Abstract
Objective:
To test the hypothesis that platelet-rich fibrin glue (PR-FG) can be used clinically as a scaffold to deliver autologous culture-expanded bone marrow mesenchymal stem cells (BM-MSCs) for cartilage repair and to report clinical results 1 y after implantation of MSCs PR-FG.
Patients and Methods:
Autologous BM-MSCs were culture expanded, placed on PR-FG intraoperatively, and then transplanted into 5 full-thickness cartilage defects of femoral condyles of 5 patients and covered with an autologous periosteal flap. Patients were evaluated clinically at 6 and 12 mo by the Lysholm and Revised Hospital for Special Surgery Knee (RHSSK) scores and radiographically by x-rays and magnetic resonance imaging (MRI) at the same time points. Repair tissue in 2 patients was rated arthroscopically after 12 mo using the International Cartilage Repair Society (ICRS) Arthroscopic Score.
Study Design:
Case series; level of evidence 4.
Results:
All patients’ symptoms improved over the follow-up period of 12 mo. Average Lysholm and RHSSK scores for all patients showed statistically significant improvement at 6 and 12 mo postoperatively (P < 0.05). There was no statistically significant difference between the 6 and 12 mo postoperative clinical scores (P = 0.18). ICRS arthroscopic scores were 8/12 and 11/12 (nearly normal) for the 2 patients who consented to arthroscopy. MRI of 3 patients at 12 mo postoperatively revealed complete defect fill and complete surface congruity with native cartilage, whereas that of 2 patients showed incomplete congruity.
Conclusion:
Autologous BM-MSC transplantation on PR-FG as a cell scaffold may be an effective approach to promote the repair of articular cartilage defects of the knee in human patients.
Keywords: mesenchymal stem cells, platelet-rich plasma, fibrin glue, cartilage, repair
Introduction
Human articular cartilage has limited repair potential.1 Marrow-stimulation procedures as microfracture for cartilage repair rely on the formation of a primitive mesenchymal blood clot that forms fibrous tissue with variable outcome. Limitations of osteochondral grafts include limited donor site, morbidity, questionable cell viability, and fibrocartilage formation in between osteochondral plugs.2,3 Cell-based strategies have explored chondrocytes and mesenchymal stem cells (MSCs) with extensive basic science and preclinical studies.4-6 However, clinical strategies have focused on autologous chondrocyte implantation, yet the concept itself is not ideal; it is a 2-step procedure with donor site morbidity, the quantity of harvested chondrocytes is limited, and chondrocytes dedifferentiate into fibroblasts when cultured ex vivo. Because of these limitations, the focus in cartilage repair is shifting toward bone marrow mesenchymal stem cells (BM-MSCs) and biodegradable scaffolds with or without growth factors. BM-MSCs are available in larger quantities, are easier to isolate and expand, and demonstrate strong chondrogenic potential.7
Platelet-rich preparation such as platelet-rich plasma (PRP) and platelet-rich fibrin glue (PR-FG) is a novel biological scaffold that has not been widely tested by itself as an MSC carrier in clinical chondrogenesis. It is nonimmunogenic, bioabsorbable, sterile, easily prepared, and set intraoperatively. The α-secretory granules of platelets contain transforming growth factor (TGF)-β1 and insulin growth factor-1 (IGF-1), which stimulate cartilage regeneration.8-11 It is expected that the biological effect of multiple growth factors on tissue regeneration is greater than that of a single growth factor. PR-FG has also been shown to provide sustained release and protection against proteolytic degradation of endogenous growth factors.12,13 Because the average baseline blood platelet count in an individual is 200,000 ± 75,000/µL, a platelet concentrate count of 1,000,000/µL (5-fold) has been commonly described for therapeutic platelet-rich preparations.14 Concentrations of growth factors in PR-FG are found to be in supraphysiological levels (5- to 27-fold, according to different processing methods).15 The concentrations of TGF-β1 and IGF-1 in PR-FG are in the range of 90 to 400 ng/mL and 50 to 200 ng/mL, respectively.16-18
This pilot study was conducted for the following aims: first, to test the hypothesis that PR-FG can be used clinically as a scaffold to deliver cultured MSCs for cartilage repair. The 2nd aim is to report on the clinical results 1 y after implantation of MSCs-PR-FG.
