Abstract
Background and Aim
Allergic bronchopulmonary aspergillosis (ABPA) is classified radiologically based on the findings of central bronchiectasis (CB) and other radiologic features (ORF). However, the long-term clinical significance of these classifications remains unknown. We hypothesized that the immunological activity and outcomes of ABPA could be predicted on HRCT chest finding of high-attenuation mucus (HAM), a marker of inflammatory activity. In this study, we evaluate the severity and clinical outcomes of ABPA with different radiological classifications.
Methods
Patients were classified based on CT chest findings as: (a) serologic ABPA (ABPA-S) and ABPA-CB; (b) ABPA-S, ABPA-CB, and ABPA-CB-ORF; and, (c) ABPA-S, ABPA-CB and ABPA-CB-HAM. The clinical, spirometric and serological (total and A fumigatus specific IgE levels, eosinophil count) severity of the disease and clinical outcomes in various classifications were analyzed.
Results
Of the 234 (123 males, 111 females; mean age, 34.1 years) patients, 55 (23.5%) had normal HRCT, 179 (76.5%) had CB, 49 (20.9%) had HAM, and 27 (11.5%) had ORF. All immunological markers were consistently higher in the HAM classification, while in other classifications these findings were inconsistent. On multivariate analysis, the factors predicting frequent relapses were presence of HAM (OR 7.38; 95% CI, 3.21–17.0) and CB (OR 3.93; 95% CI, 1.63–9.48) after adjusting for ORF.
Conclusions
The classification scheme based on HAM most consistently predicts immunological severity in ABPA. Central bronchiectasis and HAM are independent predictors of recurrent relapses in ABPA. Hence, HAM should be employed in the radiological classification of ABPA.
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a complex immunological disorder that commonly complicates the course of patients with asthma and cystic fibrosis. The disease occurs secondary to antigens released by Aspergillus fumigatus, an ubiquitous fungi that colonizes the tracheobronchial tree in these patients.[1], [2] The condition clinically presents with poorly controlled asthma, hemoptysis, weight loss and fever. The Rosenberg-Patterson criteria are most often used for diagnosis.[3], [4] High-resolution computed tomography (HRCT) of thorax is the imaging modality of choice for the diagnosis of ABPA. The findings on HRCT chest include central bronchiectasis (CB), high-attenuation mucus (HAM), air trapping (mosaic attenuation) and centrilobular nodules. The prevalence of ABPA in asthma is as high as 19% whereas the prevalence in cystic fibrosis ranges from 6–10%.[2], [5] The first case of ABPA was reported in 1952 by Hinson from the United Kingdom (UK)[6] whereas in the United States (US) it was identified in 1967.[7] There was an initial belief that the disorder is rare in North America[8] but subsequent reports disproved this myth.[9], [10] In fact, amongst the four largest series of ABPA in the world (111 cases [UK][11]; 118 cases [US][4]; 155 cases [India][12], 164 [India][13]), one is from the United States.
There are patients of ABPA who otherwise fulfill all diagnostic criteria, but lack demonstrable abnormalities on CT chest. They are labeled as seropositive ABPA (ABPA-S) compared with the more common presentation with CB (ABPA-CB).[14] Another CT classification scheme categorizes ABPA into mild (ABPA-S), moderate (ABPA-CB) and severe (ABPA-CB with other radiologic findings [ABPA-CB-ORF]).[15] The active inflammatory component of ABPA clinically manifests as excess mucus secretion and radiologically as mucoid impaction.[16] On HRCT images, the CT attenuation of mucoid impaction in ABPA is similar to or less than the attenuation of paraspinal skeletal muscle (CT Hounsfield values, 10–40).[17], [18], [19] However, in many patients, mucoid impaction manifests with high attenuation CT values (CT Hounsfield values, 70–170),[12], [18], [20] and is visually denser than the paraspinal skeletal muscle.[12], [18], [20], [21], [22], [23], [24] The criterion for HAM is based on the visual appearance of the density of mucus being greater than the paraspinal skeletal muscle. The corresponding high CT attenuation values wherever available further strengthens the diagnosis. The clinical importance of HAM lies in the fact that it has been shown to be associated with recurrent relapses.[12], [25]
Over the last two decades there is an increased awareness that CT findings can predict outcomes in many pulmonary disorders including CF.[26], [27], [28], [29] We hypothesized that the presence of HAM on CT chest at diagnosis would not only correlate with immunological severity, but could also predict outcome in ABPA. Herein, we assess the severity of the disease and clinical significance of different radiological classifications based on HRCT chest findings, and also propose a new radiologic classification for ABPA.
Materials and Methods
The present study is a post hoc analysis of prospectively collected data, and includes 234 consecutive patients of ABPA diagnosed between January 2004 and December 2008 and followed till December 2009. The clinical characteristics of 205 patients have been previously described. [12], [25], [30] The study was approved by the Institute Ethics Committee, PGIMER, Chandigarh, and a written informed consent was taken from all patients.
