Abstract
For years, the field of drug delivery has focused on (1) controlling the release of a therapeutic and (2) targeting the therapeutic to a specific cell type. These research endeavors have concentrated mainly on the development of new degradable polymers and molecule-labeled drug delivery vehicles. Recent interest in biomaterials that respond to their environment have opened new methods to trigger the release of drugs and localize the therapeutic within a particular site. These novel biomaterials, usually termed "smart" or "intelligent", are able to deliver a therapeutic agent based on either environmental cues or a remote stimulus. Stimuli-responsive materials could potentially elicit a therapeutically effective dose without adverse side effects. Polymers responding to different stimuli, such as pH, light, temperature, ultrasound, magnetism, or biomolecules have been investigated as potential drug delivery vehicles. This review describes the most recent advances in "smart" drug delivery systems that respond to one or multiple stimuli.
Introduction
Polymeric materials that respond to a stimulus are often called "smart" or "intelligent" due to their intrinsic ability to alter their physical or chemical properties. For the majority of the polymers that fall into this category, the response to a change in the surrounding environment is not only quick, on the order of minutes [1,2] to hours [3,4], but also reversible, mimicking the dynamics observed in natural polymers, such as proteins, polysaccharides, and nucleic acids in living organic systems [5]. The response to stimuli is manifested in many forms: individual chain dimension/size, shape, surface characteristics, secondary structure, solubility, and degree of intermolecular association. These unique capabilities have been applied to a diverse range of applications, including: drug delivery [4,6-8], diagnostics [9,10], biological coating technologies [11,12], biosensing [10,13], and microfluidics [14].
Conventional drug delivery methods physically entrap molecules within a polymer lattice; drug is released slowly by diffusion or upon degradation of the network. These methods typically result in an early peak in plasma drug concentration followed by a steady, linear release. This is far from ideal because the local drug concentration and location of delivery is not precisely controlled. Below the therapeutic dose, the drug is ineffective whereas high concentrations of drug may be toxic or lead to undesirable side effects. Polymers have been used to tailor drug release, which maintains the drug concentration within the desired therapeutic range. However, such controlled release systems are insensitive to metabolic changes in the body and are unable to modulate drug release nor target the drug to diseased tissue. This lack of control has motivated the exploitation of bioresponsive polymers as drug carriers.
As early as the 1950 s, stimuli responsive hydrogels have been studied for drug release [15]. Since then, polymers that react to different stimuli have been developed. These stimuli include pH [16-20], ionic strength [21], and the presence of metabolic chemicals (e.g., enzymes or antigens) [22,23]. Such stimuli may enable a drug carrier to distinguish between diseased and healthy tissue. More recently, drug carriers that respond to magnetic fields [24], light [25], radiation [26], and ultrasound [27] have also been developed. These external stimuli allow for greater control over when and where the drug is released. By tuning the formulation or chemical moieties of the polymer, the sensitivity to the stimuli can be precisely controlled. This review aims to provide an overview of how responsive polymers may be used to improve drug delivery.
Stimuli-responsive materials for drug delivery
pH-sensitive drug delivery
pH-sensitive polymers (see Table 1) have garnered much attention in the fields of drug delivery [28,29], gene delivery [30], and insulin delivery [31]. Generally, pH-sensitive polymers have weak acids or bases with pKa values between 3 and 10 [32]. Carboxylic, sulfonate, and primary or tertiary amino groups exhibit a change in ionization state as a function of pH. Transitions in solubility, conformation, and swelling arise due to changes in ionization, where specific polymer groups switch between a neutral and charged state (e.g., poly(N,N-dimethylaminoethyl methacrylate (DMAEMA) [33-35]) or a hydrophilic and hydrophobic state (e.g., poly(N-iso-propylacrylamide) (PNIPAm) [36-38]).
Table 1.
Stimuli-sensitive drug delivery.
| Stimulus | Carrier type | Payload | Reference |
|---|---|---|---|
| pH | PPADK | Dexamethasone | [45] |
| DMAEMA/HEMA | Paclitaxel | [3] | |
| PC/DAP liposomes | siRNA | [51] | |
| Temperature | PNIPAm/PLGA | Paclitaxel | [36] |
| MPPC/DPPC/HSPC/DSPEC-PEG-2000 | Doxorubicin | [54] | |
| FA/PDMA/PNIPAm | Dipyridamole | [57] | |
| Light | Cu chlorophyllin/PNIPAm | None reported | [60] |
| Quinone-methide | Nile Red | [63] | |
| Au/meso porous silica | Paclitaxel | [61] | |
| Ultrasound | Pluronic P105/N,N-diethylacrylamide | Doxorubicin | [66] |
| DPPC:DPPE-PEG2000 liposomes | Calcein | [67] | |
| Glucose | poly(methacrylic acid-g-ethylene glycol) with glucose oxidase, PNIPAm with phenylboronic acid | Insulin | [69-72] |
| PNIPAm or carboxymethyl dextran with con. A | None reported | [73,74] | |
| Enzyme | PEG diacrylate/human neutrophil elastase-sensitive peptide | None reported | [75] |
| Fibrin/β-nerve growth factor fusion proteins | β-nerve growth factor | [76] | |
| sPLA2-degradable retinoid lipid pro-drug | Retinoid lipid pro-drug | [77] | |
| Magnetic | Magnetite | Squalenoyl gemcitabine (SQdFdC) | [78] |
| Poly[aniline-co-N-(1-one-butyric acid)] aniline (SPAnH)/iron oxide | Epirubicin | [79] | |
| Polylactide/nanocrystalline magnetite | Paclitaxel | [80] | |
| Egg-PC/maghemite/PAH/PSS | Calcein | [81] | |
Eisenberg et al. investigated pH-sensitive polymer swelling to control the release of drug molecules [15]. Since then, several biocompatible and biodegradable pH-sensitive polymers have been developed. Unfortunately, few pH-sensitive polymers have been used for drug delivery systems because of their limited sensitivity near the pH of blood (pH 7.4). For example, natural polymers (alginate [39,40], chitosan [41,42], and carrageenan [43]) and synthesized polymers (poly(acrylic acid) (AA) [44] and poly(methacrylic acid) (MAA) [45]) exhibit a high swelling property at high pH due to ionizable functional groups on the polymer backbone or side chain. These polymers are not responsive under most physiological conditions, albeit the gastrointestinal system.
