The localization of polycystin (PC)1) to the plasma membrane requires coexpression with PC2 and cleavage at the PC1 G protein-coupled receptor proteolytic site. Neither the PC1 binding capacity of PC2 nor its channel function is required for this effect.
Abstract
Polycystin (PC)1 and PC2 are membrane proteins implicated in autosomal dominant polycystic kidney disease. A physiologically relevant cleavage at PC1's G protein-coupled receptor proteolytic site (GPS) occurs early in the secretory pathway. Our results suggest that PC2 increases both PC1 GPS cleavage and PC1's appearance at the plasma membrane. Mutations that prevent PC1's GPS cleavage prevent its plasma membrane localization. PC2 is a member of the trp family of cation channels and is an important PC1 binding partner. The effect of PC2 on PC1 localization is independent of PC2 channel activity, as tested using channel-inhibiting PC2 mutations. PC1 and PC2 can interact through their C-terminal tails, but removing the C-terminal tail of either protein has no effect on PC1 surface localization in human embryonic kidney 293 cells. Experiments in polarized LLC-PK cells show that apical and ciliary PC1 localization requires PC2 and that this delivery is sensitive to PC2 truncation. In sum, our work shows that PC2 expression is required for the movement of PC1 to the plasma and ciliary membranes. In fibroblast cells this localization effect is independent of PC2's channel activity or PC1 binding ability but involves a stimulation of PC1's GPS cleavage before the PC1 protein's surface delivery.
INTRODUCTION
Most membrane proteins must be targeted to specific and restricted subcellular locations to function optimally. Consequently, cells have developed intricate signaling pathways and trafficking machinery to guarantee the proper establishment and maintenance of these localizations. Mislocalization of functionally important proteins can be detrimental at the cellular and organismal levels, and some genetic diseases are attributable to pathogenic mutations that alter a particular protein's distribution (Seabra et al., 2002; Muth and Caplan, 2003). This correlation between abnormal pathology and protein localization is certainly true in the case of an interesting subset of mutant alleles of the genes PKD1 and PKD2, which encode the membrane proteins polycystin (PC)1 and PC2, respectively. Mutations in PKD1 or PKD2 cause autosomal dominant polycystic kidney disease (ADPKD), a common genetic disease affecting approximately one in a thousand individuals. The disease causes progressive cyst formation in the adult kidney, resulting in end-stage renal failure in approximately half of all ADPKD patients by their sixth decade of life (Gabow, 1993). Many pathogenic mutations in the gene encoding PC1 result in the production of little or no full length PC1 protein. Other disease-causing mutations of PC1, however, lead to the generation of PC1 protein that does not accumulate in at least one of its sites of functional residence (Roitbak et al., 2004; Russo et al., 2005). Furthermore, mutations in the tuberous sclerosis 2 protein, which may play a role in ensuring the proper trafficking of PC1, also is associated with a polycystic kidney phenotype (Kleymenova et al., 2001). The connection between mislocalization of PC1 and severe kidney pathology reveals the critical importance of the cellular mechanisms that ensure the proper subcellular trafficking and retention of the PC1 protein.
There is robust expression of PC1 and PC2 in the epithelial cells of the developing and mature renal tubules, as well in the cells of a variety of other somatic tissues, including the heart, liver, bone, and endocrine glands (Ward et al., 1996; Markowitz et al., 1999; Peters et al., 1999). Within the cell, polycystin-1 and -2 colocalize to the primary cilia where they play roles in calcium signaling, mechano- and chemosensation, and the modification of signaling cascades such as the Janus tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT), activator protein (AP)-1, and the Wnt pathways (reviewed in Veland et al., 2009. Polycystin-2 is a transient receptor potential channel family member that acts as a calcium-activated calcium release channel and is most abundantly localized to the endoplasmic reticulum (Cai et al., 1999; Koulen et al., 2002). PC2 and PC1 are thought to interact primarily through their C-terminal cytoplasmic tails (Qian et al., 1997; Tsiokas et al., 1997; Casuscelli et al., 2009). Several investigations have suggested that PC1 and PC2 may reciprocally affect each other's surface membrane or ciliary localizations, although the precise nature of this interdependence has varied somewhat among experimental systems (Hanaoka et al., 2000; Grimm et al., 2003; Babich et al., 2004). An interaction between these two proteins also has been suggested to be important in creating a functional ion channel, whether through activation of the PC2 protein's intrinsic channel properties or through emergent channel properties attributable to formation of the complex (Hanaoka et al., 2000; Delmas et al., 2004).
PC1 is a very large protein that undergoes several cleavages during its processing and movement through the secretory pathway. One of these cleavages occurs at a G protein-coupled receptor proteolytic site (GPS), which is located at a position in the PC1 protein's extracellular N-terminal domain just before the beginning of the first transmembrane domain (Qian et al., 2002). Cleavage at this site involves a cis-autoproteolytic event that occurs early in the secretory pathway (Wei et al., 2007). Missense mutations in PC1 residues that are required for the autocatalytic GPS cleavage severely impair the normal functions of the PC1 protein, as evidenced by the capacity of these mutations to cause ADPKD (Qian et al., 2002). In addition, the ability of the PC1 protein to undergo successful GPS cleavage seems to be a prerequisite for proper kidney development, because evidenced by the cystic phenotype observed in mice that are homozygous for the expression of noncleaving PC1 alleles (Yu et al., 2007). Two other cleavages take place at the cytoplasmic C-terminal tail of PC1, releasing fragments that translocate to the nucleus and modify the AP-1 and JAK/STAT signaling pathways (Chauvet et al., 2004; Low et al., 2006). The extent of C-terminal cleavage is modulated by changes in fluid flow, consistent with the hypothesis that production of soluble cytoplasmic fragments contributes to the PC1 protein's postulated role as a participant in mechanosensory functions (Chauvet et al., 2004).