Patients and Methods
The study included 5 patients: 4 men and 1 woman (range = 21-37 y; mean = 25.4 y). Inclusion criteria were a full-thickness cartilage lesion in weightbearing areas of the femoral condyle (Outerbridge grade III or IV), size 3 to 12 cm2, disabling symptoms not responding to conservative treatment, age between 18 and 50 y, and patients agreeing to comply with strict rehabilitation. Exclusion criteria were complex ligamentous knee instability, kissing lesions, axial malalignment (>10° varus/valgus) assessed clinically, osteochondral neoplasia, inflammatory joint disease, immune suppression, and symptomatic vascular/neurologic disorder of the lower extremities. All of the patients provided written informed consent according to regulations and after approval of the Ethical Committee and were operated on by the same surgeon (the 1st author). Patient details are provided in Table 1.
Table 1.
Characteristics of the 5 Patients with Cartilage Defects Treated with Bone Marrow Mesenchymal Stem Cells Transplanted in Platelet-Rich Fibrin Glue
| Case | Gender | Age | Disease | Site of lesion | Size of lesion (cm × cm) | Side | No. of previous operations | Associated surgery | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 24 | Degenerative OA due to neglected OCD | MFC | 3.5 × 3 | Rt | 1 | NA | 16 |
| 2 | F | 21 | OCD | LFC | 2 × 1.5 | Rt | 1 | NA | 16 |
| 3 | M | 24 | OCD | LFC | 1.8 × 2.1 | Lt | 1 | NA | 15 |
| 4 | M | 21 | Traumatic | LFC | 2 × 3.2 | Rt | 1 | NA | 12 |
| 5 | M | 37 | Traumatic + ACL tear | MFC | 2.2 × 2.4 | Lt | 2 | ACLR + MF | 12 |
| Mean = 25.4 | Mean = 5.8 cm2 | Mean = 14.2 |
Note: OCD = osteochondritis dissecans; OA = osteoarthritis; MFC = medial femoral condyle; LFC = lateral femoral condyle; Rt = right; Lt = left; NA = non applicable; ACL = anterior cruciate ligament; ACLR = anterior cruciate ligament reconstruction; MF = microfracture.
For clinical evaluation, we used 2 scores collected by an independent observer: the Lysholm score (subjective) and the Revised Hospital for Special Surgery Knee Score (subjective and objective). In addition, plain x-rays (AP, lateral ± tunnel views) and magnetic resonance images (MRIs; T1 weighted, T2-fat-suppressed weighted, proton density imaging) of the involved knee were performed using a 1.5T MRI machine (Philips, Amsterdam, the Netherlands) and evaluated by an independent senior musculoskeletal radiologist. The scores, x-rays, and MRIs were taken preoperatively and 6 and 12 mo postoperatively. Second-look arthroscopy was performed after 12 mo on 2 patients who consented to the procedure. The repair tissue was rated according to the International Cartilage Repair Society (ICRS) Arthroscopic Grading System. None of the patients consented to an arthroscopic biopsy.
Bone marrow (20 mL) was aspirated from the iliac crest under aseptic standard operative procedures and placed in heparinized tubes. Cell cultures were performed at the Molecular Biology and Tissue Engineering Unit at the same university. Briefly, nucleated cells were isolated with a density gradient (Ficoll-Paque; GE HealthCare, Waukesha, WI) and resuspended in culture medium (Delbecco’s Modified Eagle’s Medium) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (10,000 µg/mL). FBS used in this study was purchased from reliable countries without bovine virus traceability, where it is furthermore subjected to serial filtration and terminal sterilization with gamma irradiation and followed by bacterial and viral testing using validated procedures to ensure pathogen freedom. Cells were incubated at 37 °C in 5% CO2 for ~14 d. Culture medium was changed every 2 to 3 d. When cells reached 80% to 90% confluence, cultures were washed twice with phosphate-buffered saline (PBS), and the cells were trypsinized with 0.25% Trypsin in 1 mM EDTA for 5 min at 37 °C. After centrifugation, cells were resuspended in medium and subcultured for ~10 d, reaching an average count of 15 × 106. MSCs in culture were characterized by their adhesiveness and fusiform shape, by flow cytometry for MSC surface markers (CD34−, CD45−, and CD73+) and for CD29 gene expression by reverse transcriptase polymerase chain reaction.19-22 On the day of implantation, cells were trypsinized, collected, and resuspended in 1 mL PBS and transferred to the operating room in sterile tubes. Potential immunogenicity from FBS proteins was furthermore minimized by repeated copious irrigation of the MSC pellet with PBS before final resuspension and transfer to the operating room.
