Abstract
Dichloroacetate (DCA) and trichloroacetate (TCA) were previously found to induce various levels of oxidative stress in the hepatic tissues of mice after subacute and subchronic exposure. The cells are known to have several protective mechansims against production of oxidative stress by different xenobiotics. To assess the roles of the antioxidant enzymes and glutathione (GSH) in DCA- and TCA-induced oxidative stress, groups of B6C3F1 mice were administered either DCA or TCA at doses of 7.7, 77, 154 and 410 mg/kg/day, by gavage for 4 weeks (4-W) and 13 weeks (13-W), and superoxide dismutase (SOD) catalase (CAT) and glutathione peroxidase (GSH-Px) activities, as well as GSH were determined in the hepatic tissues. DCA at doses ranging between 7.7-410, and 7.7-77 mg/kg/day, given for 4-W and 13-W, respectively, resulted in either suppression or no change in SOD, CAT and GSH-Px activities, but doses of 154-410 mg DCA/kg/day administered for 13-W were found to result in significant induction of the three enzyme activities. TCA administration on the other hand, resulted in increases in SOD and CAT activities, and suppression of GSH-Px activity in both periods. Except for the DCA doses of 77-154 mg/kg/day administered for 13-W that resulted in significant reduction in GSH levels, all other DCA, as well as TCA treatments produced no changes in GSH. Since these enzymes are involved in the detoxification of the reactive oxygen species (ROS), superoxide anion (SA) and H2O2, it is concluded that SA is the main contributor to DCA-induced oxidative stress while both ROS contribute to that of TCA. The increases in the enzyme activities associated with 154-410 mg DCA/kg/day in the 13-W period suggest their role as protective mechanisms contributing to the survival of cells modified in response to those treatments.
Keywords: Dichloroacetate, Trichloroacetate, Superoxide Dismutase, Catalase, Glutathione Peroxidase, Glutathione, Liver, Mice
INTRODUCTION
Dichloroacetate (DCA) and trichloroacetate (TCA) are among the haloacetates formed during the process of chlorinating municipal water supplies (Krasner et al., 1989, Richardson et al., 2008; Uden and Miller; 1983). Trichloroethylene is a widely used organic solvent that contaminates the surface water of several industrial areas, and in vivo metabolism of this solvent in humans and animals was found to generate DCA and TCA (Decant et al., 1984; Green and Prout, 1985; Hathway, 1980). Studies on the long term toxicity and carcinogenicity of DCA and TCA found the compounds to be hepatotoxic and hepatocarcinogenic in rodents in general, and in B6C3F1 male mice, in particular (Bull et al., 1990; Daniel et al., 1992; DeAngelo et al., 1991; 1999; Herren-Freund et al., 1987). Although both compounds were suggested to act as complete hepatocarcinogens in mice (Hassoun et al, 2010), two different pictures of hispathological changes were described for hepatic tissues after chronic exposure to each of them (Bull et al., 1990). While DCA treatment resulted in hepatomegaly associated with excessive accumulation of glycogen in hepatocytes with areas of focal necrosis, TCA treatment resulted in quantitative increases in cell size, accumulation of glycogen, no focal necrotic damage, but greater and marked lipofuscin accumulation (Bull et al, 1990).
Various biomarkers of oxidative stress, including lipid peroxidation (LP), superoxide anion (SA) and DNA damage were found to be induced in response to single high doses of DCA and TCA (Austin et al., 1996; Hassoun and Dey, 2008; Larson and Bull, 1992; Nelson and Bull, 1988; Nelson et al., 1989; Parrish et al., 1996). In addition to the contribution of the chemicals-induced production of reactive oxygen species (ROS) to the process of oxidative stress, studies also suggested the contribution of free radical generation by DCA and TCA through reductive dechlorination pathways after administration of single oral doses (Larson and Bull, 1992), and inhibition of glutathione S-transferase zeta1-1 (GSTZ1-1) by DCA (Blackburn et al., 2006; Tzeng et al., 2000) to that process. However, recent studies in our lab have indicated the induction of various biomarkers of oxidative stress, including SA, LP and DNA-SSBs in hepatic tissues of B6C3F1 mice by DCA and TCA doses believed to correspond to non-hepatocarcinogenic concentrations to those producing maximal hepatocarcinogenicity (Hassoun et al., 2010). The studies also demonstrated significant induction of SA, LP and DNA-SSBs after periods of exposure that were far less than those required for the production of the hepatotoxic/hepatocarcinogenic effects and suggested the role of early induction of those biomarkers in the later production of those effects (Hassoun et al., 2010). The generation of various reactive oxygen species (ROS), as well as free radicals is modulated by several antioxidant enzymes and glutathione (GSH). Superoxide dismutase (SOD) is an antioxidant enzyme that results in SA dismutation to H2O2, and catalase (CAT) and glutathione peroxidase (GSH-Px) act in concert with SOD catalyzing the conversion of H2O2 to H2O (Davies, 1995, Halliwell et al., 1992; Josephy et al., 1997; McCord, 1993). GSH in its reduced form, while serving as a substrate for GSH-Px, is also known to render several ROS and free radicals non toxic (Anderson, 1985; Reed, 1994).