In our Chest Clinic, all patients with asthma (except those with glucocorticoid intake more than three weeks in the preceding six months) are screened for Aspergillus sensitization using an intradermal skin test. Patients who demonstrate immediate cutaneous hyperreactivity to Aspergillus antigen are further investigated for ABPA with IgE levels (total and A fumigatus specific), eosinophil count, Aspergillus precipitins and HRCT chest. The detailed methodology has been previously described.[12], [25], [30] Patients are diagnosed as ABPA if they meet both the following criteria: (a) total IgE levels >1000 IU/mL; (b) A fumigatus specific IgE levels >0.35 kUA/L; and, two of the following criteria: (a) presence of serum precipitins against A fumigatus; (b) radiographic pulmonary opacities (fixed/transient); (c) absolute eosinophil count >1000 cells/µL; (d) central bronchiectasis on HRCT.[12], [25], [30], [31]
The HRCT of the chest was categorized for the presence and extent of bronchiectasis.[32] Bronchiectasis was classified as ‘central’ when confined to the medial half (point midway between hilum and chest wall) of the lung.[33] The presence of HAM was considered if the mucus was visually denser than the normal paraspinal skeletal muscle and the corresponding CT attenuation values were noted.[12], [24] Other radiologic findings (ORF) such as pulmonary fibrosis, bleb, bullae, pneumothorax, parenchymal scarring, emphysematous change, multiple cyst, fibrocavitary lesions and pleural thickening proposed by Kumar et al. were recorded.[15] Based on the HRCT findings the patients were classified according to the following schemes:
(a) Greenberger classification: ABPA-S and ABPA-CB[14]
(b) Kumar classification: ABPA-S, ABPA-CB and ABPA-CB-ORF[15]
(c) Classification based on HAM: ABPA-S, ABPA-CB and ABPA-CB-HAM
The patients were treated with glucocorticoids and followed up with history and physical examination, chest radiograph and total IgE levels every six weeks.[2] Treatment response was classified as remission, if the IgE levels declined by >35% and there was clinical/radiological improvement after three months of glucocorticoids or relapse if there was a doubling of baseline IgE levels with clinicoradiological worsening. Patients with two or more relapses were classified as frequent relapses whereas those with no or one relapse were categorized as infrequent relapses. For the purpose of this study, we evaluated the clinical and serological severity in the three radiological classifications, and the factors predicting frequent relapses. To ascertain the clinical significance of various classifications, we systematically excluded patients with HAM, CB and ORF from each of the classifications and then re-analyzed the severity.
Statistical analysis
Data are presented as mean (SD), median (IQR) or number (percentage). Statistical significance was assumed at a p-value of less than 0.05. The differences between continuous variables were analyzed using student's t-test, Mann-Whitney U test, ANOVA (with Scheffe's test for post hoc analysis) or Kruskal-Waalis test (with Dwass-Steel-Critchlow-Fligner test for post hoc analysis) as appropriate. Categorical variables were compared using the chi-square test. A multivariate logistic regression analysis was performed to determine the CT findings predicting frequent relapses.
Results
There were 123 males and 111 females with a mean (SD) age of 34.1 (12.5) years. The baseline characteristics of these patients are shown in Table 1. The median duration of asthma was six years with the recognition of ABPA being generally delayed. In fact, 40.6% of patients had inappropriately received antitubercular therapy in the past. Fifty-five (23.5%) patients had a normal HRCT (ABPA-S) and 179 (76.5%) had CB (ABPA-CB) (Figure 1). Forty-nine (20.9%) patients were identified to have HAM impaction on HRCT chest (Figure 2) at presentation whereas 27 (11.5%) patients had ORF (Figure 3). Three patients had HAM involving a single lobe whereas the remaining had multilobar involvement. The density measurements are available in only 15 patients and ranged from 108 to 168 Hounsfield units.
Table 1. Baseline characteristics including spirometry, serological and HRCT findings (n = 234).
| Demographic details | |
| Age (years), mean (SD) | 34.1 (12.5) |
| Male Gender, No. (%) | 123 (52.6) |
| History | |
| Duration of Asthma (years), median (IQR) | 6 (4–13) |
| Hemoptysis, No. (%) | 86 (36.8) |
| Expectoration of brownish black mucous plugs, No. (%) | 93 (39.7) |
| Cigarette smoking, No. (%) | 14 (6) |
| History of anti-tuberculous therapy, No. (%) | 95 (40.6) |
| Spirometry, No. (%) | |
| Normal | 55 (23.5) |
| Mild obstruction | 59 (25.2) |
| Moderate obstruction | 75 (32.1) |
| Severe obstruction | 45 (19.2) |
| Bronchodilator reversibility | 103 (44) |
| Serological findings | |
| Aspergillus skin test, No. (%) | |
| Type I | 234 (100) |
| Type III | 181 (77.4) |
| Absolute eosinophil count (cells/µL), median (IQR) | 847 (480–1551) |
| Aspergillus precipitins, No. (%) | 194 (82.9) |
| Total IgE levels (IU/mL), median (IQR) | 5015 (2839–10000) |
| A fumigatus specific IgE levels (kUA/L), median (IQR) | 4.6 (1.4–16.1) |
| HRCT findings, No. (%) | |
| Normal | 55 (23.5) |
| Central bronchiectasis | 179 (76.5) |
| No. of lobes, median (IQR) | 3 (2–4) |
| No. of segments, median (IQR) | 7 (5–10) |
| Other radiologic findings (pulmonary fibrosis, bleb, bullae, pneumothorax, parenchymal scarring, emphysematous change, multiple cyst, fibrocavitary lesions and pleural thickening) | 27 (11.5) |
| Centrilobular nodules and/or tree-in-bud opacities | 69 (29.5) |
| High attenuation mucus | 49 (20.9) |
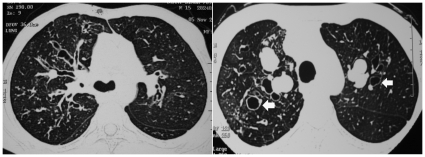
Figure 1. Presence of central bronchiectasis in two different patients with allergic bronchopulmonary aspergillosis.
The presence of classic signet ring appearance of dilated bronchi is easily appreciable (arrows). The bronchiectasis is located predominantly in the inner half of the lung fields.
Figure 2. High-resolution computed tomographic images of patients with allergic bronchopulmonary aspergillosis demonstrating the presence of high-attenuation mucus.
(A) Mediastinal window showing the presence of hyperattenuated mucus within dilated bronchi. The mucus is denser than the paraspinal skeletal muscle (asterisk) (B) Lung window shows that hyperdense mucus can occasionally be appreciated even with the parenchymal sections (circle); (C) CT Hounsfield values of mucus in dilated bronchi: mucus in the left lung is hyperdense with higher CT attenuation values compared to mucoid impaction in the right lung; (d) Hyperattenuated (bold arrow) and normal attenuation mucus (thin arrow) in the same mediastinal window.