Platelet concentrate was prepared in the hospital blood bank (El-Shabrawishi Hospital Blood Bank, Cairo, Egypt) according to standardized protocols previously reported elsewhere.23 Briefly, using a cell saver centrifuge system, 500 mL volume of blood from healthy donors was collected into 70 mL of citrate-phosphate-dextrose anticoagulant. This was delivered into the 125-mL disposable bowl at 60 mL/min, with the centrifuge spinning at 2039g until the red blood cells reached 1.25 cm from the collar of the bowl. The centrifuge speed was then reduced to 368g, and a blood flow rate of 60 mL/min was used to continuously collect the PRP, from which 1 mL of platelet-rich concentrate was used with FG to form PR-FG. This method has been shown to yield a platelet count of 7.7 ± 1.1 × 108/mL.23
In a 2nd-stage implantation procedure, under a tourniquet, the knee joint was exposed via medial or lateral parapatellar arthrotomy. The defect was excised to a stable rim, and sclerotic bone was curetted. Multiple 3- to 4-mm-deep perforations in the subchondral bone were performed using a 1.2 Kirschner wire at 3- to 4-mm intervals. An autologous periosteal flap was harvested from the anteromedial ipsilateral proximal tibia and tailored to the defect size. The flap was sutured to surrounding cartilage with the cambium layer facing toward the subchondral bone using absorbable 4-0 sutures, leaving the 12 o’clock position open for introduction of the BM-MSCs/PR-FG mixture. The periosteal flap was additionally secured by FG. Allogenous PR-FG was supplied as 1 mL platelet concentrate, 1 mL fibrinogen, and 1 mL thrombin in separate syringes from the aforementioned blood bank. BM-MSCs were mixed with the PR-FG, and the mixture was left to gel and then introduced through the 12 o’clock position and subsequently sutured and sealed by FG (Fig. 1). The inoculum density of BM-MSCs at the time of implantation was 2 × 106 cells/cm2. The surgical wound was closed in layers. One drain without suction was placed intra-articularly.24-26
Figure 1.

Autologous mesenchymal stem cell (MSCs) platelet-rich fibrin glue (PR-FG) implantation. (a) Cartilage defect curetted to a peripheral stable rim of cartilage and subchondral bone drilled. (b) Periosteal flap harvested from the proximal tibia. (c) Defect covered with periosteal flap, sutured, and then sealed with fibrin glue. (d) MSCs mixed with PR-FG intraoperatively to be introduced through the 12 o’clock position of the defect.
Continuous passive motion was initiated on the 2nd day following surgery. Weightbearing was restricted, whereas passive, active-assisted, and active range-of-motion exercises were initiated toward a full range equal to the contralateral knee. After 4 to 6 wk, partial weightbearing was initiated as tolerated. By 8 wk, full weightbearing, unlimited gym work (except treadmill jogging) was permitted. Light jogging was started at 5 mo.24-26
Statistical analyses were done using Statistical Package for Social Studies (SPSS) Software, version 11 for Windows. The clinical scores were shown as the mean ± standard deviation at 3 time points: preoperatively and 6 and 12 mo postoperatively. Data were analyzed statistically by Friedman’s test and the Wilcoxon signed-rank test. P values of less than 0.05 were accepted as significant.
Results
None of the cell cultures had bacterial or fungal infection, nor were there any perioperative complications in any of the patients. The clinical scores of the 5 patients are summarized in Table 2.
Table 2.
Details of the Clinical Scores of the 5 Patients Preoperatively, 6 and 12 mo Postoperatively, as Well as the Arthroscopic Scores of the 2 Patients Who Underwent Follow-up Arthroscopy at 1 Y
| Lysholm score | Revised hospital for special surgery knee score | International Cartilage Repair Society arthroscopic score | |||||
|---|---|---|---|---|---|---|---|
| Case | Preop | 6 mo postop | 12 mo postop | Preop | 6 mo postop | 12 mo postop | 12 mo postop |
| 1 | 20 | 76 | 76 | 31 | 69 | 69 | |
| 2 | 45 | 66 | 76 | 47 | 75 | 79 | 8 |
| 3 | 53 | 95 | 95 | 71 | 90 | 90 | |
| 4 | 50 | 92 | 92 | 62 | 93 | 93 | 11 |
| 5 | 38 | 79 | 91 | 58 | 68 | 88 | |
| Mean ± SD | 41.2 ± 13.14 | 81.6* ± 11.93 (P = 0.042) |
86.0* ± 9.25 (P = 0.042) |
53.8 ± 15.39 | 79.0* ± 11.77 (P = 0.043) |
83.8* ± 9.78 (P = 0.043) |
|
Statistically significant difference.