Previously observed differences in the levels of various oxidative stress biomarkers in hepatic tissues of mice treated with DCA and TCA were noted at doses ranging between non-hepatocarcinogenic and those producing maximal hepatocarcinogenicity (Hassoun et al., 2010). Although induction of various biomarkers of oxidative stress is known to be associated with oxidative tissue damage, all aerobic organisms utilize a series of primary antioxidant defense mechanisms, such as scavengers, direct and indirect repair systems, damage removal systems and antioxidant enzymes and protein, including GSH, to protect against oxidant damage (Davies, 1995). This study was undertaken to assess the contribution of the antioxidant enzymes and GSH to the previously observed differences in the induction of various biomarkers of oxidative stress by different DCA and TCA treatments.
Materials and Methods
Chemicals
All of the chemicals used for this study were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO), and were at the highest grade available.
Animals and treatments
The long term hepatotoxicity and hepatocarcinogenicity attributed to DCA and TCA have been extensively studied in B6C3F1 male mice (Bull et al., 1990; Daniel et al., 1992; DeAngelo et al., 1991; 1999; Herren-Freund et al., 1987). Further, subacute and subchronic studies in our lab have found the compounds to induce dose- and time-dependent increases in the levels of SA, LP and DNA-SSBs in B6C3F1 male mice (Hassoun et al., 2010). In order to correlate the previously observed changes in SA, LP and DNA damage production in response to DCA and TCA with possible changes in antioxidant enzyme activities and GSH levels, the tissues used for the this study were same ones used previously (Hassoun et al., 2010). In brief, approximately, 6-weeks old B6C3F1 male mice weighing 20 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). The mice were allowed to acclimate for 3 days prior to the start of treatment and allowed free access to food and water. Hepatotoxic and hepatocarcinogenic effects were found to be produced by DCA and TCA in B6C3F1 mice at concentrations ranging from 1 to 5 g/l in the drinking water, administered for 52-75 weeks (Bull et al., 1990; deAngelo et al, 1991; 1999; Herren-Freund et al., 1987). The time-weighted mean daily doses for DCA that correspond to 0.05, 0.5, 3.5 and 5 g/l of DCA in the drinking water were calculated in one study and found to be equivalent to 7.6, 77, 410 and 486 mg /kg/day, respectively (DeAngelo et al.1991). In another lifetime exposure study; however, the time-weighed mean daily doses that correspond to 0.05, 0.5, 1, 2 and 3.5 g/l DCA in the drinking water were found to be equivalent to 8, 84, 168, 315, and 429 mg/kg/day (DeAngelo et al., 1999). DCA concentrations equivalent to 7.6, 77, and 410 mg/kg/day were identified as the doses that correspond to the non carcinogenic dose, the threshold carcinogenic dose and the dose that results in 100% tumor prevalence, respectively (DeAngelo et al., 1991). Further, Bull et al. (1990) found that 1 and 2 g/l of DCA or TCA received by the B6C3F1 mice in the drinking water for 52 weeks were hepatocarcinogenic. They also calculated the total doses of either compound that correspond to 1-2 g/l and found them to be respectively equivalent to 150-300 mg DCA/kg/day, and 165-330 mg TCA/kg/day. Accordingly, the doses of DCA and TCA for this study were chosen to be 7.7, 77, 154 and 410 mg/kg/day DCA and TCA, and were believed to resemble a range between non carcinogenic dose to a dose producing maximal carcinogenesis. The compounds were dissolved in distilled water (pH of the solutions was adjusted to 7) and administered by gavage to groups of mice (7 animals/ group) for 4 weeks (4-W), (subacute treatment) or 13 weeks (13-W), (subchronic treatment). Control animals received distilled water (pH adjusted to 7) at a rate of 5 ml/kg body weight/day. Animals were euthanized 24 hours after the end of the 4-W and 13-W treatment periods, using carbon dioxide anesthesia, followed by cervical dislocation.
Homogenization of the hepatic tissues
After the animals were euthanized, the livers were removed and weighed. The livers were frozen at −80° C until they were used for the different assays. Experience in our lab indicates insignificant changes in antioxidant enzyme activities when tissues are frozen for at least 1 year at the specified temperature. A portion of each liver was homogenized in sucrose buffer containing 0.32 M sucrose, 1 mM EDTA, and 10 mM Tris-HCL, to produce 10% (w/v) homogenates. The homogenates were centrifuged at 9,000 x g for 30 min and supernatants were used for the determination of SOD, CAT and GSH-Px activities (Jiang et. al., 1998). Another portion of the liver was homogenized in 5% sulfosalicylic acid to generate 20% (w/v) homogenates. The homogenates were centrifuged at 900 x g for 5 min and supernatants were used for the determination of GSH.
Determination of SOD activity
SOD activity was determined according to the method of Marklund and Marklund (1974), with modification, and is based on the inhibition of pyrogallol autooxidation by SOD. An aliquot of the supernatant (200 μl) was mixed with 750 μl Tris-cacodylic buffer, containing 50 mM Tris-HCL, 50 mM cacodylic acid and 1 mM EDTA, pH 8.2, followed by the addition of 250 μl of 0.5 mM pyrogallol. The reactions were carried out at a temperature of 25° C and absorbances of the mixtures were recorded at 420 nm immediately, and then every 30 sec for 3 min using a Spectronic 20 spectrophotometer (Spectronic Instrument, Rochester, NY). Changes in the rates of absorbances were calculated and converted into units of SOD activity per mg protein, where one unit is equivalent to the quantity of SOD that is needed to produce 50% inhibition of pyrogallol autooxidation (Marklund and Marklund, 1974).
