI] [PubMed] [Google Scholar]
- Morris R. G. M., & Spooner R. I. W. (1990). Watermaze software [Computer software]. Edinburgh, Scotland: Watermaze Software. [Google Scholar]
- O'Keefe J., & Burgess N. (1996). Geometric determinants of the place fields of hippocampal neurons. Nature, 381, 425–428. [DOI] [PubMed] [Google Scholar]
- Paxinos G., & Watson C. (1996). The rat brain in stereotaxic coordinate (3rd ed.). New York, NY: Academic Press. [Google Scholar]
- Pearce J. M. (2009). The 36th Sir Frederick Bartlett lecture: An associative analysis of spatial learning. Quarterly Journal of Experimental Psychology, 62, 1665–1684. [DOI] [PubMed] [Google Scholar]
- Pearce J. M., Good M. A., Jones P. M., & McGregor A. (2004). Transfer of spatial behavior between different environments: Implications for theories of spatial learning and for the role of the hippocampus in spatial learning. Journal of Experimental Psychology: Animal Behavior Processes, 30, 135–147. [DOI] [PubMed] [Google Scholar]
- Pearce J. M., Roberts A. D. L., & Good M. (1998). Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature, 396, 75–77. [DOI] [PubMed] [Google Scholar]
- Rescorla R. A., & Wagner A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Black A. H. & Prokasy W. F. (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York, NY: Appleton-Century-Crofts. [Google Scholar]
- Solstad T., Boccara C. N., Kropff E., Moser M., & Moser E. I. (2008). Representation of geometric borders in the entorhinal cortex. Science, 322, 1865–1868. [DOI] [PubMed] [Google Scholar]
- Swanson L. W. (1992). Brain maps: Structure of the rat brain. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- Wall P. L., Botly L. C. P., Black C. K., & Shettleworth S. J. (2004). The geometric module in the rat: Independence of shape and feature learning in a food finding task. Learning & Behavior, 32, 289–298. [DOI] [PubMed] [Google Scholar]