Compared with preoperative status, all patients experienced marked improvement in symptoms and knee function, allowing them to return to normal daily activities. All 4 patients without preexisting radiographic signs of advanced degenerative changes returned to their previous sporting levels. Clinical scores 6 mo and 12 mo postoperative were statistically different from preoperative scores (P < 0.05). The difference between the 6- and 12-mo clinical scores did not reach statistical significance (P = 0.18; Fig. 2).
Figure 2.
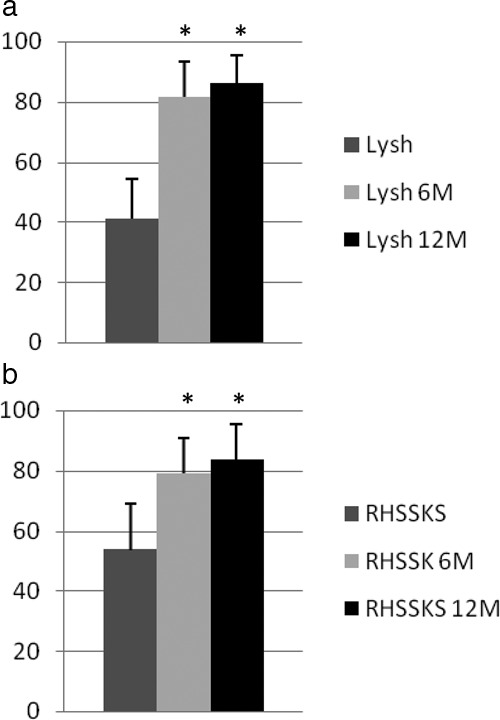
Mean clinical scores: preoperative (dark gray column), 6 mo postoperative (light gray), and 12 mo postoperative (black). (a) Lysholm (Lysh) scores 6 mo (Lysh 6M) and 12 mo (Lysh 12M) postoperative were statistically different (*) from preoperative scores (P < 0.05). The mean 6 and 12 mo postoperative Lysholm scores improved from 41.2 ± 13.14 preoperatively to 81.6 ± 11.93 at 6 mo (P = 0.042) and 86.0 ± 9.25 at 12 mo (P = 0.042). (b) Revised Hospital for Special Surgery Knee (RHSSK) scores 6 mo (RHSSK 6M) and 12 mo (RHSSK 12M) postoperative were also statistically different (*) from preoperative scores (P < 0.05). The mean 6 and 12 mo postoperative RHSSK scores improved from 53.8 ± 15.39 to 79.0 ± 11.77 at 6 mo (P = 0.043) and 83.8 ± 9.78 at 12 mo (P = 0.043). No statistically significant difference was found between scores at 6 and 12 mo postoperative (P = .18).
Radiologically, patient 1 had more advanced degenerative changes at the time of surgery (Fig. 3) than the other 4 patients, and his preoperative scores were also initially lower. Six- and 12-mo follow-up MRI of the operated knee showed partial defect fill, complete incongruity of the repair tissue with native cartilage, and lower signal intensity in relation to the surrounding subchondral bone. The MRI findings coincide with this patient’s relatively lower postoperative scores. X-rays of the patient at 6 and 12 mo showed no evidence of filling of the bony element of the defect nor restoration of the congruity of the femoral condyle. However, the patient reported significant relief of symptoms and was able to return to his laborious job after being incapacitated because of knee pain before surgery.
Figure 3.
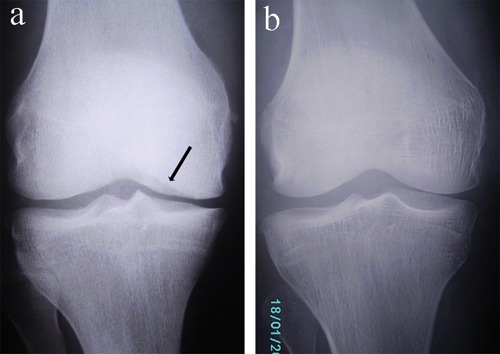
X-rays of case 1 and 4. (a) Preoperative AP radiograph of the knee of case 1 showing medial femoral condyle osteochondral erosion (black arrow), narrowing of the medial joint line, and osteophyte formation. (b) Preoperative AP radiograph of the knee of case 4 showing preserved joint line with no radiographic evidence of advanced degenerative joint changes. The patient had an osteochondral lesion in the lateral femoral condyle that was apparent on magnetic resonance imaging (see Fig. 4) and radiographic tunnel views.