Determination of CAT activity
CAT activity was determined according to the method of Cohen et al. (1970), with modification. The assay was based on enzyme-catalyzed decomposition of H2O2, using potassium permanganate (KMNO4) and had three components, the blank, standard and sample. The blank, standard, and the sample tubes contained 100 μl of sucrose buffer,100 μl of deionized water, and 100 μl of supernatant, respectively. One ml of 6mM H2O2 was added to the sample and the blank tubes, and 1 ml of deionized water was added to the standard tube. The tubes were vortexed and placed on ice for 3 min and the reactions were then stopped by adding 200 μl of 6N H2SO4 to each tube. After stopping the reactions, 1.4 ml of 2 mM KMnO4 was added to each tube and absorbances were immediately recorded at 480 nm, using a Biotek® Spectrophotometer. Absorbance values were converted into units of CAT /mg protein, where a unit is equal to k/0.00693. The first order reaction rate constant (k) = log (S0/S2) × (2.3/t). The logarithm conversion factor is 2.3 and t was equivalent to the incubation time (3 min). S0 and S2 were determined by subtracting the blank absorbance from the standard absorbance, and the sample absorbance from the standard absorbance, respectively (Jiang et. al., 1998).
Determination of glutathione peroxidase (GSH-Px) activity
The method of Lawrence and Burk (1976) was employed to measure the activity of GSH-Px. In brief, a 100 μl aliquot of supernatant was mixed with 700 μl of reaction mixture containing, 1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH and 1 mM GSH in a phosphate buffer saline, pH 7.2, and 100 μl containing 10 units of glutathione reductase. The tubes were vortexed and incubated for 5 min at room temperature. After incubation, 100 μl of 0.2 mM H2O2 was added to initiate the reaction and absorbance recorded at 340 nm, immediately and then every 30 sec over a period of 3 min, using a Spectronic-20 spectrophotometer (Spectronic Instruments, Rochester, NY). Changes in the rate of absorbance were converted into nmoles of NADPH oxidized/min/mg protein, using an extinction coefficient of 6.22 × 103 L mol −1cm −1 (Lawrence and Burk, 1976)
Determintaion of total GSH
Total GSH (GSH + GSSG, in GSH equivalents) was determined by the recycling assay described by Anderson (1985), and is based on GSH oxidation by 5,5′-dithiobis(2-nitrobenzoic acid (DTNB) to yield GSSG and the stoichiometric formation of 5-thio-2 nitrobenzoic acid (TNB). GSSG is then reduced to GSH by the action of GSSG reductase in the presence of NADPH. The assay required the stock buffer containing 143 mM sodium phosphate and 6.3 mM tertra sodium EDTA, pH 7.5, the daily buffer containing 0.248 mg/ml NADPH in stock buffer and GSSG reductase solution containing 266 units of the enzyme /ml of stock buffer. The assay mixture contained 10 μl of supernatant, 700 μl daily buffer, 100 μl DTNB solution, and 190 μl deionized water. The mixtures were incubated at 30°C for 15 min, followed by the addition of 10 μl GSSG-reductase solution. Absorbances of the mixtures were recorded at 412 nm immediately, and then every 30 sec for 3 min, using a Spectronic 20 spectrophotometer (Spectronic Instruments, Rochester, NY). A standard curve for GSH was prepared following the aforementioned method but replacing the 10 μl of sample with 10 μl solutions containing 1-4 nmole of GSH.
Determination of protein
The protein content of the hepatic tissue homogenates was determined according to the method of Lowry et al. (1951), using BSA as the standard.
Statistical methods
Data were analyzed using Microsoft Excel® data analysis tool package. Data are expressed as means of 7 samples (animals) ± S.D. A two-factor Analysis of Variance (ANOVA) with replication was used to compare between the effects of different doses and time points on each enzyme, and Scheffe’s S method was used as a post hoc test. Student’s t-test was used to compare between the effects of various doses of DCA and TCA at each period of treatment and the corresponding control at each of those periods. A significance level of p < 0.05 was employed. .