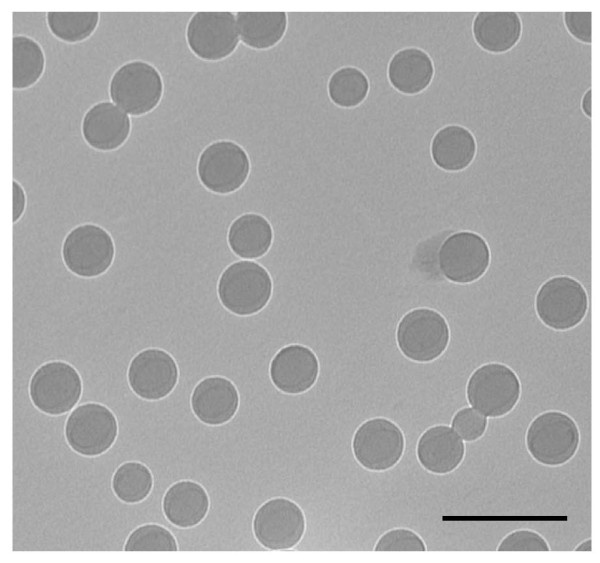
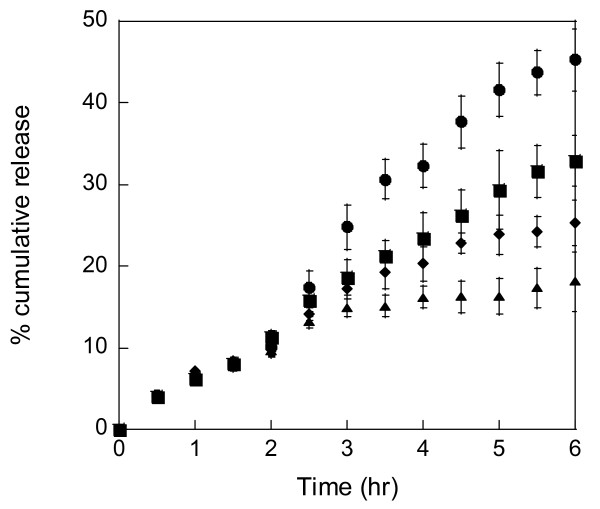
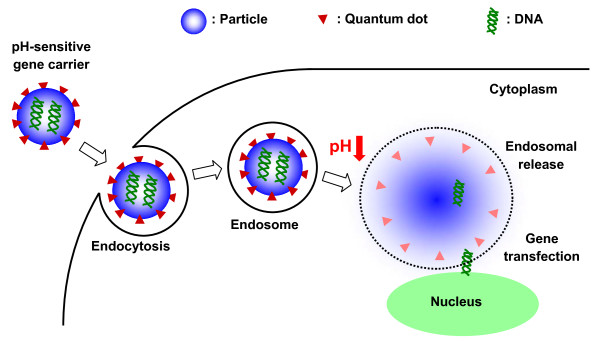
Systemic delivery requires that drug carriers respond to small changes in pH, near pH 7.4. In 2005, Heffernan and Murthy developed an acid-sensitive biodegradable drug delivery vehicle using poly(1,4-phenyleneacetone dimethylene ketal) (PPADK), which contains ketal linkages allowing for acid-catalyzed hydrolysis of the polymer into low molecular weight hydrophilic compounds. Thus, the release of drug molecules is accelerated under acidic conditions [46]. You and Auguste synthesized a series of pH-sensitive nanoparticles comprised of DMAEMA and 2-hydroxyethyl methacrylate (HEMA) (Figure 1) [3]. DMAEMA is a pH-responsive monomer that has a tertiary amine functional group with a pKa of 7.5 [47]. In vitro results support that the drug would remain within the particle during circulation; upon exposure to a low pH environment (e.g., a tumor [48]), the particle would swell resulting in release of the drug. Monodisperse, pH-sensitive DMAEMA/HEMA nanocarriers encapsulating paclitaxel exhibited pH-dependent release kinetics (Figure 2). The particles had a high volume swelling ratio at low pH, low crosslinking density, and high content of DMAEMA. A similar series of particles were used for gene delivery, where triggered release of plasmid DNA within the low pH endosome was optimized [49,50]. Plasmid DNA for green fluorescent protein was encapsulated. HeLa cells were successfully transfected with a dependence on the swelling ratio and crosslinking density (Figure 3).
Figure 1.
Transmission electron microscope image of DMAEMA/HEMA nanoparticles used for drug delivery. Scale bar is 500 nm [3].
Figure 2.
Triggered paclitaxel release was observed by incubating 10/90 (mol/mol) DMAEMA/HEMA nanoparticles crosslinked with 3 mol% TEGDMA for 2 hours at pH 7.4 (black triangle) followed by a reduction in pH to either 6.8 (black circle), 7.0 (black square), or 7.2 (black diamond) for 4 hours. The error is the standard deviation of the mean, where n = 3 [3].
Figure 3.
Schematic illustration of the delivery of pH-sensitive gene carriers. For example, the DMAEMA/HEMA nanoparticle releases DNA in the low pH endosome [49].
pH-dependent liposomes have also been used to trigger the release of drug within acidic environments. Auguste et al. formulated liposomes with variable surface charge by varying the lipid composition [51]. pH-sensitive liposomes were composed of a zwitterionic lipid (phosphatidylcholine) and a titratable lipid (dimethylammonium propane) with a pKa of 6.7. This allowed the liposome's net charge to become cationic upon decreasing the pH. pH-dependent liposomes may be shielded from the immune system using poly(ethylene glycol) (PEG)-b-polycation polymers. The polycation block electrostatically anchored the PEG polymer to the liposome surface at pH 7.4, but released the polymer at pH 5.5 due to electrostatic repulsion between the cationic polymer and cationic liposome surface. The triggered release of adsorbed PEG-b-polycation polymers from pH-dependent liposomes may protect the drug carrier from immune recognition during circulation (pH 7.4) and allow subsequent intracellular delivery of siRNA within the endosome. The bare liposome maintains the membrane disruption/fusion capability [52,53].
Temperature-sensitive drug delivery
Increases in temperature are associated with several disease states (e.g., cancer [54,55]). Thermo-responsive drug carriers have been employed to release their payload within environments above the physiological temperature. Thermo-sensitive polymers exhibit a phase transition in solution at a temperature known as the lower critical solution temperature (LCST). For example, PNIPAm, a well-studied thermo-responsive polymer, undergoes a reversible phase transition in aqueous solution from hydrophilic to hydrophobic at its LCST of approximately 32°C. Chemical modifications of PNIPAm have been effective in controlling the LCST [56]. In 2005, Liu et al. synthesized poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(D,L-lactide-co-glycolide) micelles for controlled paclitaxel delivery [36]. Paclitaxel release was accelerated when the physiological temperature was raised above the LCST. The paclitaxel-loaded micelles were more effective in killing human breast carcinoma cells at 39.5°C than 37°C. De and colleagues developed folate-conjugated, thermo-responsive block copolymer micelles. Folate is known to bind to several cancer cell types [57]. The drug release studies from folate-conjugated PNIPAm-DMA micelles demonstrated a temperature-responsive drug release. Delivery of paclitaxel at the tumor site can alter the overall drug biodistribution. Needham et al. developed temperature-sensitive liposomes containing doxorubicin [54]. Their liposome formulation, composed of 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (MPPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), hydrogenated soy sn-glycero-3-phosphocholine (HSPC), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-polyethylene glycol 2000 (DSPE-PEG-2000), was optimized to rapidly release the drug under mild hyperthermic temperatures (39°C to 40°C). Changing the drug biodistribution can increase therapeutic efficacy.
Light-sensitive drug delivery
Light (ultraviolet or visible) is a desirable external stimulus for drug delivery systems because it is inexpensive and easily controlled. Light-sensitive drug carriers are fabricated from polymers that contain photo-sensitizers such as azobenzene, stilbene, and triphenylmethane [37,58,59]. Suzuki and Tanaka have investigated visible light-responsive hydrogels using the trisodium salt of copper chlorophyllin in PNIPAm hydrogels [60]. When light is applied to the hydrogels, the chromophore absorbs the light, increasing the local temperature of the hydrogel. The resulting temperature change alters the swelling behavior. Vivero-Escoto et al. prepared gold-capped mesoporous silica nanospheres for photo-induced intracellular release of drugs in human cells [61]. The 100 nm silica nanospheres were capped with 5 nm gold nanospheres and functionalized with a cationic photo-reactive linker. Photoirradiation using ultraviolet (UV) light for 10 min at 0.49 mW/cm2 cleaved the photolabile linker, causing uncapping of the silica due to charge repulsion between the gold and silica nanospheres, allowing drug to be released [61,62]. Fomina et al. developed a novel light-sensitive polymer containing a quinone-methide moiety [63]. Nile Red, a hydrophobic dye, was released from nanoparticles after only one minute of 350 nm light exposure. Light can be effective in modulating drug release because it can be used to increase the local temperature and to cleave bonds.
