Abstract
Objectives
We investigated whether an intervention mainly consisting of a signed agreement between patient and physician on the objectives to be reached, improves reaching these secondary prevention objectives in modifiable cardiovascular risk factors six-months after discharge following an acute coronary syndrome.
Background
There is room to improve mid-term adherence to clinical guidelines' recommendations in coronary heart disease secondary prevention, specially non-pharmacological ones, often neglected.
Methods
In CAM-2, patients discharged after an acute coronary syndrome were randomly assigned to the intervention or the usual care group. The primary outcome was reaching therapeutic objectives in various secondary prevention variables: smoking, obesity, blood lipids, blood pressure control, exercise and taking of medication.
Results
1757 patients were recruited in 64 hospitals and 1510 (762 in the intervention and 748 in the control group) attended the six-months follow-up visit. After adjustment for potentially important variables, there were, between the intervention and control group, differences in the mean reduction of body mass index (0.5 vs. 0.2; p < 0.001) and waist circumference (1.6 cm vs. 0.6 cm; p = 0.05), proportion of patients who exercise regularly and those with total cholesterol below 175 mg/dl (64.7% vs. 56.5%; p = 0.001). The reported intake of medications was high in both groups for all the drugs considered with no differences except for statins (98.1% vs. 95.9%; p = 0.029).
Conclusions
At least in the short term, lifestyle changes among coronary heart disease patients are achievable by intensifying the responsibility of the patient himself by means of a simple and feasible intervention.
Background
Clinical practice guidelines recognise a series of pharmacological and hygienic-dietetic measures as being effective for secondary prevention in patients with acute coronary syndrome [1]. However, in spite of the improvement in recent years in measures to assure compliance in secondary prevention in post-infarct patients, there still exists considerable room for improvement [2-9]. Adherence to the treatment recommended has a favourable effect on the evolution of those patients who comply. In patients with coronary heart disease, following the recommendations of clinical guidelines in secondary prevention has a favourable effect on morbimortality in the follow-up period [10-12]. Multiple strategies have been developed to improve the adherence of patients to the recommendations, with more or less impact on the achievement of aims [13,14]. But, on occasion, these are focused exclusively on measures of pharmacological prescription, and in others there is no control group against which to assess the impact of the measures adopted [15].
One last important aspect is the continuity of care after hospital discharge. Measures in this respect have been shown to be more effective the sooner they are applied after the provision of care (such as when the hospital discharge report itself is issued) [16]. Many strategies to improve hospital care and recommendations at discharge are widely known, but rarely do they attempt to go beyond the time of the actual submission of the discharge report [17], even when the effectiveness of carrying out early monitoring of patients discharged after an acute coronary event is acknowledged [18].
The objective of our study is the evaluation after six months of follow-up of a programme of intervention at hospital discharge, easily embeddable in the daily clinical practice, aimed at increasing the proportion of patients that meet objectives in modifiable cardiovascular risk factors among patients who suffered an acute coronary syndrome, and which its main elements are the negotiation between the patient and the physician on the treatment objectives and steps to be taken and a reinforcement visit two months after discharge.
Methods
Open-label randomized controlled trial performed with 64 Spanish hospitals participating, in which a maximum of 30 patients were recruited at each one, all of whom were discharged consecutively after suffering acute coronary syndrome.
Finally, 1,757 patients were assigned to the Intervention Group (n = 867) or the Control Group (n = 890) by means of stratified randomization by centre and with concealment of allocation sequence. The unit of randomization was the patient and the stratification by centre was done to remove the effect of the hospital by obtaining groups of equal size (intervention and control) in every hospital. These were not selected at random but made up a convenient sample of Spanish hospitals, many of which had already taken part in the research group's previous studies [16]. All participating centers were either public or concerted with the public system. The hospitals covered a wide range of size (measured by the number of beds) and complexity. 16 hospitals had less than 400 beds, 22 between 400 and 800 and 26 hospitals had more than 800 beds. 39 hospitals (61%) had cardiac catheterization laboratory. There were hospitals from all over Spain, with sixteen out of the seventeen autonomous communities of Spain represented with at least one hospital as well as thirtyfive out of the fifty provinces in which Spain is divided.
The study was carried out during 2004 and 2006. The study was approved by the Clinical Ethics and Research Commission of Galicia (CEIC). All patients signed giving their informed consent to participate in the trial.
Inclusion criteria
Patients of both genders admitted for acute coronary syndrome with or without ST-segment elevation, discharged with a diagnosis of Q-wave or non-Q-wave acute myocardial infarction (AMI) or unstable angina with sufficient cultural level to be able to understand the intervention and the ability to fulfill the schedule of proposed further visits (at two and six months). Patients under 18 or above 80 years of age were not included, as it was the case with those presenting accompanying illnesses which would cause unfavourable prognosis in the following months or which would prevent their participation. All patients received the usual information on discharge.