Results
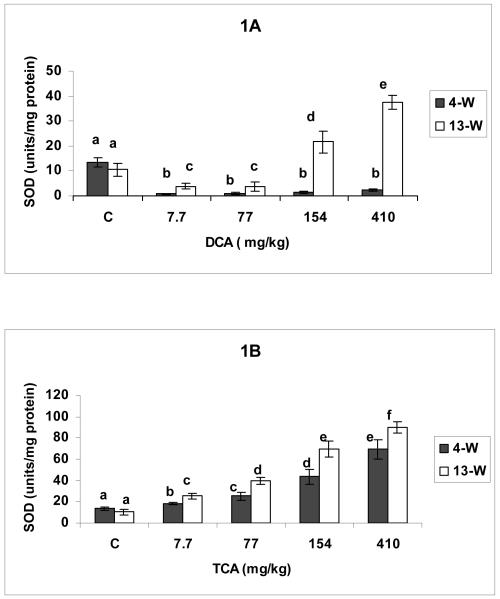
The effects of DCA and TCA on SOD activity are shown in Figures 1 A and B, respectively. DCA resulted in SOD suppression at all tested doses in the 4-W treatment period compared with corresponding controls (Figure1A). DCA doses of 7.7-77 mg/kg/day given for 13-W also resulted in SOD activity inhibition when compared with corresponding control; however, the observed decreases were significantly lower than those produced at same doses in the 4-W period (Figure 1 A). The figure also shows that DCA administration at doses of 154-410 for 13-W resulted in significant increases in SOD activity when compared with corresponding controls, and a greater rise was observed in response to 410 mg/kg/day dose compared with 154 mg/kg/day dose. TCA on the other hand resulted in dose-dependent elevation in SOD activity in both treatment periods, with significantly greater increases produced with different doses in the 13-W period compared with the corresponding doses at 4-W (Figure 1 B).
Figures 1.
A and B: SOD activity determined in hepatic tissues of DCA- (A) and TCA-treated mice (B), 4 and 13 weeks after treatment. The effects of different doses (including the controls), and different lengths of treatment were compared. Columns with non identical superscripts are significantly different (p< 0.05)
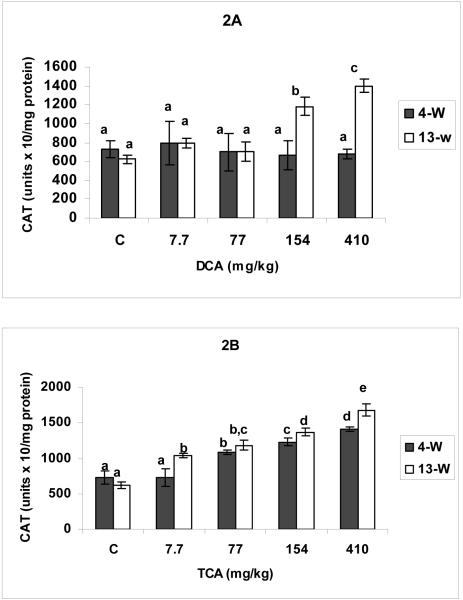
The effects of DCA and TCA on CAT activity are demonstrated in Figures 2 A and B, respectively. While all of DCA tested doses at the 4-w period, and 7.7-77 mg DCA/kg/day doses at 13-W period did not produce any significant changes in CAT activity, the 154-410 mg DCA/kg/day resulted in significant increases in CAT activity in 13-W period compared with corresponding controls (Figure 2A). Figure 2 A also shows that the rise in CAT activity in response to 410 mg DCA/kg/day was significantly greater than that associated with the 154 mg/kg/day dose. TCA administration on the other hand resulted in significant and dose-dependent elevation in CAT activity in the two tested periods, with significantly higher increases observed with different doses in the 13-W period compared with corresponding doses in the 4-W treatment period (Figure 2 B).
Figures 2.
A and B: Catalase activity determined in hepatic tissues of DCA- (A) and TCA-treated (B) mice, 4 and 13 weeks after treatment. The effects of different doses (including the controls), and different lengths of treatment were compared. Columns that do not share identical superscripts are significantly different (p < 0.05)
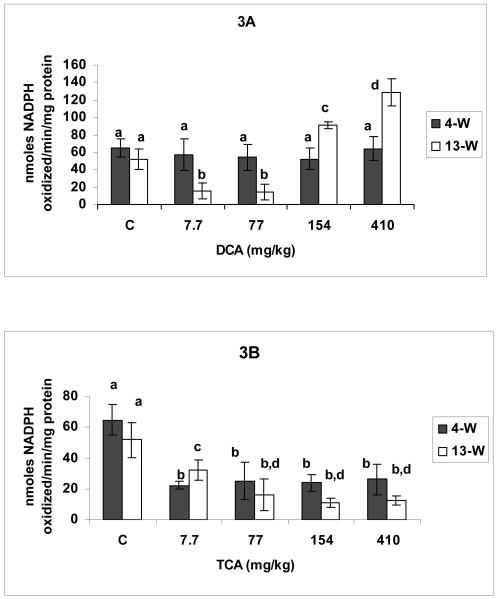
DCA and TCA effects on GSH-px activity are demonstrated in Figures 3 A and B, respectively. While none of the DCA tested doses produced significant changes in GSH-Px activity in the 4-W treatment period, doses of 7.7-77 and 154-410 mg DCA/kg/day resulted in respectively, significant suppression and induction in the 13-W treatment period compared with corresponding controls (Figure 3A). The Figure also shows a significantly greater increase produced in response to 410 mg DCA/kg/day in the 13-W treatment period compared with 154 mg/kg/day dose. TCA administration at doses ranging between 7.7-410 mg/kg/day for 4-W and 13-W produced significant inhibition of GSH-Px activity when compared with corresponding controls (Figure 3 B).
Figures 3.
A and B: Glutathione peroxidase activity in hepatic tissues of DCA- (A) and TCA-treated (B) mice, 4 and 13 weeks after treatment. The effects of different doses (including the controls), and different lengths of treatment were compared. Columns that do not share identical superscripts are significantly different (p < 0.05).