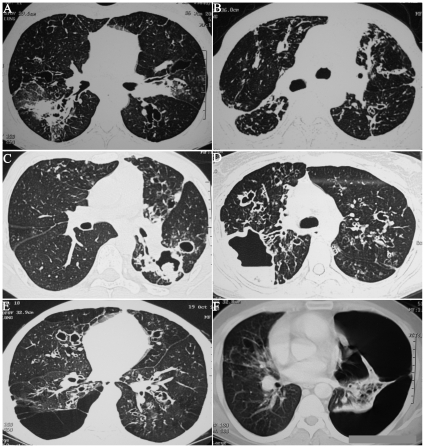
Figure 3. Other radiological features (ORF) that are encountered in patients with allergic bronchopulmonary aspergillosis:
(A) parenchymal fibrosis involving the right lower lobe; (B) parenchymal and pleural fibrosis; (C) aspergilloma within a bronchiectatic cavity; (D) large cavity with air fluid level; (E) multiple bulla with central bronchiectasis; (F) left sided pneumothorax- numerous bronchiectatic cavities can be appreciated in the collapsed lung.
Greenberger classification
The clinical features, spirometry and serological findings as per the Greenberger classification is shown in Table 2. The A fumigatus specific IgE levels and eosinophil counts but not the total IgE levels were higher in ABPA-CB compared to ABPA-S. However, the serological severity varied once patients with HAM and ORF were excluded from the analysis. On removal of ORF, both A fumigatus specific IgE levels and eosinophil counts remained significant, while on removal of HAM only the eosinophil counts retained significance (Table 2). The clinical history and the spirometry findings were similar in the two groups. The numbers of relapses were higher in the CB group irrespective of the presence or absence of HAM and ORF.
Table 2. Clinical, spirometric, serological differences and outcomes in patients with allergic bronchopulmonary aspergillosis (ABPA) based on the classification by Greenberger et al with and without the presence of high attenuation mucus (HAM) and other radiologic findings (ORF).
| All patients (n = 234) | Without HAM (n = 185) | Without ORF (n = 207) | |||||
| Characteristics | ABPA-S (n = 55) | ABPA-CB (n = 179) | P value | ABPA-CB (n = 130) | P value | ABPA-CB (n = 152) | P value |
| Clinical history | |||||||
| Age, in years | 35.9 (13.4) | 33.6 (12.2) | 0.23 | 33.9 (12.3) | 0.37 | 34.1 (11.9) | 0.36 |
| Male Gender, No.(%) | 25 (45.5) | 98 (54.7) | 0.23 | 73 (56.2) | 0.18 | 83 (54.6) | 0.24 |
| Duration of asthma, in years | 6 (5–12) | 6 (4–14) | 0.85 | 7 (4–15) | 0.75 | 7 (4–15) | 0.82 |
| Spirometry , No.(%) | |||||||
| Normal | 15 (27.3) | 40 (22.3) | 0.33 | 21 (16.2) | 0.05 | 36 (23.9) | 0.55 |
| Mild obstruction | 14 (25.5) | 45 (25.1) | 34 (26.2) | 39 (25.7) | |||
| Moderate obstruction | 20 (36.4) | 55 (30.7) | 39 (30) | 48 (31.6) | |||
| Severe obstruction | 6 (10.9) | 39 (21.8) | 36 (27.7) | 29 (19.1) | |||
| Serological findings | |||||||
| Total IgE levels, IU/mL | 3850 (2650–7800) | 5400 (2859–10313) | 0.07 | 4689 (2619–7895) | 0.65 | 5457 (2867–10314) | 0.06 |
| A fumigatus specific IgE levels, kUA/L | 2.1 (0.95–9.5) | 4.8 (1.7–18) | 0.02 | 3.4 (1.4–13.8) | 0.19 | 5.6 (1.9–17.9) | 0.01 |
| Absolute eosinophil count, cells/µL | 620 (250–962) | 983 (558–1770) | 0.001 | 850 (475–1506) | 0.03 | 1048 (650–1800) | 0.0001 |
| Type III AST, No.(%) | 45 (81.8) | 136 (76) | 0.37 | 98 (75.4) | 0.34 | 120 (79.5) | 0.2 |
| Aspergillus precipitins, No.(%) | 48 (87.3) | 146 (82) | 0.36 | 103 (79.8) | 0.23 | 117 (77) | 0.46 |
| Clinical outcome | |||||||
| Number of relapses | 0 (0–1) | 2 (0–2) | 0.0001 | 1 (0–2) | 0.004 | 2 (0–2) | 0.0001 |
| Frequent (≥2) relapses, No. (%) | 8 (14.5) | 89 (49.7) | 0.0001 | 49 (37.6) | 0.002 | 75 (49.3) | 0.0001 |
Values are expressed as mean (SD) or median (IQR) unless otherwise stated; ABPA-S - serologic ABPA; ABPA-CB - ABPA with central bronchiectasis.
The A fumigatus specific IgE levels and eosinophil counts were higher in ABPA-CB compared to ABPA-S. The serological severity varies once patients with HAM and ORF are excluded. On removal of HAM (column 4), only the eosinophil counts retain significance, whereas on removal of ORF (column 6), both A fumigatus specific IgE levels and eosinophil counts remain significant.
Kumar classification
On classifying the patients according to the Kumar classification (Table 3), the clinical history and spirometric findings were similar in the three groups. The total IgE levels were statistically insignificant between the groups. The A fumigatus specific IgE levels and eosinophil counts were significantly different between the groups. On post hoc analysis, the A fumigatus specific IgE levels were higher in ABPA-CB vs. ABPA-S and the eosinophil counts were higher in ABPA-CB compared to the other two groups (ABPA-S and ABPA-CB-ORF). Once patients with HAM were excluded, only the eosinophil counts retained significance in patients with ABPA-CB compared to ABPA-S and ABPA-CB-ORF. The number of relapses were higher in both ABPA-CB and ABPA-CB-ORF compared to ABPA-S, but was not different between ABPA-CB and ABPA-CB-ORF.