Definition of the intervention
In this experimental study the intervention is realized upon the physician/patient relationship. The intervention includes a personalised interview at discharge with patient and nearest next-of-kin, in which physician and patient discuss and sign an agreement with the patient-specific secondary prevention procedures and therapeutic aims. Both physician and patient keep a copy of the signed agreement. Written back-up information is also given, some designed for this project and other based on material available from the Spanish Society of Cardiology, the Spanish Heart Foundation and the American Heart Association ("After Your Heart Attack", "Understanding and Controlling Your Cholesterol ", "The Effects of Smoking ","Warning Signs and Actions in the Face of a Heart Attack" "Get Moving", "Understanding and Controlling Your Blood Pressure", "Controlling Your Weight", among others) (materials available upon request), as well as a phone number where to call in case of questions.
The intervention also included an interview with the patient two months after discharge in order to review the agreement, adapt treatment if needed and reinforce the intervention. Informative materials are given once again.
Every visit (both at discharge and after two months) took an average of 30-40 minutes each.
Patients assigned to the control group receive the usual care given in each centre.
Assessment of effects
The main outcome is the proportion of patients in each therapeutic objective (Table 1). These objectives form part of the intervention group's physician/patient agreement. There was direct measurement of blood pressure, blood lipids and anthropometric variables. Medication compliance, as well as smoking status and exercise were self reported by the patient.
Table 1.
Objectives and indicators
| Objectives | Indicators |
|---|---|
| Giving up smoking | % of patients who gave up smoking % of patients who continued to smoke but reduced tobacco consumption |
| Having BMI < 25 | % of patients with BMI ≥ 25 who reduced it by at least 10% % of patients with BMI ≥ 25 who reduced it by at least 5% % of patients who went from BMI ≥ 25 to BMI < 25 % of patients with BMI ≥ 30 who reduced it by at least 10% % of patients with BMI ≥ 30 who reduced it by at least 5% % of patients who went from BMI ≥ 30 to BMI < 30 |
| Doing regular exercise | % of patients who do physical exercise at least three times a week for at least 20/30 minutes % of patients who do physical exercise at least five times a week for at least 20/30 minutes (only this exercise indicator in diabetic patients) |
| Controlling lipid levels | % of patients with total cholesterol < 175 mg/dl % of patients with LDL cholesterol < 100 mg/dl (< 70 mg/dl in diabetics) % of patients with LDL cholesterol < 70 mg/dl % of patients with HDL cholesterol ≥ 40 mg/dl % of patients with triglycerides < 150 mg/dl |
| Controlling hypertension | % of patients with hypertension who have had their blood pressure controlled since discharge % of patients with blood pressure < 135/80 mmHg (< 130/80 mmHg in diabetics) |
| Taking prescribed medication | % of patients taking AAS (6 months) when prescribed at discharge % of patients taking Clopidogrel (6 months) when prescribed at discharge % of patients taking beta blockers (6 months) when prescribed at discharge % of patients taking ACE-inhibitors (6 months) when prescribed at discharge % of patients taking angiotensin receptor blocking agent Type II (ARA II) (6 months) when prescribed at discharge % of patients taking statins (6 months) when prescribed at discharge |
All data, both at baseline and follow-up, were collected directly from the patients (or from hospital records, in the case of biochemistry results) by the participating physicians.
Plan of analysis
The comparison between intervention and control groups regarding the proportion of patients who achieve each one of the objectives (smoking, arterial hypertension (AHT), cholesterol, obesity) was done by means of the chi-square test. The effect of intervention on variables indicated, adjusting for baseline differences (not expected, taking into account the design chosen) which might be confounding factors was done fitting a logistic regression model for each objective (dependent variable: achievement/non-achievement).
In order to compare average change (basal-final) in continuous variables between intervention and control groups, a model was adjusted by means of analysis of variance (ANOVA) in which the dependent variable is the change occurring between basal visit and final visit, the main independent variable being whether they belonged to the intervention or control groups, and adjustment variables are those which display significant baseline differences between control and intervention groups, among patients who completed the follow-up (analyses not presented): age group, level of education, previous history of stroke, receiving medication for high cholesterol and situation regarding the consumption of tobacco prior to admission.
Subgroup analysis
In the protocol, pre-specified subgroup analyses by age group (< 70 years of age and ≥70 years of age), gender, and the existence or not of a personal history of ischemic heart diseases were included. These are not presented in this article.
Calculation of Sample Size
The original calculation was carried out for a minimum of 40 participating centres and 1,200 patients recruited (around half of them in each group). Assuming an achievement rate of 50% in the control group (i.e. proportion of patients in LDL objectives), this sample size would give the study a power of 90% to detect differences of 10% in the achievement of objectives between the two groups for an alpha error of 0.05.
Recruitment of centres was more successful than anticipated and the large number of participating centres and patients recruited has enabled us to improve our ability to detect differences between the groups. The actual number of 1510 subjects improves the power to detect the above mentioned difference to 97,5%. Conversely, for a proportion of 50% in the control group and an alfa error of 0.05, its actual sample size gives the study a power of 90% to detect a minimum difference of 8.3% between the two groups.
Results
Baseline characteristics of the intervention and control groups are displayed in Table 2. The randomization process produced differences in a few potentially important variables from the total number assessed. Summing up, the intervention group had, on average, a slightly lower age and a greater proportion of smokers at the onset of the acute coronary syndrome, while the proportion of diabetics was smaller. The visit at two months was effectuated as planned by 726 of the 867 patients in the intervention group (84%).
Table 2.