Naturally occurring mutations in PC2 suggest important information about the protein's possible functions. One variant identified in ADPKD families substitutes the aspartic acid residue at position 511 in the protein's third predicted transmembrane domain with a valine residue (D511V) (Reynolds et al., 1999). The aspartic acid residue at position 511 is thought to constitute part of the PC2 channel's conducting pore, and the PC2-D511V protein has no channel activity and exerts dominant-negative effect on the activity of endoplasmic reticulum (ER) calcium release channels when it is expressed by transfection in cultured cells (Koulen et al., 2002; Ma et al., 2005). The localization of the D511V protein, however, is indistinguishable from that of wild-type PC2 (Koulen et al., 2002). Many investigators also have studied the properties of a PC2 protein in which a stop codon has been inserted immediately after the leucine at residue number 703 (L703X). This truncation eliminates most of the PC2 protein's cytoplasmic C terminus. The L703X truncated PC2 protein is structurally similar to the PC2 proteins produced by several naturally occurring disease-causing truncating mutations found in ADPKD patients (Koulen et al., 2002). The L703X truncating mutation eliminates a domain of the PC2 that interacts with PC1 (Qian et al., 1997), and the protein lacks the calcium- or voltage-based regulation of channel activity that is detected in the wild-type protein (Koulen et al., 2002). The extent to which PC1 undergoes at least one of its C-terminal cleavages seems to be affected by the expression of wild-type and mutated forms of PC2 (Bertuccio et al., 2009).
Clearly, the PC1 and PC2 proteins exhibit a complex and delicate interdependence in the regulation of their localizations and functions, both with respect to their independent activities and in their collaboration as members of the same complex. Much remains to be learned about the molecular and mechanistic details of this interdependence. Here, we present evidence that the localization of PC1 to the plasma membrane depends on the presence of PC2. More specifically, PC1 surface localization correlates with a PC2-stimulated increase in GPS cleavage. This cleavage and localization effect in human embryonic kidney (HEK)293 cells is independent of any stable interaction between the two proteins. It is also independent of PC2's channel function, but the surface localization effect is disrupted by the PC2-D511V mutation. This evidence links PC2's regulation of PC1 cleavage to an effect on PC1 localization, and, by extension, to PC1 function.
MATERIALS AND METHODS
Cell Culture and Transfection
HEK293 and LLC-PK cells were grown at 37°C in DMEM or α-minimal essential medium, respectively (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% l-glutamine. Transient transfections were performed using the Lipofectamine 2000 reagent (Invitrogen), and the quantities of each of the PC2 cDNA constructs used for transfection were adjusted to yield similar levels of protein expression as assayed by Western blot. Cells were assayed 24 h after transfection. Stable expression of PC1 was established in HEK293 and LLC-PK cells by using selection with G418 (Invitrogen). Stable PC2 expression was generated using Zeocin (Invitrogen). Ionomycin and A23187 (Sigma-Aldrich, St. Louis, MO) were added at the indicated concentrations for 30 min before processing the cells for immunofluorescence. To visualize cilia, LLC-PK cells were grown on 0.4-μm polycarbonate filters (Costar, a division of Corning Life Sciences, Lowell, MA) for 5 d after reaching confluence before being processed for immunofluorescence.
We used a full-length cDNA encoding the mouse Pkd1 that had been modified to contain both an N-terminal FLAG and a C-terminal triple-hemagglutinin (HA) tags (Grimm et al., 2003). Construction of cDNA plasmids expressing PC2, PC2-D511V, and PC2-L703X, all with c-Myc tags, have been described previously (Cai et al., 1999). To generate cell lines stably expressing these constructs, the coding sequence of tagged PC2 was moved to a vector that carries Zeocin resistance (pcDNA3.1; Invitrogen). PC2-T712A, which lacks a c-Myc tag, also has been characterized previously (Cai et al., 2004).
Antibodies
PC1 was detected using a polyclonal anti-FLAG antibody (Sigma-Aldrich) against the N-terminal epitope tag, or monoclonal anti-HA antibody (Covance Research Products, Princeton, NJ) against the C-terminal epitope tags. Monoclonal anti-HA antibodies conjugated to agarose beads were used for immunoprecipitation (Sigma-Aldrich). Polycystin-2 was detected using anti-c-Myc antibody (polyclonal from Sigma-Aldrich; monoclonal from Santa Cruz Biotechnology, Santa Cruz, CA), or a polyclonal antibody against the native PC2 protein's N terminus (antibody B9, a gift from Dr. Stefan Somlo, Yale University, New Haven, CT). Cilia were marked using a monoclonal anti-acetylated tubulin antibody (clone 6-11B-1; Sigma-Aldrich). Goat anti-mouse and anti-rabbit immunoglobulin G secondary antibodies were conjugated to Alexa Flour 594 and 488 dyes, respectively (Invitrogen). All dilutions were 1:200 for immunofluorescence and 1:2000 for Western blotting. For Western blots, we used horseradish peroxidase-conjugated secondary antibodies; anti-mouse was diluted 1:10,000 and ant-rabbit was diluted 1:5000 (Jackson ImmuoResearch Laboratories, West Grove, PA).