Ultrasound-sensitive drug delivery
Ultrasound has been shown to trigger drug release by raising the local temperature or causing cavitation [64]. Both processes can increase the permeability of cell membranes and accelerate polymer degradation [65]. Ultrasound-sensitive vehicles have the potential to treat tumorigenic cancers due to their invasive character, ability to penetrate deeply into the human body, and ease of control. In 2002, Pruitt and Pitt investigated ultrasound-mediated doxorubicin release using stabilized Pluronic P105 micelles [66]. Doxorubicin was encapsulated within polymeric micelles composed of 10% Pluronic P105 and N,N-diethylacrylamide and delivered systemically to rats. Application of low-frequency ultrasound at the tumor site resulted in doxorubicin release; this resulted in a significant reduction in tumor volume. Lin et al. have investigated the physical and chemical properties of lipid membranes subjected to ultrasound treatment [67]. They showed that high permeability resulting from ultrasound treatment is correlated with lipid packing and can be useful for efficient drug release and ultrasound-mediated DNA transfection. In 2007, Ferrara et al. reviewed that small gas bubbles, used to enhance ultrasound contrast, can be used for drug delivery applications and monitoring [68]. When driven by an ultrasonic pulse, small gas bubbles oscillate with a wall velocity on the order of tens to hundreds of meters per second and can be deflected to a vessel wall or fragmented into particles on the order of nanometers. Also, a focused ultrasound beam can be used for disruption of delivery vesicles and blood vessel walls, which offer the opportunity to locally deliver a drug or gene. Ultrasound does not damage the surrounding tissue, making it attractive for triggering drug release.
Biomolecule-responsive drug delivery
The presence of biomolecules specific to an organ or disease state may be useful to regulate the release of drugs. Biomolecules can either participate in a chemical reaction or result in cleavage of a chemical bond. In this section, we will discuss the use of hydrogels that are (1) responsive to glucose and (2) use enzymes to facilitate hydrogel degradation.
Glucose-responsive hydrogels have been investigated for self-regulating the release of insulin for the treatment of diabetes. Early studies have been largely based on the combination of glucose oxidase with polyelectrolyte hydrogels that exhibit pH-responsive swelling or shrinking behavior [69,70]. As glucose diffuses within the hydrogel, entrapped glucose oxidase catalyzes its conversion to gluconic acid, lowering the local pH of the gel and resulting in swelling and the subsequent release of insulin. However, the efficiency of glucose oxidase decreases with pH. PNIPAm coupled with phenylboronic acid has been investigated as a glucose-responsive system [71,72]. Introduction of phenylboronic acid decreases the volume phase transition temperature. The hydrogel swells in the presence of glucose. More recently, Miyata et al. demonstrated that biomolecular complexes such as the carbohydrate-binding lectin, concanavalin A (Con. A), could be coupled with PNIPAm to achieve reversible swelling or shrinking in response to stepwise changes in glucose concentration [73]. Zhang et al. also utilized Con. A as the cross-linker for carboxymethyl dextran (CM-dextran) based hydrogels. Competitive displacement between Con. A and terminal glucose moieties on dextran by free glucose changed both the morphology and permeability of the gel [74]. Although these systems triggered insulin release through volumetric changes, regulating the rate and reliability of release remains a challenge.
Researchers have also exploited the presence of site or disease specific enzymes in drug delivery by incorporating enzyme-cleavable peptides within hydrogels. For example, Aimetti et al. prepared a PEG hydrogel drug delivery system which incorporated human neutrophil elastase (HNE)-sensitive linkers for the treatment of inflammation. HNE is a serine protease secreted by neutrophils, which accumulates at sites of inflammation. HNE-sensitive peptides were synthesized using solid phase Fmoc chemistry and their degradation kinetics were characterized. The rate of substrate degradation can be tailored by the incorporation or substitution of specific amino acids. Local, controlled release from hydrogels containing HNE-sensitive peptides was achieved in the presence of HNE as visualized by fluorescence energy resonance transfer (FRET) [75].
Growth factor delivery, controlled by enzymes involved in tissue regeneration, has also been investigated. Sakiyama-Elbert et al. designed the delivery of beta-nerve growth factor (β-NGF) from fibrin matrices as a nerve regeneration therapy. They synthesized β-NGF fusion proteins with an enzymatically degradable linker that served as the covalent anchor to the fibrin matrix and thus prevented a potential loss of enzymatic activity [76]. Researchers have also exploited the enzyme-triggered degradability of certain prodrugs. Pedersen et al. investigated anti-cancer retinoid lipophilic drugs that are covalently attached to phospholipids. These prodrugs have a lipid backbone that is degradable by secretory phospholipase A2 (sPLA2) IIA. The prodrugs self-assembled into liposomes, which were susceptible to degradation by (sPLA2) IIA. In vitro studies utilizing MT-3 breast carcinoma and HT-29 colon adenocarcinoma cell lines demonstrated high cytotoxicity of prodrug liposomes only in the presence of the (sPLA2) IIA enzyme [77].
Magnetic-sensitive drug delivery
Magnetic drug delivery systems possess three main advantages: (1) visualization of drug delivery vehicles, (2) ability to guide and control movement of drug carriers through magnetic fields, and (3) thermal heating which has been used to control drug release or produce tissue ablation. Magnetic drug carriers like magnetite, maghemite, cobalt ferrite, and carbonyl iron are biocompatible, non-toxic and non-immunogenic [78]. Arias et al. utilized magnetite to produce magnetic core/shell nanoparticles for drug delivery. The nanoparticles consisted of a magnetite core with a self-assembling squalenoyl gemcitabine, an amphiphilic anti-cancer drug, shell. Optical microscopy images showed the alignment of the core/shell nanoparticles under the influence of a 0.2 T magnetic field [78]. Liu et al. reported in vitro and in vivo studies of poly[aniline-co-N-(1-one-butyric acid)] aniline (SPAnH) nanoparticles encapsulating iron oxide (Fe3O4). To overcome the blood-brain barrier, the authors utilized focused ultrasound to temporarily disrupt the barrier and increase permeability. Their results showed that an estimated 0.16 ± 0.03 mM of magnetic nanoparticles were delivered to brain tumors in Sprague-Dawley rats; this was estimated to be 15-fold higher than the therapeutic range [79]. Magnetic nanoparticles have also been proposed as a component in drug eluting stents for the treatment of vascular diseases. Chorny et al. reported the use of polylactide nanoparticles incorporating magnetite nanocrystals and encapsulating paclitaxel. In vitro studies demonstrated cell growth inhibition with a relatively low dose and brief (five minute) magnetic field exposure. In vivo studies performed in a rat carotid artery model of stent restenosis showed a significant benefit over the control group. Additionally, 13.2 ± 2.0 μg of magnetic nanoparticles delivered to the stented carotid segment under a magnetic field were retained in the artery compared to only 3.4 ± 1.9 μg of particles delivered without a magnetic field [80].
Magnetic nanoparticles have also been encapsulated within liposomes. da Silva Gomes et al. synthesized liposomes encapsulating magnetic nanoparticles with an outer polyelectrolyte shell using a layer by layer deposition technique. The lipid vesicles were characterized by dynamic light scattering, cryo-transmission electron microscopy and atomic force microscopy. Polyelectrolyte coated-liposomes were highly stable as they showed no significant membrane disruption or leakage of encapsulated contents in the presence of detergent Triton TX-100 [81].
Multiple responsive-matrices in drug delivery
Substantial benefits may be gained from the development of polymeric macromolecules that are responsive to small environmental changes and consequently elicit a response. Despite the many advances that have been accomplished, the field of stimuli-responsive biomaterials still faces many challenges. Most of the "smart" materials that have been investigated are primarily focused on a single type of stimulus. Developing a material that is responsive to more than one stimulus may combine the delivery of a drug with other capabilities such as detection, imaging, or feedback. Attention has been focused on materials that respond to more than one stimulus (Table 2).
Table 2.