Table 3. Clinical, spirometric, serological differences and outcomes in patients with allergic bronchopulmonary aspergillosis (ABPA) based on the classification by Kumar et al with and without the presence high attenuation mucus (HAM).
| All patients (n = 234) | Without HAM (n = 185) | ||||||
| Characteristics | ABPA-S (n = 55) | ABPA-CB (n = 152) | ABPA-CB-ORF (n = 27) | P value | ABPA-CB (n = 111) | ABPA-CB-ORF (n = 19) | P value |
| Clinical history | |||||||
| Age, in years | 35.9 (13.4) | 34.1 (11.9) | 30.4 (13.7) | 0.16 | 34.2 (11.9) | 32 (14.9) | 0.49 |
| Male Gender, No.(%) | 25 (45.5) | 83 (54.6) | 15 (55.6) | 0.48 | 61 (55) | 12 (63.2) | 0.33 |
| Duration of asthma, in years | 6 (5–12) | 7 (4–15) | 5 (3–10) | 0.18 | 8 (4–15) | 5 (3–8) | 0.08 |
| Spirometry , No.(%) | |||||||
| Normal | 15 (27.3) | 36 (23.7) | 4 (14.8) | 0.21 | 20 (18) | 3 (15.8) | 0.25 |
| Mild obstruction | 14 (25.5) | 39 (25.7) | 6 (22.2) | 30 (27) | 4 (21.1) | ||
| Moderate obstruction | 20 (36.4) | 48 (31.6) | 7 (25.9) | 34 (30.6) | 5 (26.3) | ||
| Severe obstruction | 6 (10.9) | 29 (19.1) | 10 (37) | 27(24.3) | 7 (36.8) | ||
| Serological findings | |||||||
| Total IgE levels, IU/mL | 3850 (2650–7800) | 5457 (2867–10314) | 5400 (2320–10220) | 0.18 | 4588 (2500–7860) | 5800 (2659–11000) | 0.36 |
| A fumigatus specific IgE levels, kUA/L | 2.1 (0.95–9.5) | 5.6 (1.9–17.9)c | 2.3 (1.4–18.3) | 0.03 | 3.9 (1.4–13) | 1.6 (1.1–18.3) | 0.35 |
| Absolute eosinophil count, cells/µL | 620 (250–962) | 1048 (650–1800)c , d | 450 (200–985) | 0.0001 | 983 (593–1540)c , d | 370 (161–650) | 0.0001 |
| Type III AST, No.(%) | 45 (81.8) | 117 (77) | 19 (70.4) | 0.5 | 84 (75.7) | 14 (73.7) | 0.62 |
| Aspergillus precipitins, No.(%) | 48 (87.3) | 120 (79.5) | 26 (96.3) | 0.06 | 85 (77.3) | 18 (94.7) | 0.09 |
| Clinical outcome | |||||||
| Number of relapses | 0 (0–1) | 2 (0–2)c | 2 (0–2)a | 0.0001 | 1 (0–2)c | 1 (0–2) | 0.03 |
| Frequent (≥2) relapses, No. (%) | 8 (14.5) | 75 (49.3)c | 14 (51.8)a | 0.0001 | 42 (37.8)c | 7 (36.8)a | 0.007 |
Values are expressed as mean (SD) or median (IQR) unless otherwise stated; ABPA-S - serologic ABPA; ABPA-CB - ABPA with central bronchiectasis; ABPA-CB-ORF - ABPA with CB and other radiologic findings;
ABPA-CB-ORF value significant compared to ABPA-s;
ABPA-CB-ORF value significant compared to ABPA-CB;
ABPA-CB value significant compared to ABPA-s;
ABPA-CB value significant compared to ABPA-CB-ORF;
The A fumigatus specific IgE levels and eosinophil counts, but not the total IgE levels are different between the groups (columns 1 to 3). On post hoc analysis, the A fumigatus specific IgE levels are higher in ABPA-CB (column 2) compared to ABPA-S (column 1) and the eosinophil counts are higher in ABPA-CB (column 2) in comparison to the other two groups (columns 1 and 3). Once HAM is excluded (columns 5 and 6), only the eosinophil counts remain significant in ABPA-CB (column 5) compared to ABPA-S (column 1) and ABPA-CB-ORF (column 6).
New classification based on HAM
On classifying patients based on HAM (Table 4), the eosinophil counts and the IgE levels (total and A fumigatus specific) were higher in ABPA-CB-HAM compared to both ABPA-S and ABPA-CB, and remained significant even after exclusion of patients with ORF (Table 5). The clinical history and the spirometric findings were similar in the three groups. The numbers of relapses were not only higher in both ABPA-CB and ABPA-CB-HAM compared to ABPA-S but were also higher in ABPA-CB-HAM vs. ABPA-CB.