Surface Immunofluorescence
HEK293 cells were grown on glass coverslips coated with poly-l-lysine, whereas LLC-PK cells were grown on polycarbonate filters. To stain surface protein the cells were incubated for 1 h in a humidified chamber at 4°C with polyclonal anti-FLAG antibody diluted in a blocking buffer of 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) with 100 nM CaCl2 and 1 mM MgCl2 (PBS++). Cells were then washed with PBS++, fixed for 20 min in 4% paraformaldehyde, permeabilized in PBS++ with 0.3% TritonX-100 and 0.1% BSA, and blocked for 30 min in goat serum dilution buffer (GSDB; 16% goat serum, 120 mM sodium phosphate, 0.3% Triton X-100, and 450 mM NaCl). They were then incubated for 1 h in a humidified chamber with the monoclonal anti-HA primary antibody diluted in GSDB, washed, and incubated for 1 h with the Alexa Fluor-conjugated secondary antibodies diluted in GSDB. For a more detailed description, see Chapin et al. (2009). The protocol for immunofluorescence without surface labeling takes cells straight to paraformaldehyde fix after washing with PBS++ and then continues as described above.
Image Acquisition and Quantification
Images used for illustration were taken with Zeiss LSM510 Meta confocal microscope. A single 0.5-μm scan slice is shown, except in those images where it is noted that a vertical z-stack of images was compressed to provide a single view of the entire cell surface. For quantification, 10 representative images of each experimental condition were taken using an Axiophot microscope with AxioVision software (Carl Zeiss, Thornwood, NY) by using identical exposure conditions. In one experiment, the calculations were based on compressed vertical z-stacks of confocal images, as noted. The images were then analyzed with ImageJ (National Institutes of Health, Bethesda, MD) to calculate the sum of the pixel intensities above a cut-off threshold determined to eliminate background noise. This threshold was chosen based on analysis of the fluorescence intensity histograms associated with images of fields of coverslips lacking cells. This pixel intensity total was then divided by the number of cells that were positive for surface PC1 staining above the given threshold, yielding a calculation of average pixel intensity per cell. To quantify conditions with no PC1 surface expression, cells were counted using the internal anti-HA antibody immunofluorescence. A two-tailed t test was used to compare conditions.
Immunoprecipitation, Biotinylation, Western Blots, and Quantification
Biotinylation was performed by incubating the cells with sulfo-NHS-SS-biotin (Pierce Chemical from Thermo Fisher Scientific, Rockford, IL) in buffer (10 mM triethanolamine, 2 mM CaCl2, and 125 mM NaCl at pH 8.9) at 4°C for a total of 40 min, followed by quenching with 100 mM glycine for a further 20 min. Lysis proceeded as described below, and labeled protein was precipitated using streptavidin-coated agarose beads by rotating overnight at 4°C (Gottardi et al., 1995). Washes and elution were performed as described for the immunoprecipitation protocol.
Cell lysis was accomplished by sonication with three 5-s pulses at 40% intensity in lysis buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris, pH 7.5, 1% Triton X-100, and protease inhibitors [Complete Protease Inhibitor Tablet, Roche Diagnostics, Basel, Switzerland]), and the lysates were cleared by centrifugation at 18,000 × g for 15 min at 4°C. Lysates were then mixed with SDS-containing sample buffer and heated to 55°C for 10 min. For immunoprecipitation from cleared lysates from a 12-well cell culture plate, monoclonal anti-HA was added, either preconjugated to agarose beads (15 μl, total volume) or simultaneously with the addition of protein G beads (30 μl of beads with 1 μl of anti-HA antibody). After rotating overnight at 4°C the samples were washed four times with lysis buffer, once with PBS, and eluted off the beads with sample buffer at 55°C.
Prepared samples were run in an SDS buffer in a polyacrylamide gel electrophoresis gel and elecrophoretically transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). After blocking for one hour in 150 mM NaCl, 20 mM Tris, 5% (wt/vol) powdered milk and 0.1% Tween, the blots were incubated with the specified primary antibody followed by the species-appropriate horseradish peroxidase-conjugated secondary antibody. Signal was visualized using chemiluminescence (GE Healthcare, Piscataway, NJ). ImageJ software (National Institutes of Health) was used for densitometry.