Comparison of Basal Characteristics of Intervention and Control Groups*
| Intervention (n = 867) |
Control (n = 890) |
p-value | |
|---|---|---|---|
| Males | 77.7 | 75.6 | 0.301 |
| Age (years old) (mean ± sd) | 62.1 ± 11.6 | 63.6 ± 11.4 | 0.007 |
| Present diagnosis AMI | 65.9 | 64.6 | 0.304 |
| Family history of ischemic cardiomyopathy | 18.7 | 17.9 | 0.657 |
| Certain previous diagnosis of ischemic cardiomyopathy | 31.5 | 29.1 | 0.276 |
| Personal history: | |||
| Stroke | 4.8 | 6.4 | 0.156 |
| Peripheral arteriopathy | 8.8 | 9.3 | 0.683 |
| Renal failure | 5.3 | 5.8 | 0.624 |
| Arterial hypertension | 58.9 | 60.1 | 0.616 |
| Hypercholesterolemia | 51.4 | 50.7 | 0.748 |
| Diabetes | 25.3 | 29.6 | 0.044 |
| Obesity | 31.3 | 34.2 | 0.195 |
| Smokers | 33.3 | 26.1 | 0.001 |
| BMI (kg/m2) (average ± sd) | 28.2 ± 4.2 | 28.3 ± 4.3 | 0.627 |
| BMI ≥ 30 kg/m2 | 29.6 | 30.4 | 0.932 |
| Abdominal perimeter (men/women) ≥ 102/88 cm | 47.8 | 51.8 | 0.172 |
| Abdominal perimeter (men/women) ≥ 94/80 cm | 74.0 | 74.3 | 0.929 |
* Results expressed as % unless otherwise indicated
During the six-month follow-up period, 17 patients died in the intervention group (2%) and 22 in the control group (2.5%), a non-significant difference. Another 88 patients from the intervention group and 120 from the control group failed to realize the follow-up visit. This resulted in a difference in the proportion of patients who completed the follow-up visit (87,9% vs 84,0, p = 0,041), mainly due to "unknown cause" (Figure 1).
Figure 1.
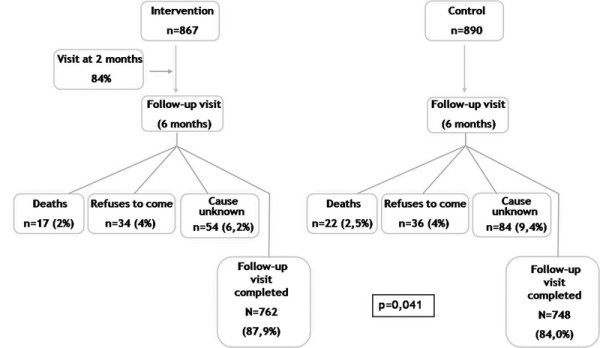
Flow-chart of follow-up of patients included in trial.
The main results of the study are summarized in Table 3, which presents the proportion of patients achieving the various objectives defined and the degree of improvement in the related quantitative variables. It also presents the odds ratios of achieving different objectives, adjusted for those variables which showed differences between the two groups at the beginning of the study (age group, educational level, previous history of stroke, medication for cholesterol and tobacco consumption prior to admission). As achieving treatment objectives is something desirable, odds ratio above 1 are good in this context. For continuous variables, instead of adjusted odds ratios, the table presents adjusted differences (for the same variables) in the mean changes between the two groups.
Table 3.
Degree of Achievement of Objectives at Six Months in Both Groups
| Objectives (nI/nC) | Intervention (n = 762) |
Control (n = 748) |
Adjustedγ Odds ratio |
Adjusted OR 95% CI |
p-value |
|---|---|---|---|---|---|
| Given up smoking (253/200) | 76.3 | 71.0 | 1.25 | 0.81-1.94 | 0.309 |
| BMI ≥ 25 kg/m2 (567/547) | |||||
| < 25 kg/m2 | 9.9 | 7.8 | 1.21 | 0.79-1.86 | 0. 373 |
| ⇓ BMI ≥ 10% | 7.4 | 6.8 | 1.15 | 0.72-1.84 | 0.564 |
| ⇓ BMI ≥ 5% | 25.0 | 19.7 | 1.31 | 0.98-1.76 | 0.068 |
| BMI ≥ 30 kg/m2 (212/209) | |||||
| < 30 kg/m2 | 25.9 | 24.9 | 1.04 | 0.66-1.63 | 0.860 |
| ⇓ BMI ≥ 10% | 11.3 | 10.5 | 1.15 | 0.61-2.17 | 0.666 |
| ⇓ BMI ≥ 5% | 32.1 | 27.8 | 1.26 | 0.82-1.93 | 0.300 |
| Changes in BMI (average) (kg/m2)* | - 0.5 | - 0.2 | 0.35* | 0.16-0.55* | < 0.001 |
| Changes in abdominal perimeter (average) (cm)* | - 1.6 | - 0.6 | 1.03* | 0.00-2.06* | 0.05 |
| Exercise 5 times/week | 29.4 | 23.4 | 1.29 | 1.02-1.64 | 0.033 |
| Exercise 3 times/week | 48.4 | 41.0 | 1.29 | 1.05-1.59 | 0.017 |
| Cholesterol < 175 md/dl | 64.7 | 56.5 | 1.46 | 1.17-1.81 | 0.001 |
| LDL < 100 mg/dl (70 in diabetic patients) | 47.9 | 44.8 | 1.09 | 0.87-1.35 | 0.449 |
| BP < 135/80 mmHg (130/80 in diabetic patients) | 46.7 | 42.4 | 1.14 | 0.91-1.41 | 0.253 |
| Taking of medication (percentage who take it on final visit, out of total given medication on discharge from hospital) | |||||
| Aspirin (712/698) | 97.5 | 96.0 | 1.54 | 0.84-2.83 | 0.161 |
| Clopidogrel (571/533) | 72.9 | 74.3 | 0.91 | 0.69-1.20 | 0.499 |
| Beta-blockers (621/590) | 94.4 | 94.6 | 0.93 | 0.56-1.54 | 0.769 |
| ACE-inhibitors (400/387) | 86.8 | 84.0 | 1.31 | 0.87-1.98 | 0.192 |
| Angiotensin receptor blocking agent Type II (ARA II) (86/97) | 84.9 | 86.6 | 0.83 | 0.35-1.95 | 0.664 |
| Statins (677/635) | 98.1 | 95.9 | 0.83 | 0.35-1.95 | 0.029 |
Results in intervention and control columns expressed as % of total number of patients affected by this objective as indicated in the first column, unless otherwise indicated
γ Differences in means (ANOVA model) or odds ratios [OR, logistic regression] adjusted for age group, education level, history of stroke, medication for cholesterol and tobacco consumption prior to admission, variables with statistical significant differences at baseline in the group of patients included in this analysis. Reference group for all estimates: control group). See interpretation of OR in the results section.