Multiple stimuli-sensitive drug delivery.
| Stimuli | Carrier type | Payload | Reference |
|---|---|---|---|
| Temperature/pH | PNIPAm/MAA | Vitamin B12 | [82] |
| PNIPAm/AA | Isoniazid | [6] | |
| PNIPAm/AAm/VP | Naltrexone | [94] | |
| Light/pH or light/temperature | Polyacrylamide/Salicylideneaniline | None reported | [101] |
| PSS/PAH/Au | FITC-dextran | [58] | |
| Magnetic/temperature or magnetic/pH | PEO/PPO/PEO/Fe2O3 | Ibuprofen and Eosin Y | [102] |
| PNIPAm/γ-Fe2O3/SiO2 | None reported | [105] | |
| MPEG-b-PDEAEMA-b-PGMA, MPEG-b-PDMAEMA-b-PGMA, PDEAEMA-b-PGMA and MPEG-b-PGMA/Fe3O4 | Chlorambucil and indomethacin | [109] | |
Temperature- and pH-responsive matrices
Temperature- and pH-responsive matrices have been extensively studied in drug delivery because these two parameters often deviate from the norm in diseased tissue. Both environmental changes offer the ability for self-regulated control over the delivery of a drug, avoiding the need for external stimuli. Poly(N-isopropylacrylamide-co-methacrylic acid) and PNIPAm are well-established thermo-responsive polymers [6,38,82-94]. These polymers may be combined with pH-responsive polymers, like AA and its alkyl esters such as MAA [6,82,85,94,95]. Zhang et al. prepared temperature and pH-responsive nanoparticles from combinations of PNIPAm and MAA at different molar ratios [82]. The relative permeability of the nanoparticles increased significantly when the temperature was increased from 37°C to 43°C and when the pH decreased from 6 to 4. Nanoparticles encapsulating vitamin B12 exhibited a partition coefficient that decreased from 0.8 to 0.3 with increasing temperature and decreased from 0.8 to 0.6 with decreasing pH. Gu et al. prepared PNIPAm-co-AA hydrogels with hollow "cages" [6]. They showed that isoniazid (INH), an antitubercular drug, was located inside the cavity of the gel "cages" and also within the shell. The "cages," which were synthesized by SiO2-templated polymerization, had a silica core that was subsequently etched away by hydrofluoric acid leaving a hollow interior. The hydrodynamic diameter of the hydrogel "cages" decreased from 367 nm to 200 nm with increasing temperature and decreasing pH. Salehi et al. synthesized an injectable hydrogel system composed of PNIPAm, acrylamide (AAm), and vinyl pyrrolidone (VP) to encapsulate naltrexone, an opiate receptor antagonist [94]. The swelling ratios of the gel increased when the pH decreased from 8.5 to 7.4 and decreased when the temperature increased from 25°C to 37°C. They also performed in vitro release studies where a low burst effect and a slow release profile of naltrexone was observed over the course of 28 days.
In addition to PNIPAm, other temperature-responsive polymers have been investigated in dual-responsive drug delivery systems [41,96-100]. Moon et al. prepared and characterized amphiphilic, pH- and temperature-responsive polyaspartamide derivatives, which formed micelles with an average diameter of 100 nm at 25°C [41]. A sol-gel transition was observed when the temperature was increased from 15°C to 25°C and when the pH was increased from 6 to 10. Ding et al. fabricated injectable hydrogels based on glycol chitosan and benzaldehyde-capped poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEO-PPO-PEO) [98]. In vivo tests using a rat model demonstrated that the hydrogel underwent a sol-gel transition at physiological conditions. These hydrogels have the ability to encapsulate both hydrophilic and hydrophobic drugs and can control the release profile by varying temperature or pH.
Light- and pH- or temperature-responsive matrices
Light-responsive materials are combined with a secondary stimulus such as temperature or pH to increase control over drug release. Light responsiveness is usually introduced to a temperature or pH-sensitive material by conjugating a photo-reactive moiety [95]. Jochum et al. synthesized a thermo- and light-responsive polyacrylamide copolymer having salicylideneaniline as a photo-sensitive group [101]. Salicilideneaniline isomerizes from the enol to keto form and changes its dipole moment upon exposure to UV light. To synthesize the polymer, the authors performed a double polymer analogous reaction of poly(pentafluorophenyl acrylate) (PPFPA) and varied the molar composition of salicylideneaniline from 1 to 15 mol%.
Light- and pH-responsive materials have also been investigated. Angelatos et al. designed light- and pH-responsive polyelectrolyte/gold nanoparticle microcapsules via the layer by layer colloid-templating method [58]. Microcapsules were prepared by depositing the electrolytes poly(sodium 4-styrenesulfonate) (PSS) and poly(allylamine hydrochloride) (PAH) in a layer by layer fashion onto a template of melamine formaldehyde (MF) microparticles. The MF core was subsequently etched away with hydrochloric acid, and gold nanoparticles were introduced into the microcapsule shell. Fluorescein isothiocyanate (FITC)-dextran was encapsulated and was shown to be released after a decrease in pH and upon irradiation of 10 ns pulses of light at 1064 nm.
Magnetic- and pH- or temperature-responsive matrices
Magnetic fields can be remotely applied to localize drug carriers and to induce a temperature change. There have not been an extensive number of studies focusing on magnetic- and temperature-responsive materials, but this area has received increasing attention within the last few years [102-106]. Luo et al. prepared microspheres by encapsulating silica-coated superparamagnetic magnetite nanoparticle clusters with a crosslinked PNIPAm shell [105]. The microspheres exhibited a temperature-dependent swelling ratio; the hydrodynamic diameter decreased from 750 nm to 500 nm when the temperature increased from 20°C to 60°C. Additionally, the microspheres had greater magnetic responsivity at temperatures higher than the volume phase transition temperature due to the decrease in size at higher temperatures. A faster separation-redispersion behavior of the microspheres was observed at 60°C, above the volume phase transition temperature, compared to that at 25°C. In another study, a different temperature-responsive material, poly(ethyleneimine)-modified poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) block copolymer, was used instead of PNIPAm to coat iron oxide nanoparticles [102]. The nanoparticle hydrodynamic diameter decreased from 45 to 25 nm when the temperature increased from 20°C to 35°C. One of the most attractive features of this drug delivery system is the ability to reversibly control the payload release by changing the PEO-PPO-PEO polymer shell conformation. The polymer shell acts as a gate; it is in an extended conformation at room temperature but changes to a coiled conformation upon heating to 37°C. In vitro release of hydrophobic and hydrophilic model drugs was achieved by switching the temperature from 37°C to 20°C. In addition, the nanoparticles showed good biocompatibility and effective nerve regeneration when loaded with a ganglioside in a spinal cord injury rat model.
Magnetic- and pH-responsive materials have also been investigated [107-110]. Superparamagnetic Fe3O4 nanoparticles were coated with different pH-responsive block copolymers [109]. Four different block copolymers, methoxypoly(ethylene glycol)-b- (N,N-diethylamino)ethyl methacrylate-b-poly(glycidyl methacrylate) (MPEG-b-PDEAEMA-b-PGMA), methoxypoly(ethylene glycol)-b-(N,N-diethylamino)methyl methacrylate-b-poly(glycidyl methacrylate) (MPEG-b-PDMAEMA-b-PGMA), PDEAEMA-b-PGMA and MPEG-b-PGMA, were synthesized. The block copolymers were conjugated to the surface of Fe3O4 nanoparticles stabilized with HClO4 via a ligand exchange method. The authors performed drug release studies with MPEG-b-PDEAEMA-b-PGMA-Fe3O4 and MPEG-b-PDMAEMA-b-PGMA-Fe3O4 nanocarriers encapsulating chlorambucil (CLB), an anti-cancer agent, and indomethacin (IND), an anti-inflammatory agent. Their results showed that upon a decrease in pH (below the pKa of each drug), the percentage of drug release increased up to 90% for CLB and 70% for IND. At pH 7.4 the percent of drug release for both IND and CLB was approximately 25%.
Concluding remarks
The ability to alter the biodistribution of a drug by modulating its release profile through the use of smart polymers could transform drug delivery from passive controlled release to active stimuli-regulated delivery. Altering the drug biodistribution has the ability to reduce toxicity and side effects while improving therapeutic outcomes due to the ability to deliver higher doses of drug to the site of interest. The stimuli-responsive polymers reviewed here serve to provide a snapshot of the utility and complexity of polymers that can sense, process, and respond to stimuli in modulating the release of a drug. Stimuli-responsive drug delivery vehicles come in the form of polymersomes [111,112], liposomes [113-115], micelles [116-118], and dendrimers [119,120]. All of these systems aim to deliver an effective dose of drug at a specific time and place.