Table 4. Clinical, spirometric, serological differences and outcomes in patients with allergic bronchopulmonary aspergillosis (ABPA) based on the proposed staging on the basis of high attenuation mucus (HAM) with and without the presence of other radiologic findings (ORF).
| All patients (n = 234) | Without ORF (n = 207) | ||||||
| Characteristics | ABPA-S (n = 55) | ABPA-CB (n = 130) | ABPA-CB-HAM (n = 49) | P value | ABPA-CB (n = 107) | ABPA-CB- HAM (n = 45) | P value |
| Clinical history | |||||||
| Age, in years | 35.9 (13.4) | 33.9 (12.3) | 32.6 (12) | 0.33 | 34.6 (11.9) | 33 (11.9) | 0.37 |
| Male Gender, No.(%) | 25 (45.5) | 73 (56.2) | 25 (51) | 0.4 | 59 (55.1) | 24 (53.3) | 0.5 |
| Duration of asthma, in years | 6 (5–12) | 7 (4–15) | 5 (3–11) | 0.28 | 7 (4–15) | 5 (3.5–12) | 0.27 |
| Spirometry , No.(%) | |||||||
| Normal | 15 (27.3) | 21 (16.2) | 10 (20.4) | 0.25 | 19 (17.8) | 10 (22.2) | 0.48 |
| Mild obstruction | 14 (25.5) | 34 (26.2) | 11 (22.4) | 29 (27.6) | 10 (22.2) | ||
| Moderate obstruction | 20 (36.4) | 39 (30) | 16 (32.7) | 33 (30.8) | 15 (33.3) | ||
| Severe obstruction | 6 (10.9) | 36 (27.7) | 12 (24.5) | 26 (24.3) | 10 (22.2) | ||
| Serological findings | |||||||
| Total IgE levels, IU/mL | 3850 (2650–7800) | 4689 (2619–7895) | 10220 (5310–12200)a , b | 0.0001 | 4590 (2698–7860) | 10314 (5556–12435) a , b | 0.0001 |
| A fumigatus specific IgE levels, kUA/L | 2.1 (0.95–9.5) | 3.4 (1.4–13.8) | 7.8 (4.7–23.7)a , b | 0.0001 | 3.9 (1.4–13) | 9.3 (4.7–23.9) a , b | 0.0001 |
| Absolute eosinophil count, cells/µL | 620 (250–962) | 850 (475–1506) | 1200 (800–2506)a , b | 0.0001 | 983 (593–1540) | 1200 (824–2056) a , b | 0.0001 |
| Type III AST, No.(%) | 45 (81.8) | 98 (75.4) | 38 (77.6) | 0.63 | 82 (76.6) | 35 (77.8) | 0.75 |
| Aspergillus precipitins, No.(%) | 48 (87.3) | 103 (79.8) | 43 (87.8) | 0.3 | 81 (76.4) | 39 (86.7) | 0.15 |
| Clinical outcome | |||||||
| Number of relapses | 0 (0–1) | 1 (0–2)c | 3 (2–3)a , b | 0.0001 | 1 (0–2)c | 3 (2–3)a , b | 0.0001 |
| Frequent (≥2) relapses, No. (%) | 8 (14.5) | 49 (37.7)c | 40 (81.6)a , b | 0.0001 | 39 (36.4)c | 36 (80)a , b | 0.0001 |
Values are expressed as mean (SD) or median (IQR) unless otherwise stated; ABPA-S - serologic ABPA; ABPA-CB - ABPA with central bronchiectasis;
ABPA-CB-HAM value significant compared to ABPA-s;
ABPA-CB-HAM value significant compared to ABPA-CB;
ABPA-CB value significant compared to ABPA-s;
ABPA-CB value significant compared to ABPA-HAM.
Table 5. Effects of HRCT findings (HAM, number of bronchiectatic segments and ORF) on occurrence of frequent relapses - multivariate logistic regression model.
| Variables | Adjusted odds ratio (95% confidence intervals) | P value |
| High-attenuation mucus | 6.86 (3.03 to 15.52) | 0.0001 |
| Other radiologic findings | 1.34 (0.56 to 3.21) | 0.505 |
| Central bronchiectasis | 3.41 (1.45 to 8.01) | 0.005 |
| Constant | 1.043 | 0.901 |
Impact of serological and CT findings on frequent relapses
There was clinical and radiological improvement in all the patients receiving glucocorticoid therapy. The mean (SD) duration of follow-up was 31.1 (17.6) months. 138 (59%) patients experienced relapse of ABPA with the median (range) relapses being 1 (0–7). There were 97 (41.5%) patients with frequent relapses. On multivariate logistic regression analysis, the factors predicting frequent relapses were presence of HAM and CB after adjusting for other CT finding of ORF (Table 5).
Discussion
The results of this study suggest that HRCT chest findings correlate with immunological severity in ABPA, and the most consistent marker of serological severity was HAM. The classification of ABPA based on other criteria was inconsistent with some features being severe in one classification but not in the other. The CT findings of HAM and CB at diagnosis were also independent predictors of recurrent relapses.
The Rosenberg-Patterson criteria (Table 6) are most frequently used in the diagnosis of ABPA.[3], [4] However, there is lack of consensus on the number of criteria required for diagnosis or the specific cut-off values for the various tests utilized in diagnosis.[34] An immediate cutaneous hypersensitivity to A fumigatus antigen is the most reliable method for screening for ABPA.[5], [31], [35], [36], [37] A positive test is virtually seen in all patients although 40% of asthmatics without ABPA can also demonstrate skin test positivity.[5] However, the type III and IV cutaneous reactions do not have diagnostic or prognostic value.[2], [12], [25], [30] The total IgE level is another useful test in the diagnosis of ABPA, and normal values exclude active ABPA from the work-up of patients with respiratory symptoms. Neither the initial levels nor the quantum decline in IgE values have any prognostic significance.[12], [25] Elevated A fumigatus specific IgG/IgE levels greater than twice the pooled serum samples from patients with Aspergillus hypersensitive asthma is paramount in the differential diagnosis of ABPA from Aspergillus sensitized asthma.[30], [38] Serum precipitins to A fumigatus although present in 69–90% of patients with ABPA,[11], [12], [39], [40], [41] are also seen in 10% of other pulmonary disorders including asthma,[41], [42], [43] and thus represent supportive not diagnostic evidence for ABPA.