* In these continuous outcome variables, (changes in body mass index [BMI] and abdominal perimeter) the coefficients of the ANOVA model adjusted for the same variables, instead of odds ratios, are presented.
nI/nC: Number of patients in intervention/control groups on which the percentages presented are based when they are not the same as the total indicated in the corresponding column.
CI: Confidence interval
To sum up, the tendency is always towards a greater number of people achieving objectives within the intervention group, but differences are only statistically significant in the case of mean differences in body mass index, waist measurement, patient's own declaration of exercise realized and total cholesterol level. With regard to body mass index, even though the mean of this variable is lower in the intervention group, the proportion of patients achieving the predetermined objectives is small, both among those who are overweight and those who are obese, and there are no significant differences between the intervention and control groups, even in the case of the less ambitious objectives (reduction of at least 5% of initial body mass index).
Discussion
This trial carries out a short-term assessment of a simple intervention in the relationship between physician and patient in which its main element is the negotiation between the two and the ability to reach agreements regarding which steps to take in relation to care and the objectives to be fulfilled. The findings can be summed up as a moderate but potentially important effect on variables relating to lifestyle (physical exercise and body mass index), which are usually the most difficult aspects to modify. It also demonstrates that the vast majority of patients state that they take the medication and that an intervention such as that described above contributes nothing in this respect, at least as far as we can judge from the statements of the patients themselves, with all the limitations that this implies.
Although many studies have attempted to improve compliance levels in activities in secondary prevention after an acute coronary syndrome, firstly, many of these are focused exclusively on the moment of hospital discharge, and secondly, the intervention takes place only on the physician [14-17]. Thus, as a new element, our study prolongs the time of the intervention beyond hospital discharge, and moreover includes the patient as a participant in the achievement of objectives. The agreement signed by the patient, and early monitoring in the clinic (two months after discharge) to reinforce the message, were found to be related to a reduction in BMI, in exercise carried out continuously and in the objective of reaching levels of total cholesterol. These results suggest that this type of interventions can result in an improvement on the quality of care in this type of patients.
Intervention type and results
Early monitoring of coronary patients after discharge subsequent to a coronary event has been related, in various studies, both with higher rates of pharmacological compliance and with a favourable impact on patients' evolution, with a reduction in hospitalizations and mortality. Guidelines also recommend early monitoring (2 to 6 weeks) even in low-risk patients, after an acute coronary syndrome [19,20]. In our study, the intervention performed is compared with common practice. This is variable among centres, but common practice is to refer a high proportion of patients to the general practitioner on hospital discharge. In-depth interviews between cardiologist and patient prior to discharge are uncommon, due to the progressive reduction in time spent in hospital by these patients. Our study, unlike others, failed to demonstrate an improvement in levels of pharmacological compliance. In part, this could be explained by the absence of a control group in these studies, so that the benefit reflected in these studies may always be overrated. The positive effect of an early visit, that took place after two months in our study, is related to the fact that hospital discharge is usually a critical moment where the change between hospital and out-patient care (whether primary or specialized care) is a period of vulnerability, when high rates of errors related to medication and incomplete or inappropriate information are present [21,22]. However, an early follow-up visit can help prevent problems of primary compliance, which in post-acute myocardial infarct patients can be as high as 17.7% in cardiology medication [23].