Despite many advances, there are still numerous challenges and opportunities that exist to translate responsive polymers from the laboratory the clinic. There is a need to develop polymers with greater sensitivity to a more diverse range of stimuli. In terms of biochemical signals or biomarkers, this is usually in the range of nano to picomolar concentrations [121]. This may require both a highly sensitive sensing mechanism and/or an amplification system to elicit a response from the polymer. In terms of the physical microenvironment, there are only minor differences in pH and temperature between diseased and normal tissues. Therefore, smart polymers must be able to accurately sense the changes in their surroundings to target drug release.
There is also a significant opportunity for smart polymers to respond to multiple stimuli. Hybrid polymers created in this manner will offer more parameters to tune drug delivery, which may be necessary for more complex and dynamic environments. It is worth noting that in addition to drug delivery applications, smart polymers in general have broad applications in tissue engineering and regenerative medicine (e.g. as injectable systems for delivery of cells or self-regulating scaffolds for cell growth or infiltration), and in actuators (e.g. as smart valves and coating in microfluidics or shape memory devices). Given the continuous development of new responsive polymer compositions, we expect increasingly elaborate and versatile drug carriers to be introduced in the future.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JY performed the experiments of transmission electron microscope (TEM), paclitaxel release study, and drafted the manuscript. JY, DA, and GY wrote the manuscript. DTA is responsible for the overall content. All authors read and approved the final manuscript.
Contributor Information
Jin-Oh You, Email: jyou@seas.harvard.edu.
Dariela Almeda, Email: dalmeda@fas.harvard.edu.
George JC Ye, Email: jcye@fas.harvard.edu.
Debra T Auguste, Email: auguste@seas.harvard.edu.
Acknowledgements
The authors gratefully acknowledge research support from the MRSEC program of the National Science Foundation under Award Number DMR-0820484. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under NSF award no. ECS-0335765. CNS is part of the Faculty of Arts and Sciences at Harvard University.
References
- Gemeinhart RA, Chen J, Park H, Park K. pH-sensitivity of fast responsive superporous hydrogels. J Biomater Sci Polym Ed. 2000;11:1371–1380. doi: 10.1163/156856200744390. [DOI] [PubMed] [Google Scholar]
- Gemeinhart RA, Park H, Park K. Effect of compression on fast swelling of poly(acrylamide-co-acrylic acid) superporous hydrogels. J Biomed Mater Res. 2001;55:54–62. doi: 10.1002/1097-4636(200104)55:1<54::AID-JBM80>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- You JO, Auguste DT. Feedback-regulated paclitaxel delivery based on poly(N,N-dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate) nanoparticles. Biomaterials. 2008;29:1950–1957. doi: 10.1016/j.biomaterials.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Jhaveri SJ, Hynd MR, Dowell-Mesfin N, Turner JN, Shain W, Ober CK. Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules. 2009;10:174–183. doi: 10.1021/bm801101e. [DOI] [PubMed] [Google Scholar]
- Galaev IY, Mattiasson B. 'Smart' polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999;17:335–340. doi: 10.1016/S0167-7799(99)01345-1. [DOI] [PubMed] [Google Scholar]
- Gu JX, Xia F, Wu Y, Qu XZ, Yang ZZ, Jiang L. Programmable delivery of hydrophilic drug using dually responsive hydrogel cages. J Controlled Release. 2007;117:396–402. doi: 10.1016/j.jconrel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Shim WS, Kim JH, Kim K, Kim YS, Park RW, Kim IS, Kwon IC, Lee DS. pH- and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. Int J Pharm. 2007;331:11–18. doi: 10.1016/j.ijpharm.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Dufresne MH, Le Garrec D, Sant V, Leroux JC, Ranger M. Preparation and characterization of water-soluble pH-sensitive nanocarriers for drug delivery. Int J Pharm. 2004;277:81–90. doi: 10.1016/j.ijpharm.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Pichot C, Taniguchi T, Delair T, Elaissari A. Functionalized thermosensitive latex particles: Useful tools for diagnostics. J Disper Sci Technol. 2003;24:423–437. doi: 10.1081/DIS-120021799. [DOI] [Google Scholar]
- Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Peptide-based stimuli-responsive biomaterials. Soft Matter. 2006;2:822–835. doi: 10.1039/b607706d. [DOI] [PubMed] [Google Scholar]
- Luzinov I, Minko S, Tsukruk VV. Responsive brush layers: from tailored gradients to reversibly assembled nanoparticles. Soft Matter. 2008;4:714–725. doi: 10.1039/b718999k. [DOI] [PubMed] [Google Scholar]
- Motornov M, Minko S, Eichhorn KJ, Nitschke M, Simon F, Stamm M. Reversible tuning of wetting behavior of polymer surface with responsive polymer brushes. Langmuir. 2003;19:8077–8085. doi: 10.1021/la0343573. [DOI] [Google Scholar]
- Alarcon CDH, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- Beebe DJ, Moore JS, Bauer JM, Yu Q, Liu RH, Devadoss C, Jo BH. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature. 2000;404:588–590. doi: 10.1038/35007047. [DOI] [PubMed] [Google Scholar]
- Kuhn W, Hargitay B, Katchalsky A, Eisenberg H. Reversible dilation and contraction by changing the state of ionization of high-polymer acid networks. Nature. 1950;165:514–516. doi: 10.1038/165514a0. [DOI] [Google Scholar]
- Ramkissoon-Ganorkar C, Liu F, Baudys M, Kim SW. Modulating insulin-release profile from pH thermosensitive polymeric beads through polymer molecular weight. J Controlled Release. 1999;59:287–298. doi: 10.1016/S0168-3659(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Suedee R, Jantarat C, Lindner W, Viernstein H, Songkro S, Srichana T. Development of a pH-responsive drug delivery system for enantioselective-controlled delivery of racemic drugs. J Controlled Release. 2010;142:122–131. doi: 10.1016/j.jconrel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Du JZ, Tang YP, Lewis AL, Armes SP. pH-sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J Am Chem Soc. 2005;127:17982–17983. doi: 10.1021/ja056514l. [DOI] [PubMed] [Google Scholar]
- Lomas H, Massignani M, Abdullah KA, Canton I, Lo Presti C, MacNeil S, Du J, Blanazs A, Madsen J, Armes SP, Lewis AL, Battaglia G. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discussions. 2008;139:143–159. doi: 10.1039/b717431d. [DOI] [PubMed] [Google Scholar]
- Griset AP, Walpole J, Liu R, Gaffey A, Colson YL, Grinstaff MW. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive Polymeric drug delivery system. J Am Chem Soc. 2009;131:2469–2471. doi: 10.1021/ja807416t. [DOI] [PubMed] [Google Scholar]
- Tan BH, Tam KC, Lam YC, Tan CB. Microstructure and rheology of stimuli-responsive nanocolloidal systems - Effect of ionic strength. Langmuir. 2004;20:11380–11386. doi: 10.1021/la0481290. [DOI] [PubMed] [Google Scholar]
- Meyers SR, Kenan DJ, Grinstaff MW. Enzymatic release of a surface-adsorbed RGD therapeutic from a cleavable peptide anchor. Chemmedchem. 2008;3:1645–1648. doi: 10.1002/cmdc.200800205. [DOI] [PubMed] [Google Scholar]