Table 6. Rosenberg-Patterson criteria for the diagnosis of allergic bronchopulmonary aspergillosis [3], [4].
| Major criteria |
| • Bronchial asthma |
| • Immediate cutaneous hypersensitivity to A fumigatus antigen |
| • Serum total IgE levels (>1000 IU/mL) |
| • Serum A fumigatus specific IgG and/or IgE levels more than twice the mean plus two standard deviation values in patients with Aspergillus hypersensitive asthma |
| • Central bronchiectasis on HRCT chest |
| • Serum precipitins against A fumigatus |
| • Fleeting or fixed pulmonary opacities on chest radiograph |
| • Peripheral blood eosinophil count >1000 cells/µL |
| Minor criteria |
| • Sputum cultures demonstrating growth of A fumigatus |
| • Expectoration of brownish-black mucus plugs |
| • Type III skin reactions to A fumigatus antigen |
The presence of six out eight major criteria makes the diagnosis almost certain.
Similarly, there is no agreement on the severity classification, and they continue to be modified and updated.[31], [34] Greenberger et al. classified ABPA into ABPA-S or ABPA-CB respectively depending on the absence or presence of bronchiectasis.[14] They proposed that patients with ABPA-S represent the earliest stage of the disease with less severe immunologic findings compared to ABPA-CB.[14] However, in this study only the A fumigatus specific IgG levels and precipitins were higher in patients with ABPA-CB.[14] The other immunologic parameters namely total IgE and A fumigatus specific IgE were similar in the two groups.[14] Thereafter Kumar et al. divided ABPA into three categories and suggested that ABPA-CB-ORF represents clinically and serologically the severest form of ABPA.[15] However, this study had included only 18 patients (six in each group). Moreover, the findings included in the categorization of ABPA-CB-ORF were all changes representing the fibrotic stage of the disease. Both the studies were limited by the sample size and the fact that the clinical significance of these classifications was not investigated.
In this study, we evaluated the clinical and serological severity of both these classifications using data from a large set of patients. The classification scheme by Greenberger et al. showed immunological severity in some parameters (eosinophil count and A fumigatus specific IgE levels) but not in others (total IgE levels). Moreover, on excluding patients with HAM, the immunological severity was restricted only to eosinophil counts. Interestingly, in the Kumar classification, the immunological markers were most severe in patients with ABPA-CB and not ABPA-CB-ORF. This suggests that ORF does not determine serological severity and probably represents the fibrotic, burnt out phase of the disease. In addition to evaluating these two classifications, we proposed a new classification based on HAM.[16], [44], [45] The HAM classification was the most consistent with immunological severity persisting even after removal of patients with ORF.
High attenuation mucous plugging is described as the most characteristic finding of ABPA.[22], [46], [47] The finding is attributed either to the presence of calcium salts, iron and manganese,[48] or desiccated mucus.[49] The association of HAM with poorer outcomes in ABPA remains unclear, but one obvious reason is that the higher attenuation points to a more inspissated type of mucus. It is probable that the presence of HAM defines a subgroup of patients with more severe inflammation. Numerous genetic alterations have been described with ABPA,[2] and it may well be hypothesized that patients with HAM have certain genetic abnormalities that dictate a disease with more severe inflammation and poorer outcomes. However, more research is needed to investigate the exact reason for this association. There was no specific anatomical/geometric occurrence and distribution of HAM observed in this study. In contrast to previous series that found HAM to be more prevalent in unilobar mucus plugging,[18] most of our patients had multilobar hyperdense mucus plugging.
We also assessed the clinical significance of all classifications in terms of relapse. The presence of CB was a consistent marker of relapse in all classifications irrespective of whether patients with HAM or ORF were included or not. Moreover, in the Kumar classification, there was no statistical significance in the relapse rates between ABPA-CB and ABPA-CB-ORF, suggesting that it is the CB and not ORF that predicts relapse. However, in the HAM classification, the relapse rates were higher in ABPA-CB-HAM compared to ABPA-CB even after excluding patients with ORF suggesting that HAM independently predicts relapses. Finally, this was also confirmed in the multivariate model, wherein CB and HAM were independent predictors of frequent relapses.
Few studies have described factors predicting outcome in patients with ABPA. In two earlier studies, we had observed that HAM and CB are independent markers of relapse.[12], [25] Greenberger et al. and Kumar et al. reported that patients with ABPA-S developed fewer exacerbations and no patient progressed to irreversible lung disease.[14], [15], [50] However, the studies by Greenberger and Kumar did not include all the CT findings. Also, these studies did not perform multivariate analysis to determine factors predicting frequent relapses, which was performed in our study.[14], [15], [50]
The current study is different from our previous reports in numerous aspects, although it does contain majority of patients from these previous studies.[12], [25], [30] The outcome used for defining relapses is different from our previous reports.[12], [30] Previously, we had defined relapse as doubling of the baseline IgE levels irrespective of the patient's symptoms or appearance of radiological opacities.[12], [25], [30] However, we have observed that doubling of IgE levels could also occur non-specifically and hence in this study we have used a definition that includes doubling of IgE levels as well as clinicoradiological worsening. Another important difference is the definition of ORF used in this study. In our previous study, we had defined ORF as that originally described by Kumar et al. plus other CT features such as centrilobular nodules, tree-in-bud opacities and HAM.[30] However, in this study the definition of ORF is strictly retained as defined by Kumar et al.[15] This strict compartmentalization has been performed to clearly ascertain the significance of ORF vis-à-vis HAM in defining the radiological severity and their significance in ABPA. Finally the multivariate regression model in this study includes only CT findings and not serological findings, because we had previously demonstrated that serological findings do not predict clinical outcome.[12], [25]
What are the clinical implications of this study? Based on the results of this study, a patient with ABPA and hyperattenuated mucus at diagnosis represents an immunologically severe disease with increased propensity of recurrent relapses. Whether these group of patients require more intensive treatment protocols or closer monitoring remains to be answered. Hence, HAM should be incorporated in the classification of ABPA. These conclusions are valid and particularly relevant, not only because of the large sample size and long duration of follow-up, but also due to the fact that we analyzed the clinical, serological and long-term outcomes in terms of relapses.