RESULTS
Surface Localization of PC1
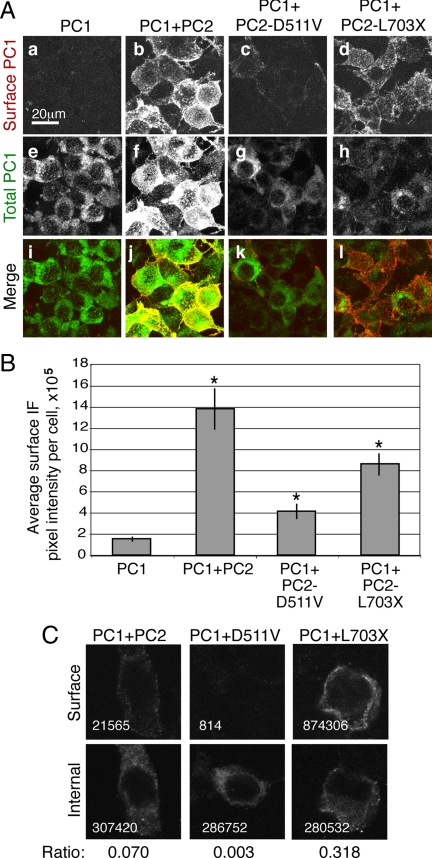
To visualize the pool of PC1 at the surface of cells, we used a live-cell labeling protocol in which cells were incubated at 4°C with an antibody against the N-terminal FLAG epitope of PC1, thereby labeling proteins that had reached the membrane and were exposed to the extracellular space. The total pool of protein was visualized using the immunofluorescence signal produced by an antibody directed against the C-terminal HA tag on the PC1 construct, applied to cells after fixation and permeabilization. This immunofluorescence protocol revealed that HEK293 cells transfected with full-length PC1 alone showed a primarily intracellular localization of the protein. When PC2 was transiently expressed with PC1, there was a sevenfold increase in the size of the surface pool of PC1, as quantified by measuring average per-cell pixel intensity (Figure 1). When PC1 was coexpressed with PC2-L703X there was an increase in plasma membrane localization similar to that seen with wild-type PC2, but coexpression with PC2-D511V resulted in the appearance of substantially less PC1 at the surface (Figure 1, A and B). PC1 reached the plasma membrane only in cells that coexpressed PC2 (Supplemental Figure 1).
Figure 1.
Coexpression of channel-active PC2 constructs increases the amount of PC1 at the surface of cultured cells. Unfixed, unpermeabilized cells transiently transfected with PC1 were labeled using an antibody directed against the extracellular FLAG epitope tag appended to the N terminus of PC1 (A, a–d). The composites presented here are compressed stacks of 15 confocal images. Internal PC1 was detected after fixation and permeabilization using an antibody directed against the C-terminal HA epitope on PC1 and the images were merged to show colocalization at the plasma membrane (i–l). There was minimal expression of PC1 on the surface when it was expressed alone (a). These cells, however, expressed PC1 internally, as seen with the antibody directed against the C-terminal HA epitope tag (e). Coexpression of PC2 was associated with the appearance of PC1 protein at the surface (b). PC2-D511V had a substantially smaller effect on PC1 surface localization (c), whereas PC2-L703X caused a distribution of PC1 similar to that seen with PC2 (d). Quantification of five representative compressed stacks showed the extents of the increases in surface PC1 that occurred in the presence of each of the PC2 constructs; asterisk indicates p < 0.05 compared with PC1 alone (B). To address the issue of variable PC1 expression individual cells were imaged and analyzed to determine the immunofluorescence intensity of surface, anti-FLAG antibody (C, top) and internal, anti-HA antibody (C, bottom). For analysis, images were grouped so that images exhibiting similar levels of total PC1 expression could be compared across conditions. Representative images from one such grouping are shown, with the total above-background pixel intensity given in the lower left corner of each image and the ratio of surface to internal immunofluorescence given below the pictures. (C). Averaging the ratios of six such groupings revealed that cells cotransfected with PC2 had a 3.95 ± 0.4-fold increase in surface PC1 compared with those cotransfected with PC2-D511V.
It was clear that the coexpression of PC2 caused an increase in the total amount of PC1 protein in the cells, as seen by anti-HA antibody immunofluorescence. To ensure that the difference between PC1 localization associated with wild-type PC2 and PC2-D511V coexpression was not due to this difference in the size of the cellular PC1 protein pool, we performed a titration analysis in which varying quantities of PC1 cDNA were cotransfected with the various PC2 constructs. Confocal images of individual cells were obtained and the surface staining detected in these cells was then compared among cells that exhibited similar anti-HA antibody immunofluorescence pixel intensities (Figure 1C). This more refined comparison confirmed that the surface localization of PC1 in the presence of PC2 was fourfold higher than the surface localization in the presence of PC2-D511V.