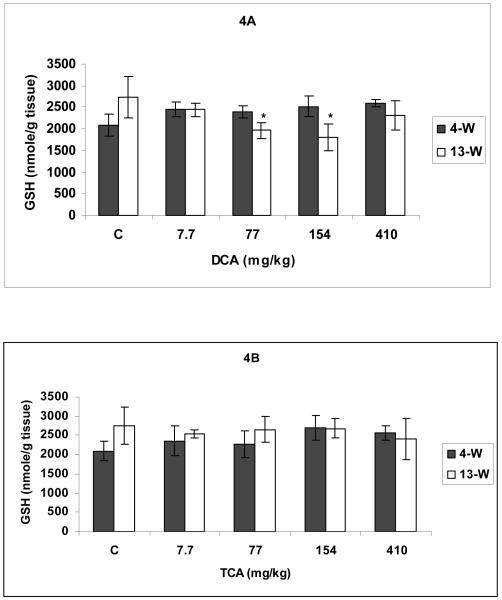
Figures 4A and 4B demonstrate the effects of DCA and TCA on GSH levels. Except for the DCA doses of 77-154 mg/kg/day at 13-W treatment period that resulted in significant lower GSH levels (Figure 4A), all other DCA and TCA treatments caused no significant effects on GSH levels in the hepatic tissues of treated mice (Figures 4A &B).
Figures 4.
A and B Total glutathione (GSH) determined in hepatic tissues of DCA- (A) and TCA-treated (B) mice, 4 and 13 weeks after treatment. * Indicates significant difference when compared with the corresponding control (p< 0.05), using t-test.
Discussion
Table 1 summarizes the results of previous studies in our lab conducted on the same tissues that were used for this study (Hassoun et al., 2010). As indicated in the table, the results of the studies demonstrated dose-dependent increases in SA production in response to DCA doses of 7.7-410, and 7.7-154 mg/kg/day in the 4-W and 13-W treatment periods, respectively (Hassoun et al., 2010). The studies also demonstrated a fall in the level of SA occurring in response to 410 mg DCA/kg/day in the 13-W period (Hassoun et al., 2010). SOD is responsible for SA dismutation to H2O2 (Davies, 1995; Josephy et al., 1997). Therefore, SOD suppression in response to DCA treatment for 4-w and to 7.7-77 mg DCA/kg/day for 13-w indicates insignificant SA dismuation to H2O2, while SOD marked increases in activity in response to 154-410 mg DCA/kg/day indicate significant SA dismutation to that ROS. CAT and GSH-Px act in concert with SOD and catalyze H2O2 conversion to water (Davies, 1995; Josephy et. al., 1997). The observed no marked changes in CAT activity, together with either no changes or significant suppression of GSH-Px activity that are associated with SOD inhibition by certain DCA treatments may all confirm insignificant SA dismutation to H2O2. Data may also demonstrate the main contribution of DCA-induced SA production to the oxidative damage that was previously reported in liver, in response to DCA treatments (Hassoun et al., 2010). Knowing that DCA can induce peroxisome proliferation after 14-days of exposure to concentrations falling in the range of doses used for this study (DeAngelo et al., 1989), the contribution of H2O2 from this source to DCA-induced hepatotoxicity should not be excluded. This is especially true for DCA doses associated with no change or suppression of CAT and/ or GSH-Px activities. Treatment with 154-410 mg DCA/kg/day for 13-w resulted in concerted increases in the three tested antioxidant enzyme activities. Studies on ROS-induced liver injuries in response to various insults demonstrated up-regulation of antioxidant enzyme activities and suggested that to be a compensatory mechanism of normal cells in response to those injuries (Crosby et al., 2008; Fella et al, 2005; Pey et al., 2003; Tsai et al., 2009). Knowing that extensive cellular modification, including cancer production was previously observed (Bull et al., 1990; Daniel et al., 1992; DeAngelo et al., 1991; 1999; Herren-Freund et al., 1987) with treatments equivalent to DCA doses associated with enhanced antioxidant enzyme activities, the observed enzymic rise may contribute to the survival of those modified cells, rather than normal cells. This can be also confirmed by the previously reported reduction in LP and DNA damage production that was associated with hepatomegaly, in response to 410 mg/kg/day of DCA in the 13-W treatment period compared with responses to lower doses of this compound in that treatment period (Hassoun et al, 2010). In addition, if the enzymic increases reflected compensatory up-regulation mechanisms to resist hepatic damage by DCA, the rise in enzymic activity would have likely been associated with 7.7 and 77 mg DCA/kg/day, which corresponded to a non carcinogenic dose and threshold dose for carcinogenesis, respectively (DeAngelo et al., 1991).
Table 1.