In conclusion, the classification scheme based on high attenuation mucus most consistently predicts immunological severity in ABPA. CB and HAM are independent predictors of frequent relapses in ABPA. Hence, HAM should be employed in the radiological classification of ABPA.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Tillie-Leblond I, Tonnel AB. Allergic bronchopulmonary aspergillosis. Allergy. 2005;60:1004–1013. doi: 10.1111/j.1398-9995.2005.00887.x. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135:805–826. doi: 10.1378/chest.08-2586. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg M, Patterson R, Mintzer R, Cooper BJ, Roberts M, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med. 1977;86:405–414. doi: 10.7326/0003-4819-86-4-405. [DOI] [PubMed] [Google Scholar]
- 4.Patterson R, Greenberger PA, Halwig JM, Liotta JL, Roberts M. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med. 1986;146:916–918. doi: 10.1001/archinte.146.5.916. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2009;13:936–944. [PubMed] [Google Scholar]
- 6.Hinson KFW, Moon AJ, Plummer NS. Broncho-pulmonary aspergillosis; a review and a report of eight new cases. Thorax. 1952;7:317–333. doi: 10.1136/thx.7.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson R, Golbert TM. Hypersensitivity disease of the lung. Univ Mich Med Cent J. 1968;34:8–11. [PubMed] [Google Scholar]
- 8.Slavin RG, Stanczyk DJ, Lonigro AJ, Broun GO. Allergic bronchopulmonary aspergillosis—a North American Rarity. Clinical and immunologic characteristics. Am J Med. 1969;47:306–313. doi: 10.1016/0002-9343(69)90156-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoehne J, Reed C, Dickie H. Allergic bronchopulmonary aspergillosis is not rare. J Lab Clin Med. 1971;78:1007–1008. [PubMed] [Google Scholar]
- 10.Hoehne JH, Reed CE, Dickie HA. Allergic bronchopulmonary aspergillosis is not rare. With a note on preparation of antigen for immunologic tests. Chest. 1973;63:177–181. doi: 10.1378/chest.63.2.177. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy DS, Pepys S. Allergic broncho-pulmonary aspergillosis. Clinical immunology: (1) Clinical features. Clin Allergy. 1971;1:261–286. doi: 10.1111/j.1365-2222.1971.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Chakrabarti A, et al. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest. 2007;132:1183–1190. doi: 10.1378/chest.07-0808. [DOI] [PubMed] [Google Scholar]
- 13.Shah A, Kala J, Sahay S, Panjabi C. Frequency of familial occurrence in 164 patients with allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol. 2008;101:363–369. doi: 10.1016/S1081-1206(10)60311-0. [DOI] [PubMed] [Google Scholar]
- 14.Greenberger PA, Miller TP, Roberts M, Smith LL. Allergic bronchopulmonary aspergillosis in patients with and without evidence of bronchiectasis. Ann Allergy. 1993;70:333–338. [PubMed] [Google Scholar]
- 15.Kumar R. Mild, moderate, and severe forms of allergic bronchopulmonary aspergillosis: a clinical and serologic evaluation. Chest. 2003;124:890–892. doi: 10.1378/chest.124.3.890. [DOI] [PubMed] [Google Scholar]
- 16.Slavin RG, Bedrossian CW, Hutcheson PS, Pittman S, Salinas-Madrigal L, et al. A pathologic study of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1988;81:718–725. doi: 10.1016/0091-6749(88)91044-5. [DOI] [PubMed] [Google Scholar]
- 17.Bulcke JA, Termote JL, Palmers Y, Crolla D. Computed tomography of the human skeletal muscular system. Neuroradiology. 1979;17:127–136. doi: 10.1007/BF00339869. [DOI] [PubMed] [Google Scholar]
- 18.Logan PM, Muller NL. High-attenuation mucous plugging in allergic bronchopulmonary aspergillosis. Can Assoc Radiol J. 1996;47:374–377. [PubMed] [Google Scholar]
- 19.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 20.Morozov A, Applegate KE, Brown S, Howenstine M. High-attenuation mucus plugs on MDCT in a child with cystic fibrosis: potential cause and differential diagnosis. Pediatr Radiol. 2007;37:592–595. doi: 10.1007/s00247-007-0471-8. [DOI] [PubMed] [Google Scholar]
- 21.Goyal R, White CS, Templeton PA, Britt EJ, Rubin LJ. High attenuation mucous plugs in allergic bronchopulmonary aspergillosis: CT appearance. J Comput Assist Tomogr. 1992;16:649–650. [PubMed] [Google Scholar]
- 22.Karunaratne N, Baraket M, Lim S, Ridley L. Case quiz. Thoracic CT illustrating hyperdense bronchial mucous plugging: allergic bronchopulmonary aspergillosis. Australas Radiol. 2003;47:336–338. doi: 10.1046/j.1440-1673.2003.01192.x. [DOI] [PubMed] [Google Scholar]
- 23.Molinari M, Ruiu A, Biondi M, Zompatori M. Hyperdense mucoid impaction in allergic bronchopulmonary aspergillosis: CT appearance. Monaldi Arch Chest Dis. 2004;61:62–64. [PubMed] [Google Scholar]
- 24.Agarwal R, Aggarwal AN, Gupta D. High-attenuation mucus in allergic bronchopulmonary aspergillosis: another cause of diffuse high-attenuation pulmonary abnormality. AJR Am J Roentgenol. 2006;186:904. doi: 10.2214/AJR.05.0125. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Saikia B, et al. Clinical significance of decline in serum IgE Levels in allergic bronchopulmonary aspergillosis. Respir Med. 2010;104:204–210. doi: 10.1016/j.rmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Logan PM, O'Laoide RM, Mulherin D, O'Mahony S, FitzGerald MX, et al. High resolution computed tomography in cystic fibrosis: correlation with pulmonary function and assessment of prognostic value. Ir J Med Sci. 1996;165:27–31. doi: 10.1007/BF02942797. [DOI] [PubMed] [Google Scholar]