PC1 Surface Localization Correlates with GPS Cleavage
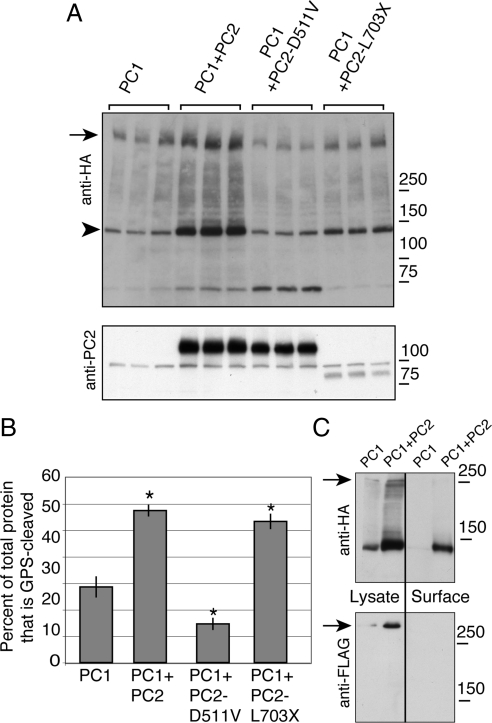
Cleavage of PC1 at its GPS site produces two main fragments: an N-terminal piece comprised of the protein's ∼3000 amino acid extracellular N terminus and a 145-kDa piece corresponding to the PC1 protein's 11 transmembrane domains and its cytoplasmic C-terminal tail, termed the C-terminal fragment (CTF; Qian et al., 2002). The CTF band was clearly visible on a Western blot of lysates prepared from transfected HEK293 cells probed with an antibody directed against the HA epitope tag that is appended to the C terminus of our PC1 construct (arrowhead in Figure 2A). Coexpressing PC2 with PC1 resulted in a twofold increase in the relative amount of PC1 CTF present in the cells, as quantified by densitometry and normalized to the total expression of PC1. Coexpressing PC2-L703X caused a similarly significant increase in PC1 cleavage, but PC2-D511V decreased the relative amount of the CTF fragment compared with the total amount of PC1 (Figure 2, A and B). It is worth noting that there was an overall increase of PC1 protein levels in cells that coexpress PC2, as seen in the immunofluorescence experiment depicted in Figure 1 and as documented previously (Tsiokas et al., 1997). The fact that PC2-L703X changes the ratio of GPS cleavage without dramatically increasing PC1 protein levels, however, suggests that these two effects of PC2 expression are mechanistically distinct.
Figure 2.
Cleavage of PC1 at the GPS is correlated with PC2-induced surface localization. Lysates from cells expressing PC1 alone or cotransfected with PC2, PC2-D511V, or PC2-L703X were immunoblotted with an antibody directed against the C-terminal HA tag of PC1, revealing the full-length PC1 band (arrow) and the large fragment resulting from cleavage at the GPS site (arrowhead, A). Expression of PC2 was verified using anti-PC2 antibody (A, bottom). The proportion of GPS-cleaved PC1 was calculated as the intensity of the GPS-cleaved protein band divided by the quantity of total PC1 protein expression, which was itself determined by summing the densities of the three major bands visible by Western blot: full-length, 150-kDa, and 70-kDa bands (B). Western blotting revealed that the 70-kDa band is not detected in untransfected cells (data not shown). Asterisk indicates significant increase or decrease in comparison to PC1 expressed alone (p < 0.05), and error bars indicate SE. Comparing the lysate and the surface protein in HEK cells expressing PC1 and PC2, as detected using surface biotinylation and streptavidin pull-down (lanes labeled Surface), showed that most of the surface PC1 had been GPS-cleaved (C, top). There was no full-length PC1 detected in the biotinylated fraction (arrow, C). Blotting with an anti-FLAG antibody showed that FLAG-positive full-length PC1 protein was not present in the pool of surface PC1 (C, bottom).
The pool of PC1 brought to the plasma membrane by each of the PC2 constructs was predominantly GPS-cleaved, as detected by cell surface biotinylation. Essentially all of the PC1 protein that could be labeled by cell surface biotinylation had undergone GPS cleavage; whereas both the full-length and CTF forms of PC1 were readily detected on Western blots of the lysate from HEK293 cells expressing PC1 and PC2, protein found in streptavidin pull-downs prepared from surface biotinylation of these cells contained CTF (Figure 2C). Consistent with the conclusion that uncleaved PC1 is largely absent from the cell surface, blotting with the antibody directed against the N-terminal FLAG epitope did not detect any full-length protein in the material recovered through surface biotinylation. Perhaps surprisingly, the cleaved N-terminal fragment was also not detected among the biotinylated proteins. It is possible that the structure of this domain of the PC1 protein precludes its lysine residues from being readily accessible to the NHS-SS-biotin reagent. Our preliminary observations, however, suggest the interesting alternative possibility that the conditions used in the biotinylation assay actually elute the N-terminal fragment from its noncovalent attachment to the membrane-associated PC1 C-terminal tail fragment (data not shown). Were this the case, then the absence of the N-terminal fragment from the pool of proteins collected after biotinylation would be consistent with the possibility that the majority of the surface population of PC1 has undergone GPS cleavage. We conclude that the presence of PC2 enhances GPS cleavage of the PC1 protein and that this stimulation of cleavage is strongly correlated with the surface delivery of PC1.