Another tool used in our study is an agreement signed by both physician and patient on the objectives to be achieved during follow-up. This strategy is an attempt to involve the patient as jointly responsible for his/her care and avoid the relinquishment of his own function within the health service which cares for him. This is justified by the fact that the patient's collaboration and responsibility are fundamental in aspects such as long-term modification of lifestyle. Our study demonstrates that this tool can be useful in achieving modifications in lifestyle. However, though we consider that it is a key element in the intervention, our study does not allow us to evaluate the different components of the intervention independently.
In our study we carried out an analysis, after 6 months, of whether the intervention influenced patients' self-reported estimation of their pharmacological compliance. We found no difference between the two groups at six months except in the percentage of use of statins, with a loss of only 1.9% in the intervention group, as against 3.9% in the control group. The absence of this effect could be explained by the short follow-up period involved (6 months), especially because the time that elapses since the acute event is recognized as a factor contributing to non-compliance with treatment. Despite a high self-reported adherence to the prescribed medication, there is still a low proportion of patients achieving clinical goals. A similar observation has been reported among coronary heart disease patients in a recent survey in eight European countries [24], and in other studies where achievement of lipid lowering goals may be as low as 30% among high risk coronary heart disease patients [25].
In summary, it is important to point out that the improvements of the intervention concentrated in hygienic-dietetic measures (regular practice of exercise and weight loss and a non-significant tendency in the reduction in smoking) variables traditionally with lower compliance and greater difficulties for improvement than the pharmacological ones in other cardiovascular conditions (i.e. heart failure) [26,27]. It is easier for a patient to take medication than to change his lifestyle. Our study suggests that such changes are possible, at least in the short term, by means of a simple and feasible intervention that includes a component to increase the compromise from the patient (patient-physician agreement) and a visit to reinforce the messages. Nevertheless, the results also show that performance is still suboptimal, and that there is a need of additional improvements.
Limitations
Compliance rates are typically higher among patients with acute conditions compared with those suffering from chronic pathologies, especially from 6 months on [28,29]. This could be a limitation to the scope of our study, given that it is a post-acute coronary syndrome study and only covers monitoring up to 6 months. An additional potential limitation is that physical activity is declared by the patient him/herself, and there are no mechanisms within the study that could make this more objective. This is the usual practice in a clinical environment, but in this study poses the doubt that the differences might be due to a few patients with a contract not wanting to admit breaking it. This does not seem to be the reason because the patient-physician agreement includes other self-reported variables besides lifestyle (i.e medication adherence) where the effect is not seen.
On the other hand, it has been affirmed that collaborative follow-up protocols which actively involve primary care can be more effective than those performed by one professional on his/her own involved in care of the patient. The design of our study did not include involvement of the primary care physician, a fact which could limit the generalization of our results [30,31].
Conclusion
Taking medication is easier for a patient than changing his/her lifestyle. As a conclusion and as possible summary message with clinical application, our study suggests that, at least in the short term, lifestyle changes are achievable by intensifying the responsibility of the patient himself.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JM conceived of the study and participated in its design, acquisition of funding, coordination and statistical analysis, and drafted the manuscript. JJGD participated in the design of the study, recruitment and follow-up of patients and drafted the manuscript. MISP performed the statistical analysis of data and revised critically the manuscript. ILG participated in the design of the study, recruitment and follow-up of patients and revised critically the manuscript.
NME participated in the design of the study, recruitment and follow-up of patients and revised critically the manuscript. EdTG participated in the design of the study and revised critically the manuscript. JMCF participated in the design of the study and revised critically the manuscript. ACB conceived of the study and participated in its design, acquisition of funding and coordination, and revised critically the manuscript. All authors read and approved the final manuscript.
Contributor Information
Javier Muñiz, Email: javmu@udc.es.
Juan J Gómez-Doblas, Email: jjgomezdoblas@secardiologia.es.
María I Santiago-Pérez, Email: soly.santiago.perez@sergas.es.
Iñaki Lekuona-Goya, Email: inaki.lekuonagoya@osakidetza.net.
Nekane Murga-Eizagaetxebarría, Email: nekanemurga@gmail.com.
Eduardo de Teresa-Galván ß, Email: edeteresa@secardiologia.es.
José M Cruz-Fernández, Email: jmcruzfer@telefonica.net.
Alfonso Castro-Beiras, Email: alfonso.castro.beiras@sergas.es.
Acknowledgements
Funding
This study has been carried out thanks to funding from Merck, Sharp & Dohme (Spain) given unconditionally to the Instituto Universitario de Ciencias de la Salud, Universidad de A Coruña. The funding institution did not participate in the design, implementation or analysis of the study, nor in the writing of this manuscript.
JM, JJGD, ETG and ACB are members of the Spanish network for cardiovascular research (RECAVA) of the Instituto de Salud Carlos III, as are the various researchers in the working group.