- Lomadze N, Schneider HM. Ternary complex formation inducing large expansions of chemomechanical polymers by metal chelators, aminoacids and peptides as effectors. Tetrahedron Lett. 2005;46:751–754. doi: 10.1016/j.tetlet.2004.12.032. [DOI] [Google Scholar]
- Zrinyi M. Intelligent polymer gels controlled by magnetic fields. Colloid Polym Sci. 2000;278:98–103. doi: 10.1007/s003960050017. [DOI] [Google Scholar]
- Harris KD, Cuypers R, Scheibe P, van Oosten CL, Bastiaansen CWM, Lub J, Broer DJ. Large amplitude light-induced motion in high elastic modulus polymer actuators. J Mater Chem. 2005;15:5043–5048. doi: 10.1039/b512655j. [DOI] [Google Scholar]
- Juodkazis S, Mukai N, Wakaki R, Yamaguchi A, Matsuo S, Misawa H. Reversible phase transitions in polymer gels induced by radiation forces. Nature. 2000;408:178–181. doi: 10.1038/35041522. [DOI] [PubMed] [Google Scholar]
- Kwok CS, Mourad PD, Crum LA, Ratner BD. Self-assembled molecular structures as ultrasonically-responsive barrier membranes for pulsatile drug delivery. J Biomed Mater Res. 2001;57:151–164. doi: 10.1002/1097-4636(200111)57:2<151::AID-JBM1154>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- Dai JD, Nagai T, Wang XQ, Zhang T, Meng M, Zhang Q. pH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int J Pharm. 2004;280:229–240. doi: 10.1016/j.ijpharm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lim DW, Yeom YI, Park TG. Poly(DMAEMA-NVP)-b-PEG-galactose as gene delivery vector for hepatocytes. Bioconjug Chem. 2000;11:688–695. doi: 10.1021/bc000014u. [DOI] [PubMed] [Google Scholar]
- Napoli A, Boerakker MJ, Tirelli N, Nolte RJM, Sommerdijk NAJM, Hubbell JA. Glucose-oxidase based self-destructing polymeric vesicles. Langmuir. 2004;20:3487–3491. doi: 10.1021/la0357054. [DOI] [PubMed] [Google Scholar]
- Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Traitel T, Cohen Y, Kost J. Characterization of glucose-sensitive insulin release systems in simulated in vivo conditions. Biomaterials. 2000;21:1679–1687. doi: 10.1016/S0142-9612(00)00050-8. [DOI] [PubMed] [Google Scholar]
- Herber S, Eijkel J, Olthuis W, Bergveld P, van den Berg A. Study of chemically induced pressure generation of hydrogels under isochoric conditions using a microfabricated device. J Chem Phys. 2004;121:2746–2751. doi: 10.1063/1.1773153. [DOI] [PubMed] [Google Scholar]
- Guice KB, Loo YL. Azeotropic atom transfer radical polymerization of hydroxyethyl methacrylate and (dimethylamino)ethyl methacrylate statistical copolymers and block copolymers with polystyrene. Macromolecules. 2006;39:2474–2480. doi: 10.1021/ma052667h. [DOI] [Google Scholar]
- Liu SQ, Tong YW, Yang YY. Thermally sensitive micelles self-assembled from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(D,L-lactide-co-glycolide) for controlled delivers of paclitaxel. Mol Biosyst. 2005;1:158–165. doi: 10.1039/b501756b. [DOI] [PubMed] [Google Scholar]
- Budhlall BM, Marquez M, Velev OD. Microwave, photo- and thermally responsive PNIPAm-gold nanoparticle microgels. Langmuir. 2008;24:11959–11966. doi: 10.1021/la8019556. [DOI] [PubMed] [Google Scholar]
- Shi J, Alves NM, Mano JF. Chitosan coated alginate beads containing poly(N-isopropylacrylamide) for dual-stimuli-responsive drug release. J Biomed Mater Res B. 2008;84B:595–603. doi: 10.1002/jbm.b.30907. [DOI] [PubMed] [Google Scholar]
- You JO, Park SB, Park HY, Haam S, Chung CH, Kim WS. Preparation of regular sized Ca-alginate microspheres using membrane emulsification method. J Microencapsul. 2001;18:521–532. doi: 10.1080/02652040010018128. [DOI] [PubMed] [Google Scholar]
- You JO, Peng CA. Phagocytosis-mediated retroviral transduction: co-internatization of deactivated retrovirus and calcium-alginate microspheres by macrophages. J Gene Med. 2005;7:398–406. doi: 10.1002/jgm.695. [DOI] [PubMed] [Google Scholar]
- Moon JR, Park YH, Kim JH. Synthesis and characterization of novel thermo- and pH-responsive copolymers based on amphiphilic polyaspartamides. J Appl Polym Sci. 2009;111:998–1004. [Google Scholar]
- Felt O, Buri P, Gurny R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998;24:979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- Pourjavadi A, Barzegar S, Zeidabadi F. Synthesis and properties of biodegradable hydrogels of kappa-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React Funct Polym. 2007;67:644–654. doi: 10.1016/j.reactfunctpolym.2007.04.007. [DOI] [Google Scholar]
- Jarvinen K, Akerman S, Svarfvar B, Tarvainen T, Viinikka P, Paronen P. Drug release from pH and ionic strength responsive poly(acrylic acid) grafted poly(vinylidenefluoride) membrane bags in vitro. Pharm Res. 1998;15:802–805. doi: 10.1023/A:1011995725320. [DOI] [PubMed] [Google Scholar]
- Jones MC, Ranger M, Leroux JC. pH-sensitive unimolecular polymeric micelles: Synthesis of a novel drug carrier. Bioconjug Chem. 2003;14:774–781. doi: 10.1021/bc020041f. [DOI] [PubMed] [Google Scholar]
- Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16:1340–1342. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- van de Wetering P, Moret EE, Schuurmans-Nieuwenbroek NME, van Steenbergen MJ, Hennink WE. Structure-activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjug Chem. 1999;10:589–597. doi: 10.1021/bc980148w. [DOI] [PubMed] [Google Scholar]
- Kavetskii RE, Osinskii SP, Bubnovskaya LN. Activation of glycolysis in tumor-tissue by inorganic-phosphate at low pH values. Bull Exp Biol Med. 1979;87:277–278. doi: 10.1007/BF00801798. [DOI] [PubMed] [Google Scholar]
- You JO, Auguste DT. Nanocarrier cross-linking density and pH sensitivity regulate intracellular gene transfer. Nano Lett. 2009;9:4467–4473. doi: 10.1021/nl902789s. [DOI] [PubMed] [Google Scholar]
- You JO, Auguste DT. The effect of swelling and cationic character on gene transfection by pH-sensitive nanocarriers. Biomaterials. 2010;31:6859–6866. doi: 10.1016/j.biomaterials.2010.04.048. [DOI] [PubMed] [Google Scholar]
- Auguste DT, Armes SP, Brzezinska KR, Deming TJ, Kohn J, Prud'homme RK. pH triggered release of protective poly(ethylene glycol)-b-polycation copolymers from liposomes. Biomaterials. 2006;27:2599–2608. doi: 10.1016/j.biomaterials.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Liang E, Hughes JA. Membrane fusion and rupture in liposomes: Effect of biodegradable pH-sensitive surfactants. J Membr Biol. 1998;166:37–49. doi: 10.1007/s002329900445. [DOI] [PubMed] [Google Scholar]
- Wilschut J, Hoekstra D. Membrane-fusion - from liposomes to biological-membranes. Trends Biochem Sci. 1984;9:479–483. doi: 10.1016/0968-0004(84)90316-5. [DOI] [Google Scholar]
- Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60:1197–1201. [PubMed] [Google Scholar]
- Sutton D, Nasongkla N, Blanco E, Gao JM. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24:1029–1046. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- Wang MZ, Fang Y, Hu DD. Preparation and properties of chitosan-poly(N-isopropylacrylamide) full-IPN hydrogels. React Funct Polym. 2001;48:215–221. doi: 10.1016/S1381-5148(01)00057-8. [DOI] [Google Scholar]