Summary of the outcome of DCA and TCA treatments on the induction of superoxide anion (SA), lipid peroxidation (LP) and DNA-single strand breaks (SSBs) (Hassoun et al., 2010). Values indicate the approximate times increases over corresponding controls. INS indicates insignificant changes
| SA |
LP |
DNA-SSBs_ |
||||
|---|---|---|---|---|---|---|
| 4-W |
13-W |
4-W |
13-W |
4-W |
13-W |
|
| DCA mg/kg/day | ||||||
| 7.7 | 1.2 | 1.4 | 2.5 | 3.5 | INS | 1.6 |
| 77 | 2.5 | 3.2 | 5.0 | 12.5 | 3.5 | 5.6 |
| 154 | 4.0 | 4.3 | 7.5 | 15.0 | 7.2 | 5.6 |
| 410 | 4.3 | 2.2 | 14.0 | 4.0 | 7.2 | 4.0 |
| TCA mg/kg/day | ||||||
| 7.7 | INS | 1.2 | INS | 1.5 | INS | INS |
| 77 | INS | 1.8 | 2.0 | 7.0 | 1.8 | 2.3 |
| 154 | 1.3 | 2.5 | 2.5 | 8.5 | 2.3 | 3.3 |
| 410 | 2.8 | 2.8 | 11.0 | 13.5 | 4.3 | 4.3 |
Tissues from TCA-treated mice used in this study previously demonstrated dose-dependent increases in the production of SA, LP and DNA damage, with marked increases observed in the 13-w compared with the 4-w treatment period (Hassoun et al., 2010). The present results show dose- and time- dependent rise in SOD activity in response to TCA, indicating SA dismutation to H2O2. However, SOD elevation occurred concomitantly with increases and suppression of CAT and GSH-PX activity, respectively. This may indicate a main role of CAT in H2O2 conversion to water, as opposed to an insignificant contribution of GSH-Px to that process. This may also indicate partial SA-H2O2 detoxification, and contribution of H2O2, besides SA, to the previously observed time- and dose-dependent increases in LP and DNA damage produced by TCA (Hassoun et al., 2010). Previous studies showed enhancement of peroxisome proliferation in the mouse liver by TCA (DeAngelo et. al., 1989; Nelson et. al., 1989), which suggest contribution of H2O2 from that source to TCA-induced hepatotoxicity. However, the TCA-induced increase in CAT activity and suppression of GSH-PX may also suggest partial detoxification of H2O2 contributed by that source. While TCA treatment did not result in any marked effects on GSH levels, DCA doses of 77-154 mg/kg/day resulted in significant reduction in concentration of GSH when administered for 13-W. These results are supported by studies demonstrating DCA-induced suppression of GSH in the J774A.1 cells, and the significant role of GSH against DCA-induced cellular death when added simultaneously with DCA (Hassoun and Mehta, 2008). DCA was found to undergo more extensive metabolism through a reductive dechlorination pathway, and a more rapid rate of elimination than TCA in B6C3F1 mice and rats after oral administration of single high doses (Larson and Bull., 1992). Other studies attributed the rapid elimination of DCA to a glutathione-dependent metabolic pathway that occurs primarily in hepatic cytosol (Lipscomb, et al., 1995; James et al., 1997), and utilizes glutathione S-transferase zeta1-1 (GSTZ1-1) (Tong et al.; 1998). However, DCA is also known as a mechanism based inhibitor of GSTZ and a loss in enzyme activity was found to occur after repeated doses or prolonged drinking water exposures (Gonzalez-Leon, 1999, Cornett et al., 1999). Therefore, it is likely that repeated exposure to DCA induced GSTZ inhibition and resulted in accumulation of electrophilic intermediates. Knowing that GSH detoxifies free radicals, besides ROS (Reed, 1994), production of free radicals through aforementioned pathway may also contribute to GSH suppression by DCA. The no changes in total GSH concentrations in response to TCA and certain DCA treatments reflect non depletion of GSH. However, those unchanged concentrations may acquire differences in the ratios of oxidized: reduced GSH. Future studies that included determining GSTZ activity and the ratio of oxidized: reduced glutathione in response to different treatments with DCA and TCA are required to support our suggestions.
In summary, exposure of mice to DCA doses that were previously found to be associated with increases in SA, LP and DNA damage production results in either suppression or no marked changes in the antioxidant enzyme activities, suggesting a predominant role of SA in the previously observed DCA-induced oxidative damage. However, exposure to DCA doses that were previously found to be hepatotoxic/hepatocarcinogenic but were associated with decreases in SA, LP and DNA damage production, resulted in concomitant increases in the antioxidant enzyme activities, suggesting a role for these enzymes in the previously noted cellular resistance to oxidative damage by DCA. TCA treatment on the other hand resulted in dose- and time-dependent rise in SOD and CAT activities and dose- and time-dependent decreases in GSH-Px, activity suggesting a partial role of SOD-CAT in protection against cellular toxicity and the contribution of SA and H2O2 to the previously observed oxidative cellular damage associated with TCA treatment. GSH levels on the other hand were found to undergo a little, though significant suppression in response to some DCA doses in the 13-w but not in the 4-w period, suggesting a significant role of GSH in protecting against DCA-induced earlier effects.
Acknowledgement
The project described was supported by Grant Number R15ES013706-01A2 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences.