At 6 mo postoperative, MRI of patient 2 showed partial defect filling with incomplete congruity of the repaired articular surface. This persisted at 12-mo follow-up MRI. X-rays at 6 and 12 mo showed persistent radiolucency and sclerosis at the margins of the defect. Arthroscopic evaluation of the repair tissue showed 75% repair of defect depth, 75% graft integration, 25% with a border >1-mm wide, and a fibrillated surface all through. The overall ICRS score was 8/12 (nearly normal cartilage). The patient had described the outcome as “good.”
Patients 3, 4, and 5 described their outcomes as “excellent,” and all showed complete defect fill with no overgrowth or periosteal hypertrophy and congruity of the repair tissue with the native cartilage on 6 to 12-mo follow-up MRI (Fig. 4). X-rays showed complete filling of the defects with restoration of the congruity of the femoral condyles and no evidence of degenerative changes. In patient 4, 2nd-look arthroscopy after 12 mo showed the graft surface to be level with the surrounding cartilage, a demarcating border <1 mm at some zones (~25% of the circumference), and an intact smooth surface (Fig. 5). The overall ICRS score was 11/12 (nearly normal cartilage). The patient returned to high-impact sports after 9 mo.
Figure 4.
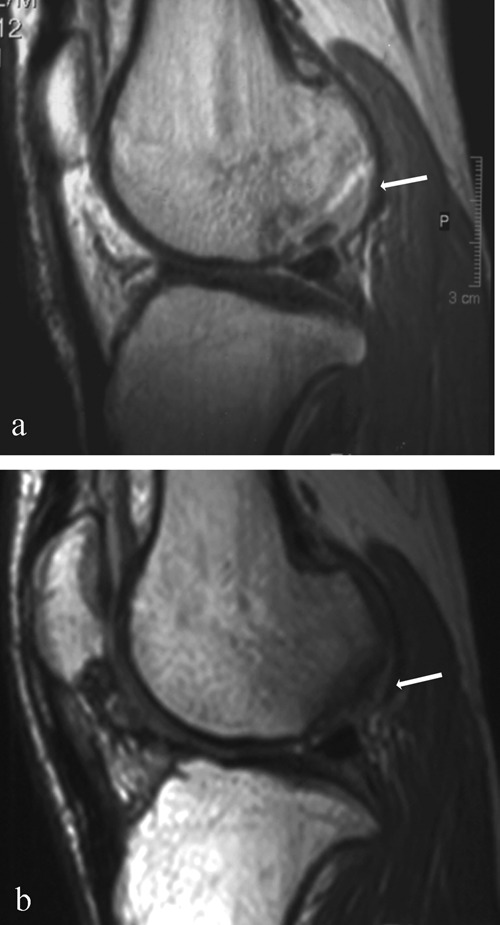
Magnetic resonance image (MRI) of sample case 4. (a) Preoperative sagittal magnetic resonance imaging (MRI) proton density sequence (PD) showing an osteochondral lesion (white arrow) in the lateral femoral condyle. This lesion was found to be completely detached on arthroscopy performed during bone marrow aspiration. (b) Sagittal MRI (PD) at 12 mo postoperative showing isointense signal of the repair cartilage (white arrow) compared with the native cartilage, complete filling of the defect, congruent surface of the repair cartilage, no delamination, and no subchondral bone marrow edema.
Figure 5.
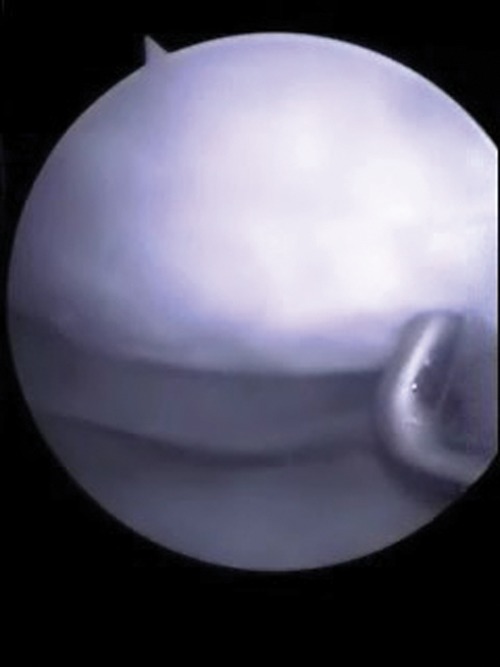
Case 4: arthroscopic view of the knee 12 mo postoperative, with the arthroscopic probe pointing to grossly appearing, smooth, hyaline-like repair tissue completely filling the defect.