- De P, Gondi SR, Sumerlin BS. Folate-conjugated thermoresponsive block copolymers: Highly efficient conjugation and solution self-assembly. Biomacromolecules. 2008;9:1064–1070. doi: 10.1021/bm701255v. [DOI] [PubMed] [Google Scholar]
- Angelatos AS, Radt B, Caruso F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J Phys Chem B. 2005;109:3071–3076. doi: 10.1021/jp045070x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Tanaka T. Phase-transition in polymer gels induced by visible-light. Nature. 1990;346:345–347. doi: 10.1038/346345a0. [DOI] [Google Scholar]
- Vivero-Escoto JL, Slowing II, Wu CW, Lin VS. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. J Am Chem Soc. 2009;131:3462–3463. doi: 10.1021/ja900025f. [DOI] [PubMed] [Google Scholar]
- Klaikherd ANC, Thayumanavan S. Multi-stimuli sensitive amphiphilic block copolymer assemblies. J Am Chem Soc. 2009;131:4830–4838. doi: 10.1021/ja809475a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and near-IR triggered release from polymeric nanoparticles. J Am Chem Soc. 2010;132:9540–9542. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini GA, de la Rosa MAD, Richardson ES, Christensen DA, Pitt WG. The role of cavitation in acoustically activated drug delivery. J Controlled Release. 2005;107:253–261. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini GA, Pitt WG. Ultrasonic-activated micellar drug delivery for cancer treatment. J Pharm Sci. 2009;98:795–811. doi: 10.1002/jps.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JD, Pitt WG. Sequestration and ultrasound-induced release of doxorubicin from stabilized Pluronic P105 micelles. Drug Deliv. 2002;9:253–258. doi: 10.1080/10717540260397873. [DOI] [PubMed] [Google Scholar]
- Lin HY, Thomas JL. Factors affecting responsivity of unilamellar liposomes to 20 kHz ultrasound. Langmuir. 2004;20:6100–6106. doi: 10.1021/la049866z. [DOI] [PubMed] [Google Scholar]
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- Hassan CM, Doyle FJ, Peppas NA. Dynamic behavior of glucose-responsive poly(methacrylic acid-g-ethylene glycol) hydrogels. Macromolecules. 1997;30:6166–6173. doi: 10.1021/ma970117g. [DOI] [Google Scholar]
- Ishihara K, Matsui K. Glucose-responsive insulin release from polymer capsule. J Polym Sci C. 1986;24:413–417. doi: 10.1002/pol.1986.140240809. [DOI] [Google Scholar]
- Kataoka K, Miyazaki H, Okano T, Sakurai Y. Sensitive glucose-induced change of the lower critical solution temperature of poly[N,N-dimethylacrylamide-co-3-(acrylamido)phenyl-boronic acid] in physiological saline. Macromolecules. 1994;27:1061–1062. doi: 10.1021/ma00082a028. [DOI] [Google Scholar]
- Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y. Totally synthetic polymer gels responding to external glucose concentration: Their preparation and application to on-off regulation of insulin release. J Am Chem Soc. 1998;120:12694–12695. doi: 10.1021/ja982975d. [DOI] [Google Scholar]
- Miyata T, Jikihara A, Nakamae K, Hoffman AS. Preparation of reversibly glucose-responsive hydrogels by covalent immobilization of lectin in polymer networks having pendant glucose. J Biomater Sci Polym Ed. 2004;15:1085–1098. doi: 10.1163/1568562041753061. [DOI] [PubMed] [Google Scholar]
- Zhang R, Tang M, Bowyer A, Eisenthal R, Hubble J. Synthesis and characterization of a D-glucose sensitive hydrogel based on CM-dextran and concanavalin A. React Funct Polym. 2006;66:757–767. doi: 10.1016/j.reactfunctpolym.2005.11.003. [DOI] [Google Scholar]
- Aimetti AA, Tibbitt MW, Anseth KS. Human neutrophil elastase responsive delivery from poly(ethylene glycol) hydrogels. Biomacromolecules. 2009;10:1484–1489. doi: 10.1021/bm9000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 2001;15:1300–1302. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- Pedersen PJ, Adolph SK, Subramanian AK, Arouri A, Andresen TL, Mouritsen OG, Madsen R, Madsen MW, Peters GH, Clausen MH. Liposomal formulation of retinoids designed for enzyme triggered release. J Med Chem. 2010;53:3782–3792. doi: 10.1021/jm100190c. [DOI] [PubMed] [Google Scholar]
- Arias JL, Reddy LH, Couvreur P. Magnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targeting. Langmuir. 2008;24:7512–7519. doi: 10.1021/la800547s. [DOI] [PubMed] [Google Scholar]
- Liu HL, Hua MY, Yang HW, Huang CY, Chu PC, Wu JS, Tseng IC, Wang JJ, Yen TC, Chen PY, Wei KC. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny M, Fishbein I, Yellen BB, Alferiev IS, Bakay M, Ganta S, Adamo R, Amiji M, Friedman G, Levy RJ. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci USA. 2010;107:8346–8351. doi: 10.1073/pnas.0909506107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes JFPD, Rank A, Kronenberger A, Fritz J, Winterhalter M, Ramaye Y. Polyelectrolyte-coated unilamellar nanometer-sized magnetic liposomes. Langmuir. 2009;25:6793–6799. doi: 10.1021/la9003142. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wu XY. Temperature and pH-responsive polymeric composite membranes for controlled delivery of proteins and peptides. Biomaterials. 2004;25:5281–5291. doi: 10.1016/j.biomaterials.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Shi J, Alves NM, Mano JF. Drug release of pH/temperature-responsive calcium alginate/poly(N-isopropylacrylamide) semi-IPN beads. Macromol Biosci. 2006;6:358–363. doi: 10.1002/mabi.200600013. [DOI] [PubMed] [Google Scholar]
- Wei H, Zhang XZ, Cheng H, Chen WQ, Cheng SX, Zhuo RX. Self-assembled thermo- and pH-responsive micelles of poly(10-undecenoic acid-b-N-isopropylacrylamide) for drug delivery. J Controlled Release. 2006;116:266–274. doi: 10.1016/j.jconrel.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Yin X, Hoffman AS, Stayton PS. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules. 2006;7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- Jiang XZ, Ge ZS, Xu J, Liu H, Liu SY. Fabrication of multiresponsive shell cross-linked micelles possessing pH-controllable core swellability and thermo-tunable corona permeability. Biomacromolecules. 2007;8:3184–3192. doi: 10.1021/bm700743h. [DOI] [PubMed] [Google Scholar]
- Wang D, Chen LW. Temperature and pH-responsive single-walled carbon nanotube dispersions. Nano Lett. 2007;7:1480–1484. doi: 10.1021/nl070172v. [DOI] [PubMed] [Google Scholar]
- Zhang LY, Guo R, Yang M, Jiang XQ, Liu BR. Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery. Adv Mater. 2007;19:2988–2992. doi: 10.1002/adma.200601817. [DOI] [Google Scholar]
- Morinloto N, Qiu XP, Winnik FM, Akiyoshi K. Dual stimuli-responsive nanogels by self-assembly of polysaccharides lightly grafted with thiol-terminated poly(N-isopropylacrylamide) chains. Macromolecules. 2008;41:5985–5987. doi: 10.1021/ma801332x. [DOI] [Google Scholar]
- Shi J, Liu LH, Sun XM, Cao SK, Mano JF. Biomineralized polysaccharide beads for dual-stimuli-responsive drug delivery. Macromol Biosci. 2008;8:260–267. doi: 10.1002/mabi.200700177. [DOI] [PubMed] [Google Scholar]
- Chang C, Wei H, Feng J, Wang ZC, Wu XJ, Wu DQ, Cheng SX, Zhang XZ, Zhuo RX. Temperature and pH double responsive hybrid cross-linked micelles based on P(NIPAAm-co-MPMA)-b-P(DEA): RAFT synthesis and "schizophrenic" micellization. Macromolecules. 2009;42:4838–4844. doi: 10.1021/ma900492v. [DOI] [Google Scholar]