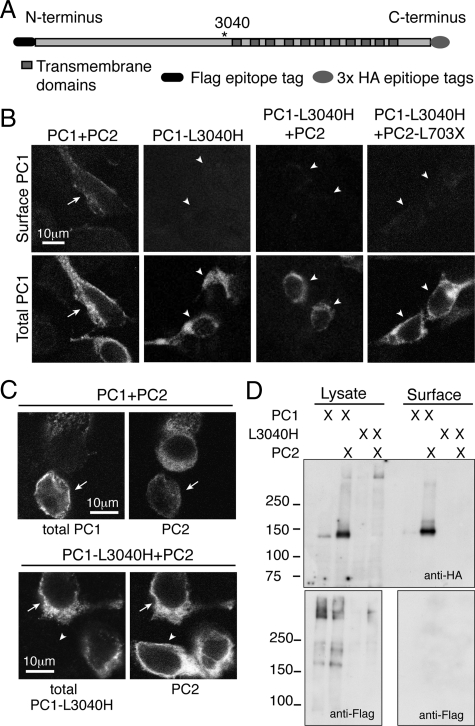
Given the correlation between GPS cleavage and increased PC1 surface localization we tested whether GPS cleavage was a prerequisite for or a consequence of surface delivery. PC1-L3040H is a form of PC1 that cannot be cleaved at the GPS site due to the mutation of the requisite leucine residue at the cleavage site (Figure 3A) (Wei et al., 2007). When expressed alone, PC1-L3040H exhibited an intracellular localization similar to that of wild-type PC1, but it failed to reach the plasma membrane when coexpressed with PC2 or PC2-L703X (Figure 3B). This lack of surface localization was not attributable to inefficient or incomplete cotransfection with PC2, because the cells expressed both PC1-L3040H and PC2 (Figure 3C). To confirm these findings, we biotinylated cells cotransfected with PC2 and either PC1 or PC1-L3040H. Under conditions in which the wild-type PC1 protein was readily detected among the recovered biotinylated cell surface proteins, no PC1-L3040H was detected in this material by using antibodies directed against either the PC1 N- or C-terminal epitope tags (Figure 3D).
Figure 3.
Impairing GPS cleavage in PC1 prevents PC1 plasma membrane localization. The GPS-mutated PC1-L3040H (A) was not delivered to the plasma membrane in association with any PC2 construct (arrowheads in B). This lack of surface delivery occurred despite robust internal expression of the PC1-L3040H protein, as seen by immunofluorescence with an internal anti-HA antibody (B, bottom). In contrast, PC1 was readily detected at the surface when it was coexpressed with PC2 and imaged under identical conditions (arrows in B, far left). PC2 was coexpressed with PC1 constructs under both cotransfection conditions (arrows, C), and any cells that expressed only one construct were most likely to express PC2 alone rather than PC1 alone (arrowheads, C). Surface biotinylation under conditions of coexpression with PC2 that revealed plasma membrane labeling of PC1 did not reveal L3040H at the surface, as detected with either anti-HA antibody (D, top) or anti-FLAG antibody (D, bottom).
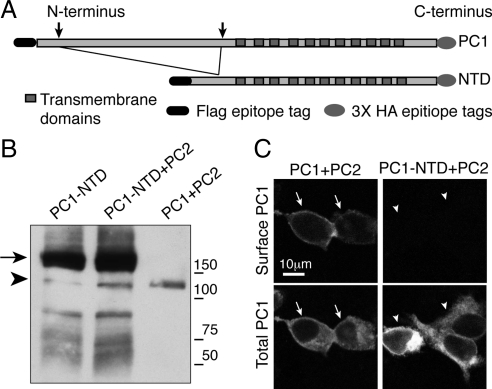
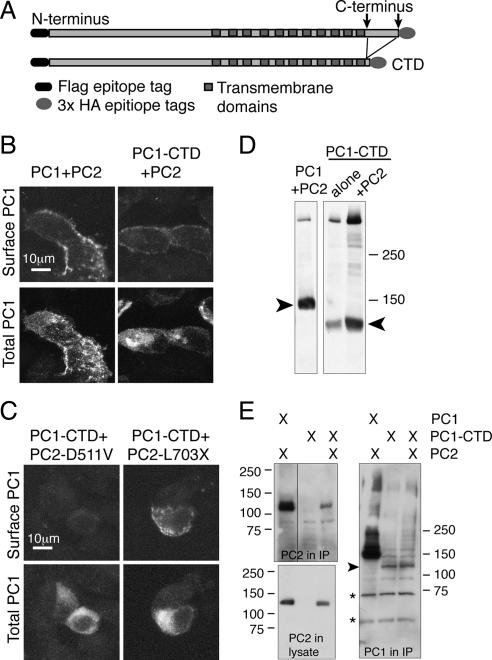
The L3040H mutation prevented the cleavage of PC1 at the GPS site, thus producing a PC1 protein in which the extracellular N-terminal domain was permanently affixed to the membrane spanning C-terminal portion of the protein. Because this mutation abrogated surface delivery, we next assessed the surface delivery of the converse mutation, a PC1-N-terminal deletion (PC1-NTD) construct lacking the majority of the extracellular N-terminal domain (Figure 4A). The deletion removed the receptor for egg jelly domain, which is necessary for optimal GPS cleavage (Qian et al., 2002), resulting in a very low amount of GPS cleavage even in the presence of PC2 (Figure 4B). Under conditions of coexpression with PC2 that produced abundant PC1 surface delivery (arrows in Figure 3C), there was little or no PC1-NTD detectable at the plasma membrane (arrowheads in Figure 3C). It is possible that the L3040H and NTD mutations cause misfolding of the PC1 protein or otherwise impair its trafficking through mechanisms independent of any effect on GPS cleavage. It is safe to conclude, however, that the essential role of cleavage in PC1's surface delivery is not attributable solely to the physical dissociation of some putative transport-inhibiting component residing in the PC1 N terminus from the C-terminal fragment, because the PC1 NTD lacks most of the N-terminal fragment but fails to reach the plasma membrane when coexpressed with PC2.
Figure 4.