Discussion
Numerous preclinical studies have reported development of hyaline-like tissue from autologous culture-expanded BM-MSCs,27,28 and some have shown superior functionality of BM-MSCs over chondrocytes.29,30 These results have further pushed MSC-based cartilage repair to clinical trials. PR-FG as a cell carrier has the advantage of being biodegradable, autologous and contains chondrogenic growth factors with sustained release profiles.7,13,31,32
In this pilot study, we found that all patients with osteochondral defects who were reconstructed with BM-MSCs transplanted on PR-FG experienced significant improvement in their functional knee scores and MRI findings as early as 6 mo and maintained over 12 mo postoperatively. This is consistent with previous published data in clinical trials of BM-MSCs.24-26,33 Although clinical improvement will be seen after most cartilage repair techniques in this short time period, this remains true only for small-sized defects (<2 cm2), not large-sized defects, as included in this small case series (average = 5.8 cm2). An example is patient 5, who had microfracture with anterior cruciate ligament reconstruction that did not relieve his cartilage-related symptoms as early as 6 mo postoperative.
There were notable variations in the results among patients with and without advanced degenerative changes in the knee prior to the surgery. This suggests that the condition of native cartilage surrounding a chondral defect affects the outcome of repair, possibly because of the decreased number and functionality of available chondrocytes as well as the decreased proteoglycan/collagen content of degenerative cartilage,34 impeding integration of repair tissue. This emphasizes early intervention in treating cartilage defects before the progression of degenerative changes that hinder better results. Further evaluation of this technique in patients with arthritic changes is required.
Although patient 2 had the smallest defect size (3 cm2) in this case series, the relatively inferior outcome in this case compared with patients 3 to 5 could be attributed to a number of causes: First, the ongoing subchondral bone pathology due to osteochondritis disssecans evident as radiolucency with surrounding bone sclerosis on plain x-rays at 1-y follow-up might have impeded proper tissue vascularization, with subsequent MSC death and poor graft integration. Second, the chondrogenic potential of BM-MSCs in this patient might have been quantitatively and qualitatively less than other patients. Payne et al.35 have recently reported that MSC chondrogenic potential shows both individual and age-dependent variations.
Although the age inclusion criterion for the study was 18 to 50 y, the patients in this pilot study were of relatively younger age (range = 21-37 y; mean = 25.4 y). This might have influenced the outcome of this cartilage repair procedure because the qualitative and quantitative difference in the metabolic activity of cells in the repair tissue is age dependent.36-41 It is well documented that MSCs age as they undergo more population doublings, and their synthetic activity and chondrogenic potential decline.42-46
The biopsy-induced injury and whether it heals spontaneously or further jeopardizes the repair limited its ethical justification to patients and remains a point of debate. This is of particular importance if long-term follow-up of the repair tissue is intended that should not be violated. MRI was used to evaluate the reparative tissue and is considered a good noninvasive method that was found to correlate well with 2nd-look arthroscopy and histology of cartilage repair tissue.47
FBS was used for in vitro culture expansion of MSCs. Steps were taken to minimize potential risks for pathogenicity and immunogenicity. Although these concerns cannot be completely eliminated, a phase 1 clinical study employing culture-expanded stem cells in FBS-containing media (at higher concentrations than used in our study) reinjected in patients with liver insufficiency similarly reported no complications or specific side effects related to the procedure.48
MSC implantation on PR-FG is a new technique and required an initial small number of patients enrolled in the pilot study, consequently limiting the sample size of this study. Because the sample size was small, we used nonparametric statistical methods, which do not require the assumptions of normality and equal variances (i.e., standard deviations). Friedman’s test was used to compare observations repeated on the same subjects as a group. It is similar to the parametric repeated-measures analysis of variance and is used to detect differences in treatments across multiple test attempts. The Wilcoxon signed-rank test was used to test for repeated measurements on a single sample. It can be used as an alternative to the paired Student’s t test when the population cannot be assumed to be normally distributed. Nonparametric tests are more conservative and are appropriate for hypothesis testing when the sample size is small. Because the sample size was small and there was no randomly assigned control group, a further randomized controlled clinical trial of this treatment modality, with larger numbers of patients and longer follow-up periods, is necessary to achieve even higher levels of validity.
This pilot study demonstrates feasibility and proof of principle that BM-MSCs PR-FG can be used clinically for the treatment of articular cartilage defects. This is consistent with basic science and preclinical in vivo studies reported in this field. We used standardized clinical outcome scores to assess the results of this treatment modality and MRI for assessment and follow up of the repair. The 2nd-look arthroscopies performed on 1 patient with partial defect fill by MRI and 1 patient with complete defect fill by MRI confirm the MRI assessments and support the use of MRI as a noninvasive postoperative assessment tool.