References
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Austin EW, Parrish JM, Kinder DH, Bull RJ. Lipid peroxidation and formation of 8-hydroxyguanosine from acute doses of halogenated acetic acids. Fundam. Appl. Toxicol. 1996;31:77–82. doi: 10.1006/faat.1996.0078. [DOI] [PubMed] [Google Scholar]
- Blackburn AC, Matthaei KI, Lim C, Taylor MC, Cappello JY, Hayes JD, Anders MW, Board PG. Deficiency of glutathione transferase zeta causes oxidative stress and activation of antioxidant response pathways. Mol. Pharmacol. 2006;69:650–657. doi: 10.1124/mol.105.018911. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology. 1990;63:341–359. doi: 10.1016/0300-483x(90)90195-m. [DOI] [PubMed] [Google Scholar]
- Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- Cornett R, James MO, Henderson GN, Cheung J, Shroads AL, Stacpoole PW. Inhibition of glutathione s-transferase zeta and tyrosine metabolism by dichloroacetate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochem. Biophys. Res. Commun. 1999;262:752–756. doi: 10.1006/bbrc.1999.1287. [DOI] [PubMed] [Google Scholar]
- Crosby LM, Simmons JE, Ward WO, Moore TM, Morgan KT, Deangelo AB. Integrated disinfection by-products (DBS) mixture research: gene expression alterations in primary hepatocyte cultures exposed to DBP mixtures formed by chlorination and ozonation/postchlorination. J. Toxicol. Environ. Health A. 2008;71:1195–1215. doi: 10.1080/15287390802182581. [DOI] [PubMed] [Google Scholar]
- Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP. Hepatocarcinogenicity of chloral hydrate, 2-chloroaldehyde and dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1992;19:159–168. doi: 10.1016/0272-0590(92)90147-a. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Oxidative stress: the paradox of aerobic life. In: Rice-Evans C, Halliwell B, Lunt GG, editors. Free Radical and Oxidative Stress: Environment, Drugs and Food Additives. Portland Press Ltd; London: 1995. pp. 3–31. [Google Scholar]
- DeAngelo AB, Daniel FB, McMillan L, Wernsing P, Savage RE. Species and strain sensitivity to the induction of peroxisome proliferation by chloroacetic acids. Toxicol. Appl. Pharmcol. 1989;101:285–298. doi: 10.1016/0041-008x(89)90277-9. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, Daniel FB, Stober JA, Olson GR. The carcinogenicity of dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 1991;16:337–347. doi: 10.1016/0272-0590(91)90118-n. [DOI] [PubMed] [Google Scholar]
- DeAngelo AB, George MH, House DE. Hepatocarcinogenicity in the male B6C3F1mouse following a lifetime exposure to dichloroacetic acid in the drinking water: dose-response determination and modes of action. J. Toxicol. Environ. Health A. 1999;58:485–507. doi: 10.1080/009841099157115. [DOI] [PubMed] [Google Scholar]
- Decant W, Metzler M, Henschler D. Novel metabolites of trichloroethylene through dechlorination reactions in mice and humans. Biochem. Pharmacol. 1984;33:2021–2027. doi: 10.1016/0006-2952(84)90568-9. [DOI] [PubMed] [Google Scholar]
- Fella K, Glückmann M, Hellman J, Karas M, Kramer PJ, Kröger M. Use of two-dimentional gel electrophoresis in predictive toxicology: identification of potential early protein biomarkers in chemically induced hepatocarcinogenesis. Proteomics. 2005;5:1914–1927. doi: 10.1002/pmic.200401067. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Leon A, Merdink JL, Bull RJ, Schultz IR. Effect of pretreatment with dichloroacetic or trichloroacetic acid in drinking water on the pharmacokinetics of a subsequent challenge dose in B6C3F1 mice. Chemico-Biol. Interact. 1999;123:239–253. doi: 10.1016/s0009-2797(99)00140-4. [DOI] [PubMed] [Google Scholar]
- Green T, Prout MS. Species differences in response to trichloroethylene. II Biotransformation in rats and mice. Toxicol. Appl. Pharmacol. 1985;79:401–411. doi: 10.1016/0041-008x(85)90138-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge MC, Cross CE. Free radicals, antioxidants, and human disease: Where are we now? J. Lab. Clin. Med. 1992;119:598–620. [PubMed] [Google Scholar]
- Hassoun EA, Cearfoss J, Spildener J. Dichloroacetate- and trichloroacetate-induced oxidative stress in the hepatic tissues of mice after long-term exposure. J. Appl. Toxicol. 2010 doi: 10.1002/jat.1516. ( www.interscinece.wiley.com)DOI 10.1002/jat.1516. NIHMSID: NIHMS189134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun EA, Dey S. Dichloroacetate- and trichloroacetate-induced phagocytic activation and production of oxidative stress in the hepatic tissues of mice after acute exposure. J. Biochem. Mol. Toxicol. 2008;22:27–34. doi: 10.1002/jbt.20210. [DOI] [PubMed] [Google Scholar]