- Fundueanu G, Constantin M, Stanciu C, Theodoridis G, Ascenzi P. pH- and temperature-sensitive polymeric microspheres for drug delivery: the dissolution of copolymers modulates drug release. J Mater Sci-Mater Med. 2009;20:2465–2475. doi: 10.1007/s10856-009-3807-0. [DOI] [PubMed] [Google Scholar]
- Liu YH, Cao XH, Luo MB, Le ZG, Xu WY. Self-assembled micellar nanoparticles of a novel star copolymer for thermo and pH dual-responsive drug release. J Colloid Interf Sci. 2009;329:244–252. doi: 10.1016/j.jcis.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Salehi R, Arsalani N, Davaran S, Entezami AA. Synthesis and characterization of thermosensitive and pH-sensitive poly (N-isopropylacrylamide-acrylamide-vinylpyrrolidone) for use in controlled release of naltrexone. J Biomed Mater Res A. 2009;89A:919–928. doi: 10.1002/jbm.a.32047. [DOI] [PubMed] [Google Scholar]
- Li YY, Dong HQ, Wang K, Shi DL, Zhang XZ, Zhuo RX. Stimulus-responsive polymeric nanoparticles for biomedical applications. Science China-Chemistry. 2010;53:447–457. doi: 10.1007/s11426-010-0101-4. [DOI] [Google Scholar]
- Guo BL, Yuan JF, Yao L, Gao QY. Preparation and release profiles of pH/temperature-responsive carboxymethyl chitosan/P(2-(dimethylamino) ethyl methacrylate) semi-IPN amphoteric hydrogel. Colloid Polym Sci. 2007;285:665–671. doi: 10.1007/s00396-006-1611-7. [DOI] [Google Scholar]
- Basset C, Harder C, Vidaud C, Dejugnat C. Design of double stimuli-responsive polyelectrolyte microcontainers for protein soft encapsulation. Biomacromolecules. 2010;11:806–814. doi: 10.1021/bm901429q. [DOI] [PubMed] [Google Scholar]
- Ding CX, Zhao LL, Liu FY, Cheng J, Gu JX, Shan-Dan, Liu CY, Qu XZ, Yang ZZ. Dually responsive injectable hydrogel prepared by in situ cross-linking of glycol chitosan and benzaldehyde-capped PEO-PPO-PEO. Biomacromolecules. 2010;11:1043–1051. doi: 10.1021/bm1000179. [DOI] [PubMed] [Google Scholar]
- Gao M, Jia XR, Li Y, Liang DH, Wei Y. Synthesis and thermo-/pH-dual responsive properties of poly(amidoamine) dendronized poly(2-hydroxyethyl) methacrylate. Macromolecules. 2010;43:4314–4323. doi: 10.1021/ma1000783. [DOI] [Google Scholar]
- Liu X, Ni PH, He JL, Zhang MZ. Synthesis and micellization of pH/Temperature-responsive double-hydrophilic diblock copolymers polyphosphoester-block-poly[2-(dimethylamino)ethyl methacrylate] prepared via ROP and ATRP. Macromolecules. 2010;43:4771–4781. doi: 10.1021/ma902658n. [DOI] [Google Scholar]
- Jochum FD, Theato P. Temperature- and light-responsive polyacrylamides prepared by a double polymer analogous reaction of activated ester polymers. Macromolecules. 2009;42:5941–5945. doi: 10.1021/ma900945s. [DOI] [Google Scholar]
- Chen S, Li Y, Guo C, Wang J, Ma JH, Liang XF, Yang LR, Liu HZ. Temperature-responsive magnetite/PEO-PPO-PEO block copolymer nanoparticles for controlled drug targeting delivery. Langmuir. 2007;23:12669–12676. doi: 10.1021/la702049d. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Srivastava RS, Misra RDK. Core-shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: Synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir. 2007;23:6342–6351. doi: 10.1021/la0636199. [DOI] [PubMed] [Google Scholar]
- Brazel CS. Magnetothermally-responsive nanomaterials: combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm Res. 2009;26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- Luo B, Song XJ, Zhang F, Xia A, Yang WL, Hu JH, Wang CC. Multi-functional thermosensitive composite microspheres with high magnetic susceptibility based on magnetite colloidal nanoparticle clusters. Langmuir. 2010;26:1674–1679. doi: 10.1021/la902635k. [DOI] [PubMed] [Google Scholar]
- Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, Banerjee R, Bahadur D, Plank C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Controlled Release. 2010;142:108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Chatterjee J, Haik Y, Chen CJ. pH-reversible magnetic gel with a biodegradable polymer. J Appl Polym Sci. 2004;91:3337–3341. doi: 10.1002/app.13545. [DOI] [Google Scholar]
- Li LL, Chen D, Zhang YQ, Deng ZT, Ren XL, Meng XW, Tang FQ, Ren J, Zhang L. Magnetic and fluorescent multifunctional chitosan nanoparticles as a smart drug delivery system. Nanotechnology. 2007;18:1–6. [Google Scholar]
- Guo M, Yan Y, Liu XZ, Yan HS, Liu KL, Zhang HK, Cao YJ. Multilayer nanoparticles with a magnetite core and a polycation inner shell as pH-responsive carriers for drug delivery. Nanoscale. 2010;2:434–441. doi: 10.1039/b9nr00244h. [DOI] [PubMed] [Google Scholar]
- Guo M, Yan Y, Zhang HK, Yan HS, Cao YJ, Liu KL, Wan SR, Huang JS, Yue W. Magnetic and pH-responsive nanocarriers with multilayer core-shell architecture for anticancer drug delivery. J Mater Chem. 2008;18:5104–5112. doi: 10.1039/b810061f. [DOI] [Google Scholar]
- Kim MS, Lee DS. Biodegradable and pH-sensitive polymersome with tuning permeable membrane for drug delivery carrier. Chem Commun. 2010;46:4481–4483. doi: 10.1039/c001500h. [DOI] [PubMed] [Google Scholar]
- Chen W, Meng FH, Cheng R, Zhong ZY. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: A comparative study with micelles. J Controlled Release. 2010;142:40–46. doi: 10.1016/j.jconrel.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Bae Y, Jang WD, Nishiyama N, Fukushima S, Kataoka K. Multifunctional polymeric micelles with folate-mediated cancer cell targeting and pH-triggered drug releasing properties for active intracellular drug delivery. Mol Biosyst. 2005;1:242–250. doi: 10.1039/b500266d. [DOI] [PubMed] [Google Scholar]
- Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Wiradharma N, Gao SJ, Tong YW, Yang YY. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomaterials. 2007;28:1423–1433. doi: 10.1016/j.biomaterials.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Fattal E, Couvreur P, Dubernet C. "Smart" delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv Drug Deliv Rev. 2004;56:931–946. doi: 10.1016/j.addr.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kirchmeier MJ, Moase EH, Zalipsky S, Allen TM. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta-Biomembr. 2001;1515:144–158. doi: 10.1016/S0005-2736(01)00409-6. [DOI] [PubMed] [Google Scholar]
- Shigeta K, Kawakami S, Higuchi Y, Okuda T, Yagi H, Yamashita F, Hashida M. Novel histidine-conjugated galactosylated cationic liposomes for efficient hepatocyte-selective gene transfer in human hepatoma HepG2 cells. J Controlled Release. 2007;118:262–270. doi: 10.1016/j.jconrel.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hui H, Fan XD, Cao ZL. Thermo- and pH-sensitive dendrimer derivatives with a shell of poly (N,N-dimethylaminoethyl methacrylate) and study of their controlled drug release behavior. Polymer. 2005;46:9514–9522. doi: 10.1016/j.polymer.2005.07.034. [DOI] [Google Scholar]
- Lai PS, Lou PJ, Peng CL, Pai CL, Yen WN, Huang MY, Young TH, Shieh MJ. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J Controlled Release. 2007;122:39–46. doi: 10.1016/j.jconrel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Stuart MAC, Huck WTS, Genzer J, Muller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nature Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]