- 27.Screaton NJ, Hiorns MP, Lee KS, Franquet T, Johkoh T, et al. Serial high resolution CT in non-specific interstitial pneumonia: prognostic value of the initial pattern. Clin Radiol. 2005;60:96–104. doi: 10.1016/j.crad.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Ghaye B, Ghuysen A, Bruyere PJ, D'Orio V, Dondelinger RF. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26:23–39; discussion 39–40. doi: 10.1148/rg.261055062. [DOI] [PubMed] [Google Scholar]
- 29.Hanak V, Golbin JM, Hartman TE, Ryu JH. High-resolution CT findings of parenchymal fibrosis correlate with prognosis in hypersensitivity pneumonitis. Chest. 2008;134:133–138. doi: 10.1378/chest.07-3005. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–448. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal R, Hazarika B, Gupta D, Aggarwal AN, Chakrabarti A, et al. Aspergillus hypersensitivity in patients with chronic obstructive pulmonary disease: COPD as a risk factor for ABPA? Med Mycol. 2010 doi: 10.3109/13693781003743148. doi: 10.3109/13693781003743148: In Press. [DOI] [PubMed] [Google Scholar]
- 32.Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995;165:261–267. doi: 10.2214/ajr.165.2.7618537. [DOI] [PubMed] [Google Scholar]
- 33.Hansell DM, Strickland B. High-resolution computed tomography in pulmonary cystic fibrosis. Br J Radiol. 1989;62:1–5. doi: 10.1259/0007-1285-62-733-1. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal R. Int J Respir Care In Press; 2010. Controversies in Allergic Bronchopulmonary Aspergillosis. [Google Scholar]
- 35.Agarwal R, Nath A, Aggarwal AN, Gupta D, Chakrabarti A. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses. 2010;53:138–143. doi: 10.1111/j.1439-0507.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 36.Malo JL, Paquin R. Incidence of immediate sensitivity to Aspergillus fumigatus in a North American asthmatic population. Clin Allergy. 1979;9:377–384. doi: 10.1111/j.1365-2222.1979.tb02496.x. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal R, Gupta D. Severe asthma and fungi: current evidence. Med Mycol. 2010 doi: 10.3109/13693786.2010.504752. doi: 10.3109/13693786.2010.504752: in Press. [DOI] [PubMed] [Google Scholar]
- 38.Wang JL, Patterson R, Rosenberg M, Roberts M, Cooper BJ. Serum IgE and IgG antibody activity against Aspergillus fumigatus as a diagnostic aid in allergic bronchopulmonary aspergillosis. Am Rev Respir Dis. 1978;117:917–927. doi: 10.1164/arrd.1978.117.5.917. [DOI] [PubMed] [Google Scholar]
- 39.Campbell MJ, Clayton YM. Bronchopulmonary aspergillosis. A correlation of the clinical and laboratory findings in 272 patients investigated for bronchopulmonary aspergillosis. Am Rev Respir Dis. 1964;89:186–196. doi: 10.1164/arrd.1964.89.2.186. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy DS, Pepys S. Allergic broncho-pulmonary aspergillosis. Clinical immunology: (2) Skin, nasal and bronchial tests. Clin Allergy. 1971;1:415–432. doi: 10.1111/j.1365-2222.1971.tb00793.x. [DOI] [PubMed] [Google Scholar]
- 41.Longbottom JL, Pepys J. Pulmonary Aspergillosis: Diagnostic and Immunological Significance of Antigens and C-Substance in Aspergillus Fumigatus. J Pathol Bacteriol. 1964;88:141–151. doi: 10.1002/path.1700880119. [DOI] [PubMed] [Google Scholar]
- 42.Greenberger PA. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1984;74:645–653. doi: 10.1016/0091-6749(84)90223-9. [DOI] [PubMed] [Google Scholar]
- 43.Vlahakis NE, Aksamit TR. Diagnosis and treatment of allergic bronchopulmonary aspergillosis. Mayo Clin Proc. 2001;76:930–938. doi: 10.4065/76.9.930. [DOI] [PubMed] [Google Scholar]
- 44.Shah A. Allergic bronchopulmonary and sinus aspergillosis: the roentgenologic spectrum. Front Biosci. 2003;8:e138–146. doi: 10.2741/944. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal R. High attenuation mucoid impaction in allergic bronchopulmonary aspergillosis. World J Radiol. 2010;2:41–43. doi: 10.4329/wjr.v2.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed tomography and magnetic resonance imaging diagnosis of allergic fungal sinusitis. Laryngoscope. 1997;107:170–177. doi: 10.1097/00005537-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Mukherji SK, Figueroa RE, Ginsberg LE, Zeifer BA, Marple BF, et al. Allergic fungal sinusitis: CT findings. Radiology. 1998;207:417–422. doi: 10.1148/radiology.207.2.9577490. [DOI] [PubMed] [Google Scholar]
- 48.Kopp W, Fotter R, Steiner H, Beaufort F, Stammberger H. Aspergillosis of the paranasal sinuses. Radiology. 1985;156:715–716. doi: 10.1148/radiology.156.3.4023231. [DOI] [PubMed] [Google Scholar]
- 49.Dillon WP, Som PM, Fullerton GD. Hypointense MR signal in chronically inspissated sinonasal secretions. Radiology. 1990;174:73–78. doi: 10.1148/radiology.174.1.2294574. [DOI] [PubMed] [Google Scholar]
- 50.Kumar R, Chopra D. Evaluation of allergic bronchopulmonary aspergillosis in patients with and without central bronchiectasis. J Asthma. 2002;39:473–477. doi: 10.1081/jas-120004905. [DOI] [PubMed] [Google Scholar]