- Hassoun E, Mehta J. Dichloroacetate-induced modulation of cellular antioxidant enzyme activities and glutathione level in the J774A.1 cells. J. Appl. Toxicol. 2008;28:931–937. doi: 10.1002/jat.1356. [DOI] [PubMed] [Google Scholar]
- Hathway DE. Consideration of the evidence for mechanisms of 1,1,2-trichloroethylene metabolism, including new identification of its dichloroacetic acid and trichloroacetic acid metabolites in mice. Cancer Lett. 1980;8:263–269. doi: 10.1016/0304-3835(80)90012-9. [DOI] [PubMed] [Google Scholar]
- Herren-Freund SL, Pereira MA, Khoury MD, Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol. Appl. Pharmacol. 1987;90:183–189. doi: 10.1016/0041-008x(87)90325-5. [DOI] [PubMed] [Google Scholar]
- James MO, Cornett R, Yan Z, Henderson GN, Stacpoole PW. Glutathione-dependent conversion to glyoxylate, a major pathway of dichloroacetate biotransformation in hepatic cytosol from humans and rats, is reduced in dichloroacetate-treated rats. Drug Metab. Dispos. 1997;25:1223–1227. [PubMed] [Google Scholar]
- Jiang J, Xu Y, Klaunig JE. Induction of oxidative stress in rat brain by acrylonitrile (CAN) Toxicol. Sci. 1998;46:333–341. doi: 10.1006/toxs.1998.2524. [DOI] [PubMed] [Google Scholar]
- Josephy PD, Mannervik B, De Montellano PO. Hydrogen peroxide, catalase and peroxidases. In: Josephy D, Mannervik B, De Montellano PO, editors. Molecular Toxicology. Oxford University Press Inc.; New York: 1997. pp. 81–89. [Google Scholar]
- Krasner SW, McGuire MJ, Jacagelo JG, Patania NL, Reagan KM, Aieta EM. The occurrence of disinfection by-products in U.S. drinking water. J. Am. Water Works Assoc. 1989;81:41–53. [Google Scholar]
- Larson JL, Bull RJ. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol. Appl. Pharmacol. 1992;115:268–277. doi: 10.1016/0041-008x(92)90332-m. [DOI] [PubMed] [Google Scholar]
- Lawrence R, Burk R. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lipscomb JC, Mahle DA, Brashear WT, Barton HA. Dichloroacetic acid: metabolism in cytosol. Drug. Metab. Dispos. 1995;23:1203–1205. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr WL, Randall RJ. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McCord JM. Human disease, free radicals, and the oxidant/antioxidant balance. Clin. Biochem. 1993;26:351–357. doi: 10.1016/0009-9120(93)90111-i. [DOI] [PubMed] [Google Scholar]
- Nelson MA, Bull RJ. Induction of strand breaks in DNA by trichloroethylene and metabolites in rat liver in vivo. Toxicol. Appl. Pharmacol. 1988;94:45–54. doi: 10.1016/0041-008x(88)90335-3. [DOI] [PubMed] [Google Scholar]
- Nelson MA, Lansing AJ, Sanchez IM, Bull RJ, Springer DL. Dichloroacetaic acid and trichloroacetic acid-induced DNA single strand breaks are independent of peroxisome proliferation. Toxicology. 1989;58:239–248. doi: 10.1016/0300-483x(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Parrish JM, Austin EW, Stevens DK, Kinder DH, Bull RJ. Haloacetate-induced oxidative damage to DNA in the liver of male B6C3F1 mice. Toxicology. 1996;110:103–111. doi: 10.1016/0300-483x(96)03342-2. [DOI] [PubMed] [Google Scholar]
- Pey A, Saborido A, Blázquez I, Delgado J, Megías A. Effects of prolonged stanozolol treatment on antioxidant enzyme activities, oxidative stress markers, and heat shock protein HSP72 levels in rat liver. J. Steroid. Biochem. Mol. Biol. 2003;87:269–277. doi: 10.1016/j.jsbmb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Reed D. Mechanisms of chemically induced cell injury and celluar protection mechanisms. In: Hodgson E, Levi PE, editors. Introduction to Biochemical Toxicology. Appleton & Lange; Connecticut: 1994. pp. 265–295. [Google Scholar]
- Richardson SD, Thruston DD, Jr., Krasner SW, Weinberg HS, miltner RJ, Schenck KM, Narotsky MG, McKague AB, Simmons JE. Integrated disinfection by products mixture research: Comprehensive characterization of water concentrates prepared from chlorinated and ozonated/postchlorinated drinking water. J. Toxicol. Environ. Health A. 2008;71:1165–1186. doi: 10.1080/15287390802182417. [DOI] [PubMed] [Google Scholar]
- Tong Z, Board PG, Anders MW. Glutathione transferase zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem. J. 1998;331:371–374. doi: 10.1042/bj3310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SM, Lin SK, Lee KT, Huang JC, Wu SH, Tsai L. y. Evaluation of redox statuses in patients with hepatitis B-virus associated hepatocellular carcinoma. Ann. Clin. Biochem. 2009;46:394–400. doi: 10.1258/acb.2009.009029. [DOI] [PubMed] [Google Scholar]
- Tzeng HF, Blackburn AC, Board PG, Anders MW. Polymorphism- and species-dependent inactivation of glutathione transferase zeta by dichloroacetate. Chem. Res. Toxicol. 2000;13:231–236. doi: 10.1021/tx990175q. [DOI] [PubMed] [Google Scholar]
- Uden PC, Miller JW. Chlorinated acids and chloral in drinking water. J. Am. Water Works Assoc. 1983;75:524–526. [Google Scholar]