Centers and investigators participating in the study
Andalucía:
1. Hospital de Poniente El Ejido, Almería: Dr. Jacinto Benítez Gil
2. Hospital Clínico Universitario de Puerta Real, Cádiz: Dr. Manuel Maestre García
3. Hospital Comarcal Infanta Margarita de Cabra, Córdoba: Dr. Joaquín Ruiz de Castroviejo
4. Hospital Reina Sofía, Córdoba: Dr. Juan Carlos Castillo Domínguez
5. Hospital Virgen de las Nieves, Granada: Dr. Joaquín Sánchez Gila
6. Hospital Juan Ramón Jiménez, Huelva: Dra. Ana Serrador Frutos, Dra. Rosa Cardenal Piris
7. Hospital Regional Carlos Haya, Málaga: Dr. Pedro A. Chinchurreta Capote, Dr. José Fco. Martínez Rivero
8. Hospital Clínico Universitario Virgen de la Victoria, Málaga: Dr. Juan José Gómez Doblas, Dr. Juan Caballero Borrego, Dra. Julia Fernández Pastor
9. Hospital Virgen del Rocío, Sevilla: Dr. Angel Martínez Martínez, Dra. Victoria Mogollón Jiménez, Dra. Rocío Pérez De La Yglesia
10. Hospital Clínico Universitario Virgen de la Macarena, Sevilla: Dr. Manuel Almendro Delia
11. Hospital Virgen de la Merced de Osuna, Sevilla: Dr. Antonio Espino
Aragón:
12. Hospital San Jorge, Huesca: Dra. María Teresa Villarroel Salcedo, Dr. Juan Pablo Gomollón García
13. Hospital Obispo Polanco, Teruel: Dr. José Mª Chopo Alcubilla
14. Hospital Miguel Servet, Zaragoza: Dr. Francisco José Monzón Lomas, Dra. Teresa Abad Montolio, Dr. Luis Zarate Duaso
15. Hospital Royo Villanova, Zaragoza: Dr. José Enrique Castillo Lueña, Dra. Marta Aliacar Muñoz, Dr. Fernando Garza Benito
Asturias:
16. Hospital Central de Asturias: Dr. Vicente Barriales Álvarez
17. Hospital Cabueñes de Gijón, Asturias: Dr. Alberto Batalla Celorio
Cantabria:
18. Hospital Marqués de Valdecilla (Santander), Cantabria: Dr. Jesús Gutiérrez Morlote
Castilla La Mancha:
19. Hospital Nuestra Señora del Prado (Talavera de la Reina), Toledo: Dr. Manuel Quintana Díaz, Dr. Marcelino Sánchez Casado, Dr. Miguel A. Taberna Izquierdo
Castilla León:
20. Hospital Universitario de Salamanca: Dr. Pedro Pavón Osuna, Dr. Víctor León Argüero
21. Hospital Universitario del Río Hortera, Valladolid: Dr. José Luis Bratos Pérez
Cataluña:
22. Centro Quirúrgico Sant Jordi, Barcelona: Dra. Nieves M. Oller Varela
23. Hospital de Terrassa (Terrassa), Barcelona: Dr. Miguel de Miguel, Dr. Ali Jaber Houbani
24. Hospital del Mar, Barcelona: Dr. Jordi Bruguera
25. Hospital Parc Taulí, Barcelona: Dr. Ignacio Anguera Camós
26. Hospital Sant Boi, Barcelona: Dr. César Romero Menor
27. Hospital Santa Creu i Sant Pau, Barcelona: Dr. Xavier García-Moll Marimon, Dr. Miguel Jauregui Abolarach, Dra. Pilar Rull Murillo
28. Hospital Valle de Hebrón, Barcelona: Dr. Jordi Soler Soler
29. Hospital Universitari Arnau de Vilanova, Lleida: Dr. Fernando Corner
30. Hospital Universitari de Tarragona Joan XXIII, Tarragona: Dr. Ramón De Castro Aritmendiz
Comunidad Valenciana:
31. Hospital General de Elche, Alicante: Dr. Fernando García De Burgos Rico, Dra. Raquel Camarasa García, Dr. David Ramos Alzamora
32. Hospital General de Alicante, Alicante: Dr. Francisco Sogorb, Dr. Sergio Aban Alique, Dra. Miriam Sandín Rollán
33. Hospital General de Castellón, Castellón: Dr. José Luis Diago Torrent
34. Hospital Arnau de Vilanova, Valencia: Dr. Juan Cosín Sales, Dra. Mª Teresa Moreno Casquete
35. Hospital Clínico Universitario de Valencia, Valencia: Dr. Angel Llacer, Dr. Julio Núñez Villota
36. Hospital Francisco de Borja (Gandía), Valencia: Dr. Salvador Bellver Bellver, Dr. Gerardo Estruch Català
37. Hospital General Universitario de Valencia, Valencia: Dr. Rafael Payá Serrano, Dra. Joaquina Belchi Navarro
38. Hospital La Fé, Valencia: Dr. Carlos Israel Chamorro Fernández, Dr. José Luis Marqués De Fez, Dr. Josep Navarro Manchón
Extremadura:
39. Hospital San Pedro de Alcántara, Cáceres: Dr. Oreste Alfredo Vaccari
Galicia:
40. Complejo Hospitalario Universitario de A Coruña, A Coruña: Dr. José Angel Rodríguez Fernández, Dra. Iris Paula Garrido Bravo
41. Hospital Clínico Universitario de Santiago de Compostela, A Coruña: Dr. Luis Manuel López Barreiro
42. Hospital Xeral Calde de Lugo, Lugo: Dr. Carlos González Juanatey, Dra. Margarita Regueiro Abel
43. Complejo Hospitalario de Ourense, Ourense: Dra. Elvira Blanco Labrador
44. Complexo Hospitalario de Pontevedra, Pontevedra: Dr. Juan Carlos Arias Castaño, Dr. Enrique Iglesias Río
45. Hospital de Meixoeiro. CHUVI (Vigo), Pontevedra: Dr. Oscar Díaz Castro, Dr. Jesús Alberto Fernández López
Islas Baleares:
46. Fundación Hospital de Manacor, Baleares: Dr. Bernardo García De La Villa Redondo