The presence of a cleavable N-terminal tail on PC1 is necessary for plasma membrane localization. An N-terminal deletion construct of PC1 (PC1-NTD) was prepared by deleting 2760 amino acids from the N terminus of PC1 (A). Expressing this construct in HEK293 cells yielded a fragment of the expected size for full-length PC1-NTD (arrow in B), with a very small amount of GPS-cleaved product (B, arrowhead denotes C-terminal cleavage fragment). PC1-NTD was not brought to the plasma membrane by coexpression with PC2, as seen using transfection and exposure conditions that are sufficient to produce localization of wild-type PC1 to the surface (C).
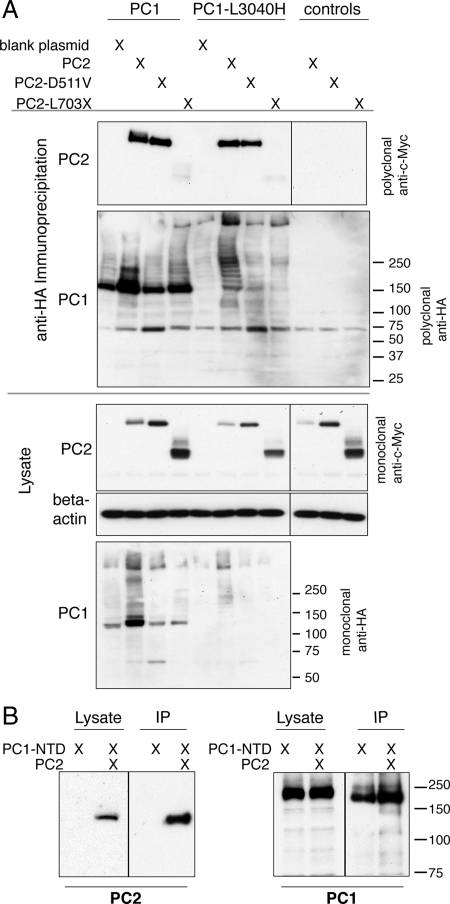
PC1 and PC2 are predicted to interact through their C termini (Qian et al., 1997; Tsiokas et al., 1997). To test whether the different effects of the PC1 and PC2 mutations could be attributed to their effects on the binding between PC1 and PC2 we performed coimmunoprecipitations of the complex that PC1 formed with each PC2 construct. Both PC1 and PC1-L3040H stably bound PC2 and PC2-D511V, whereas neither stably interacted with PC2-L703X (Figure 5A). The observation that PC1 could not form a stable complex with PC2-L703X demonstrated that the PC2 C-terminal tail is an obligate participant in the PC1-PC2 interaction. These results were in accordance with previous work that found that the domain of PC2 that interacts with PC1 resides at the protein's the C terminus (Qian et al., 1996). PC1-NTD formed a stable complex with PC2 (Figure 5B), despite its lack of plasma membrane localization when coexpressed with PC2.
Figure 5.
Both PC1 and PC1-L3040H stably bind PC2 and PC2-D511V, whereas PC2-L703X fails to coimmunoprecipitate with either PC1 construct. Protein complexes containing PC1 or L3040H were immunoprecipitated (IP'd) using an antibody against the C-terminal HA tag on PC1 and then the immunoprecipitates were Western blotted with polyclonal anti-c-Myc antibody to detect the presence of the c-Myc epitope appended to PC2 (A, top). Control conditions in which PC2 constructs were expressed in the absence of PC1 revealed that there is no nonspecific signal from the anti-c-Myc antibody (A, right-most lanes). PC2 and PC2-D511V stably interacted with both PC1 constructs, whereas PC2-L703X failed to be pulled down with PC1 or PC1-L3040H (A, top). This failure of PC2-L703X to coIP was not due to a lack of PC1 construct pull-down, as shown by blotting the IP with polyclonal anti-HA antibody (A). PC1-NTD also robustly bound to PC2, as seen by PC2's presence in the pull-down from an IP with the anti-HA antibody against PC1-NTD (B, left). The success of the IP is revealed by Western blotting with anti-HA antibody, which detects PC1-NTD in both the lysate and IP material (B, right).
This behavior highlights the fact that the PC2-binding ability of the various PC1 constructs did not parallel their surface localization patterns, suggesting the very surprising conclusion that a stable interaction with PC2 is not required for PC2-enhanced PC1 surface localization. This is a rather remarkable and unexpected finding because previous studies indicated that expressing C-terminal mutations of PC1 that prevent the interaction between PC1 and PC2 altered PC1's effect on signaling pathways (Bhunia et al., 2002; Delmas et al., 2002) and also prevented emergent physical properties of the PC1–PC2 complex, such as channel function (Hanaoka et al., 2000). Our data suggest that an as yet-uncharacterized interaction may mediate the cleavage and localization effects of PC2 on PC1, indicating that the nature and effect of the polycystin proteins' functional interrelationships may be more complex than previously appreciated.