We conclude from this study that the transplantation of autologous culture-expanded BM-MSCs in PR-FG shows great promise in the treatment of full-thickness articular cartilage defects, particularly large-sized defects (>4 cm2). We have demonstrated that PR-FG successfully fixed the cultured MSCs within the defects and provided them with a suitable environment to synthesize a hyaline-like grossly appearing cartilaginous matrix. The positive 1-y clinical outcomes support further randomized controlled clinical trials of this treatment modality with larger numbers of patients and longer follow-up periods, as previously stated.
Acknowledgments
The authors would like to acknowledge Ahmed Essam, MD, and Omar Moawya, MD, from the Radiology Department at the Cairo University School of Medicine, and Magdy El-Ekiaby, MD, director of the Shabrawishi Hospital Blood Bank, for their technical help in this study as well as Tarek Abdel Shafy, MD, and Hazem Abdel Azim, MD, previous and current chairmen of the Orthopedic Surgery Department at the Cairo University School of Medicine, for their generous support. This work was performed at Kasr El-Ainy Hospital, Cairo University School of Medicine, Cairo, Egypt.
Footnotes
Declaration of Conflicting Interests: None of the authors received grants or outside funding in support of their research or preparation of this article. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated. The authors have no conflicts of interest.
Clinical Trial Registration: www.clinicaltrials.gov ID NCT00 891501.
References
- 1. Mandelbaum BR, Browne JE, Fu F, Micheli L, Mosely JB, Jr, Erggelet C, et al. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26(6):853-61. [DOI] [PubMed] [Google Scholar]
- 2. Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation: clinical results in the knee. Clin Orthop Relat Res. 1999;360:159-68. [PubMed] [Google Scholar]
- 3. Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23-32. [DOI] [PubMed] [Google Scholar]
- 4. Chu CR, Coutts RD, Yoshioka M, Harwood FL, Monosov AZ, Amiel D. Articular cartilage repair using allogeneic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res. 1995;29(9):1147-54. [DOI] [PubMed] [Google Scholar]
- 5. Chu CR, Dounchis JS, Yoshioka M, Sah RL, Coutts RD, Amiel D. Osteochondral repair using perichondrial cells: a 1-year study in rabbits. Clin Orthop Relat Res. 1997;340:220-9. [DOI] [PubMed] [Google Scholar]
- 6. Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14(10):804-13. [DOI] [PubMed] [Google Scholar]
- 7. Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16(10):1121-30. [DOI] [PubMed] [Google Scholar]
- 8. Mankin HJ, Jennings LC, Treadwell BV, Trippel SB. Growth factors and articular cartilage. J Rheumatol Suppl. 1991;27:66-7. [PubMed] [Google Scholar]
- 9. Nair MB, Varma HK, John A. Platelet-rich plasma and fibrin glue-coated bioactive ceramics enhance growth and differentiation of goat bone marrow-derived stem cells. Tissue Eng Part A. 2009;15(7):1619-31. [DOI] [PubMed] [Google Scholar]
- 10. Phornphutkul C, Wu KY, Yang X, Chen Q, Gruppuso PA. Insulin-like growth factor-I signaling is modified during chondrocyte differentiation. J Endocrinol. 2004;183(3):477-86. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14(5):403-12. [DOI] [PubMed] [Google Scholar]
- 12. Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43(1):65-92. [DOI] [PubMed] [Google Scholar]
- 13. Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16(3):356-63. [DOI] [PubMed] [Google Scholar]
- 14. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-96. [DOI] [PubMed] [Google Scholar]
- 15. Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal: evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97(2):110-8. [DOI] [PubMed] [Google Scholar]
- 16. Su CY, Kuo YP, Nieh HL, Tseng YH, Burnouf T. Quantitative assessment of the kinetics of growth factors release from platelet gel. Transfusion. 2008;48(11):2414-20. [DOI] [PubMed] [Google Scholar]
- 17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102. [DOI] [PubMed] [Google Scholar]
- 18. Weibrich G, Kleis WK, Hafner G, Hitzler WE, Wagner W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res. 2003;14(3):357-62. [DOI] [PubMed] [Google Scholar]
- 19. Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007;40(12):893-9. [DOI] [PubMed] [Google Scholar]