47. Hospital Son Dureta, Baleares: Dr. Armando Bethencourt
Islas Canarias:
48. Hospital Universitario Dr. Negrín, Las Palmas: Dr. Ruperto Vargas García
49. Hospital Universitario Insular de Gran Canaria, Las Palmas: Dr. Carlos Culebras Cáceres, Dra. Beatriz Sáiz Udaeta
50. Hospital Clínico Universitario de Canarias. Consorcio Sanitario de Tenerife, Santa Cruz de Tenerife: Dr. Antonio Barragán Acea
51. Hospital Universitario Nuestra Sra. De la Candelaria, Santa Cruz de Tenerife: Dr. Carlos Dorta Macía
Madrid:
52. Hospital 12 de octubre, Madrid: Dr. Jesús Rodríguez García, Dra. Verónica Suberviola Sánchez Caballero, Dr. David Vaqueriza Cubillo
53. Hospital Clínico San Carlos, Madrid: Dr. Miguel Angel Cobos Gil
54. Hospital de Getafe, Madrid: Dr. Carlos Kallmeyer Martín, Dr. Oscar González Lorenzo, Dr. René Miguel Montero
55. Hospital Gregorio Marañón, Madrid: Dr. Rafael Rubio Sanz
56. Hospital La Paz, Madrid: Dr. Eduardo Ramón Armada Romero
Murcia
57. Hospital General Universitario Reina Sofía, Murcia: Dr. Tomás Vicente Vera
58. Hospital Morales Messeguer, Murcia: Dr. José A. Ruiz Ros
Navarra:
59. Hospital de Navarra, Navarra: Dra. María De Iguazel Gracia Aznarez
60. Hospital Reina Sofía (Tudela), Navarra: Dra. María Teresa Basurte Elorz, Dra. Mª Rosario Casajús Magaña, Dra. Aitziber Munarriz Arizcuren
61. Hospital Virgen del Camino, Navarra: Dra. Irene Madariaga Arnaiz
País Vasco:
62. Hospital de Txagorritxu, Álava: Dr. Fernando Aros Borau
63. Complejo Donostia, Guipúzcoa: Dr. Angel Llamas Lombardía, Dra. Silvie Bianco Mazagoll, Dr. Enrique Uribe-Etxeberría Martínez
64. Hospital Civil de Basurto (Bilbao), Vizcaya: Dra. Nekane Murga Eizagaechevarria
Centre for data management and analysis: ODDS, SL. La Coruña, Spain.
• María Isolina Santiago Pérez (data analysis)
• Luisa López Domínguez (data management)
Coordinating center: Instituto Universitario de Ciencias de la Salud. Universidad de A Coruña:
• Alfonso Castro Beiras (principal investigator)
• Javier Muñiz García
References
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. Writing Committee to Revise the 1999 ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2004;44:671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Williams SC, Schmaltz SP, Morton DJ, Koss RG, Loeb JM. Quality of care in US hospitals as reflected by standarized measures. N Engl J Med. 2005;353:255–264. doi: 10.1056/NEJMsa043778. [DOI] [PubMed] [Google Scholar]
- Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. EUROASPIRE Study Group. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II and III surveys in eight European countries. Lancet. 2009;373:929–40. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- De Velasco JA, Cosín J, López-Sendón JL, De Teresa E, De Oya M, Carrasco JL, Navarro A. en nombre del Grupo de Investigadores del estudio PREVESE. La prevención secundaria del infarto de miocardio en España. Estudio PREVESE. Rev Esp Cardiol. 1997;50:406–15. [PubMed] [Google Scholar]
- Freire Castroseiros E, Muñiz García J, Castro Beiras A. por el grupo de trabajo del estudio RIGA. Análisis del uso adecuado de los bloqueadores beta en el postinfarto. Rev Esp Cardiol. 2001;54:1277–82. [PubMed] [Google Scholar]
- EUROASPIRE I and II Group. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II. Lancet. 2001;357:995–1001. doi: 10.1016/S0140-6736(00)04235-5. [DOI] [PubMed] [Google Scholar]
- De Velasco JA, Cosín J, López-Sendón JL, De Teresa E, De Oya M, Sellers G. en nombre del Grupo de Investigadores del estudio PREVESE II. Nuevos datos sobre la prevención secundaria del infarto de miocardio en España. Resultados del estudio PREVESE II. Rev Esp Cardiol. 2002;55:801–9. doi: 10.1016/s0300-8932(02)76708-0. [DOI] [PubMed] [Google Scholar]
- Fox KA, Goodman SG, Klein W, Brieger D, Steg PG, Avezum A. Management of acute coronary syndromes. Variations in practice and outcome. Findings from the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2002;23:1177–89. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- Newby LK, LaPointe NM, Chen AY, Kramer JM, Hammill BG, DeLong ER, Muhlbaier LH, Califf RM. Long-term adherence to evidence based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term morality after acute myocardial infarction. JAMA. 2006;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease. The Heart and Soul Study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AC, Smith DH, Perrin N, Yang X, Rix M, Raebel MA, Magid DJ, Simon SR, Soumerai SB. Improved therapeutic monitoring with several interventions. A randomized trial. Arch Intern Med. 2006;166:1848–1854. doi: 10.1001/archinte.166.17.1848. [DOI] [PubMed] [Google Scholar]