- 20. Abdel Aziz MT, El-Asmar MF, Haidara M, Atta HM, Roshdy NK, Rashed LA, et al. Effect of bone marrow-derived mesenchymal stem cells on cardiovascular complications in diabetic rats. Med Sci Monit. 2008;14(11):BR249-55. [PubMed] [Google Scholar]
- 21. Kotobuki N, Hirose M, Takakura Y, Ohgushi H. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004;28(1):33-9. [DOI] [PubMed] [Google Scholar]
- 22. Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48(6):913-27. [DOI] [PubMed] [Google Scholar]
- 23. O’Neill EM, Zalewski WM, Eaton LJ, Popovsky MA, Pivacek LE, Ragno G, et al. Autologous platelet-rich plasma isolated using the Haemonetics Cell Saver 5 and Haemonetics MCS+ for the preparation of platelet gel. Vox Sang. 2001;81(3):172-5. [DOI] [PubMed] [Google Scholar]
- 24. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199-206. [DOI] [PubMed] [Google Scholar]
- 25. Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595-600. [DOI] [PubMed] [Google Scholar]
- 26. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1(1):74-9. [DOI] [PubMed] [Google Scholar]
- 27. Hui JH, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans: a comparative study of the efficacy of chondrocytes, mesenchymal stem cells, periosteal graft, and mosaicplasty (osteochondral autograft) in animal models. J Pediatr Orthop. 2004;24(4):427-33. [DOI] [PubMed] [Google Scholar]
- 28. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579-92. [DOI] [PubMed] [Google Scholar]
- 29. Cournil-Henrionnet C, Huselstein C, Wang Y, Galois L, Mainard D, Decot V, et al. Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology. 2008;45(3-4):513-26. [PubMed] [Google Scholar]
- 30. Li WJ, Chiang H, Kuo TF, Lee HS, Jiang CC, Tuan RS. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J Tissue Eng Regen Med. 2009;3(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415-28. [DOI] [PubMed] [Google Scholar]
- 32. Smith RJ, Justen JM, Sam LM, Rohloff NA, Ruppel PL, Brunden MN, et al. Platelet-derived growth factor potentiates cellular responses of articular chondrocytes to interleukin-1. Arthritis Rheum. 1991;34(6):697-706. [DOI] [PubMed] [Google Scholar]
- 33. Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15(2):226-31. [DOI] [PubMed] [Google Scholar]
- 34. Fukui N, Ikeda Y, Ohnuki T, Tanaka N, Hikita A, Mitomi H, et al. Regional differences in chondrocyte metabolism in osteoarthritis: a detailed analysis by laser capture microdissection. Arthritis Rheum. 2008;58(1):154-63. [DOI] [PubMed] [Google Scholar]
- 35. Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. Epub ahead of print, 6 February 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buckwalter JA. Evaluating methods of restoring cartilaginous articular surfaces. Clin Orthop Relat Res. 1999;367(suppl):S224-38. [DOI] [PubMed] [Google Scholar]
- 37. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] [Google Scholar]
- 38. Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(suppl 2):106-10. [DOI] [PubMed] [Google Scholar]
- 39. Nakajima H, Goto T, Horikawa O, Kikuchi T, Shinmei M. Characterization of the cells in the repair tissue of full-thickness articular cartilage defects. Histochem Cell Biol. 1998;109(4):331-8. [DOI] [PubMed] [Google Scholar]
- 40. Tew S, Redman S, Kwan A, Walker E, Khan I, Dowthwaite G, et al. Differences in repair responses between immature and mature cartilage. Clin Orthop Relat Res. 2001;391(suppl):S142-52. [DOI] [PubMed] [Google Scholar]
- 41. Wei X, Messner K. Age- and injury-dependent concentrations of transforming growth factor-beta 1 and proteoglycan fragments in rabbit knee joint fluid. Osteoarthritis Cartilage. 1998;6(1):10-8. [DOI] [PubMed] [Google Scholar]
- 42. Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67(19):9142-9. [DOI] [PubMed] [Google Scholar]
- 43. Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakahara M, Takagi M, Hattori T, Wakitani S, Yoshida T. Effect of subcultivation of human bone marrow mesenchymal stem on their capacities for chondrogenesis, supporting hematopoiesis, and telomea length. Cytotechnology. 2005;47(1-3):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163-73. [DOI] [PubMed] [Google Scholar]
- 46. Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205(2):194-201. [DOI] [PubMed] [Google Scholar]
- 47. Henderson IJ, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-6. [DOI] [PubMed] [Google Scholar]
- 48. Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24(7):1822-30. [DOI] [PubMed] [Google Scholar]