- Smith DH, Kramer JM, Perrin N, Platt R, Roblin DW, Lane K, Goodman M, Nelson WW, Yang X, Soumerai SB. A randomized trial of direct-to-patient communication to enhance adherence to beta blocker therapy following myocardial infarction. Arch Intern Med. 2008;168:477–483. doi: 10.1001/archinternmed.2007.132. [DOI] [PubMed] [Google Scholar]
- Muñiz Garcia J, Gomez Doblas JJ, Santiago Perez MI, de Teresa Galvan E, Cruz Fernandez JM, Castro Beiras A. Effect of a Simple Educational Program for Physicians on Adherence to Secondary Prevention Measures After Discharge Following Acute Coronary Syndrome. The CAM Project. Rev Esp Cardiol. 2004;57:1017–1028. doi: 10.1157/13068162. [DOI] [PubMed] [Google Scholar]
- Aronow HD, Novaro GM, Lauer MS, Brennan DM, Lincoff AM, Topol EJ, Kereiakes DJ, Nissen SE. In-Hospital Initiation of Lipid-Lowering Therapy After Coronary Intervention as a Predictor of Long-term Utilization A Propensity Analysis. Arch Intern Med. 2003;163:2576–2582. doi: 10.1001/archinte.163.21.2576. [DOI] [PubMed] [Google Scholar]
- De Velasco JA, Cosín J, Oya M, De Teresa E. en nombre del grupo de investigadores del estudio PRESENTE. Programa de intervención para mejorar la prevención secundaria del infarto de miocardio. Resultados del estudio PRESENTE. Rev Esp Cardiol. 2004;57:146–54. doi: 10.1157/13057265. [DOI] [PubMed] [Google Scholar]
- Daugherty SL, Ho PM, Spertus JA, Jones PG, Bach RG, Krumholz HM, Peterson ED, Rumsfeld JS, Masoudi FA. Association of early follow-up after acute myocardial infarction with higher rates of medication use. Arch Intern Med. 2008;168:485–491. doi: 10.1001/archinte.168.5.485. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC. Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2004;44(3):671–719. doi: 10.1016/j.jacc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, Etchells EE. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165:424–429. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- Coleman EA, Smith JD, Raha D, Min S. Post-hospital medication discrepancies: prevalence, types, and contributing system-level and patient-level factors. Arch Intern Med. 2005;165:1842–184736. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117:1028–36. doi: 10.1161/CIRCULATIONAHA.107.706820. [DOI] [PubMed] [Google Scholar]
- Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U. EUROASPIRE Study Group. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. The Lancet. 2009;373:929–940. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- Waters D, Brotons C, Chiang CW, Ferrieres J, Foody JA, Jukema J, Santos RD, Verdejo J, Messig M, McPherson R, Seung KB, Tarasenko L. for the Lipid Treatment Assessment Project 2 Investigators. Lipid Treatment Assessment Project 2. A Multinational Survey to Evaluate the Proportion of Patients Achieving Low-Density Lipoprotein Cholesterol Goals. Circulation. 2009;120:28–34. doi: 10.1161/CIRCULATIONAHA.108.838466. [DOI] [PubMed] [Google Scholar]
- Fonarow GC, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, Mehra M, O´Connor CM, Reynolds D, Walsh MN. Improving the use of evidence-based heart failure therapies in the outpatient setting: the IMPROVE HF performance improvement registry. Am Heart J. 2007;154:12–38. doi: 10.1016/j.ahj.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Fonarow GC, Yancy CW, Heywood JT. ADHERE Scientific Advisory Committee, Study Group, and Investigators. Adherence to heart failure quality-of-care indicators in US hospitals: analysis of the ADHERE Registry. Arch Intern Med. 2005;165:1469–1477. doi: 10.1001/archinte.165.13.1469. [DOI] [PubMed] [Google Scholar]
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- Rathore SS, Krumholz HM. Differences, disparities, and biases: clarifying racial variations in health care use. Ann Intern Med. 2004;141:635–638. doi: 10.7326/0003-4819-141-8-200410190-00011. [DOI] [PubMed] [Google Scholar]
- McLaughlin TJ, Soumerai SB, Willison DJ, Gurwitz JH, Borbas C, Guadagnoli E, McLaughlin B, Morris N, Chu Cheng S, Hauptman PJ, Antman E, Casey L, Asinger R, Gobel F. Adherence to national guidelines for drug treatment of suspected acute myocardial infarction: evidence for undertreatment in women and the elderly. Arch Intern Med. 1996;15:799–805. doi: 10.1001/archinte.156.7.799. [DOI] [PubMed] [Google Scholar]