PC2 enhances the C-terminal cleavage of PC1(Bertuccio et al., 2009), so to test whether the presence of the C-terminal tail affects the capacity for surface localization of PC1, we created and expressed a C-terminal deletion construct (PC1-CTD, Figure 6A). The PC1 protein harboring this deletion was retained intracellularly when expressed alone (data not shown) but was brought to the surface by coexpression with PC2 (Figure 6B). PC1-CTD also was brought to the surface by PC2-L703X but was intracellular in the presence of PC2-D511V (Figure 6C). Similar to wild-type PC1, cotransfection with PC2 increased the overall PC1-CTD protein expression, as seen by Western blot (Figure 6D). There was not a marked increase in the relative proportion of GPS-cleaved protein with PC2 coexpression, in contrast to the effect on wild-type PC1. Coimmunoprecipitation to test the stability of an interaction between the two proteins revealed that PC2 coprecipitated with PC1-CTD (Figure 6E). These results imply that domains other than the C-terminal tail of PC1 may contribute to the interaction between these two proteins, in contrast to the obligate participation of the PC2 C-terminal tail in the proteins' interaction.
Figure 6.
Removing the cytoplasmic C-terminal tail of PC1 does not inhibit either surface delivery or its interaction with PC2 constructs. A PC1 C-terminal deletion protein, PC1-CTD, was created by deleting the 179 amino acids that immediately follow the final transmembrane domain (A). Surface immunofluorescence with anti-FLAG antibody revealed PC1-CTD at the plasma membrane when it was cotransfected with PC2, similar to wild-type PC1 cotransfected with PC2 (B, top). Cotransfecting PC2-D511V failed to bring PC1-CTD to the surface (C, top left); instead, the protein was only detected intracellularly (C, bottom left). PC1-L703X, however, brought PC2-CTD to the plasma membrane (C, top right). A Western blot by using the anti-HA antibody on lysates of HEK293 cells transfected with the indicated plasmids showed that PC2 increased the abundance of PC1-CTD but did not increase the relative amount of GPS cleaved PC1-CTD protein, indicated with an arrowhead (D). Immunoprecipitating with an anti-HA antibody directed against the C-terminal tail of PC1-CTD and Western blotting with anti-c-Myc antibody against the c-Myc-tagged PC2 showed that PC1-CTD pulled down PC2 (E, top left). The coIP of PC2 with full-length PC1 is shown for control. PC2 protein was present in both lysates (E, bottom left). The amount of PC1 protein pulled down in each IP is shown by Western blotting with the polyclonal anti-HA antibody (E, right). The arrowhead points to the C-terminal tail from PC1-CTD protein, and asterisks indicate nonspecific bands produced by the polyclonal antibody.
Ion Flux and PC1 Surface Localization
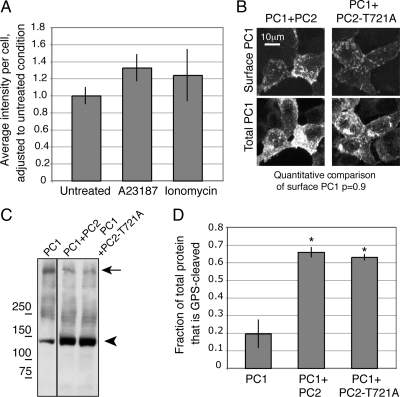
A notable characteristic of PC2-D511V is its inability to function as a calcium ion channel, distinguishing it in this regard from both PC2 and PC2-L703X. To test whether exogenously inducing an inward calcium flux would allow PC2-D511V to promote PC1 surface localization, we applied ionophores to cells expressing PC1 and PC2-D511V. Neither ionomycin nor A23187 significantly increased the amount of PC1 at the plasma membrane of HEK293 cells in the presence of PC2-D511V (Figure 7A).
Figure 7.
The surface localization and cleavage effects of PC2 coexpression on the PC1 protein are not mimicked by induced calcium ion flux and are independent of PC2's channel activity. PC2-D511V was expressed along with PC1, and cells were then treated with 1 μM of the ionophores A23187 or ionomycin. Exogenously inducing an ion flux with either drug failed to alter the localization of PC1, as quantified from images of surface localization (A). The channel-defective mutant PC2-T721A brought PC1 to the surface, as seen in a superposition of a stack of confocal images (B). Cells in both conditions exhibited PC1 at the plasma membrane as seen with the immunofluorescence signal from surface anti-FLAG antibody directed against PC1 (B, top). There was no statistical difference between the per-cell pixel intensity of surface immunofluorescence associated with cells expressing PC1 and either PC2 or PC2-T721A, with p = 0.9. Coexpression with either PC2-T721A or wild-type PC2 increased PC1 GPS cleavage to similar extents, as seen both on Western blot (C) and in quantifications of replicates of these experiments (D). Asterisk indicates p < 0.05 compared with PC1 alone.
Because the application of ionophores did not rescue the effect of PC2-D511V on PC1, we used another PC2 mutant to distinguish whether the inability of D511V to support PC1 surface delivery was due to its lack of channel activity or to an unidentified characteristic conferred by the point mutation itself. The PC2-T721A mutation abolishes calcium channel activity but does so by mutating a residue in the C-terminal tail rather than in the ion pore (Cai et al., 2004). Expressing PC2-T721A with PC1 brought PC1 to the plasma membrane (Figure 7B). Importantly, PC2-T721A expression also promoted PC1 GPS cleavage to a similar extent as wild-type PC2 (Figure 7C, quantified in D). Western blotting revealed that T721A is expressed at readily detectable levels (data not shown). The conclusion from this experiment is that PC2's channel function is not required to stimulate PC1's GPS cleavage or localization to the plasma membrane. This also suggests that the PC2-D511V mutation has a negative effect on the protein's function that is not limited to impairing its channel function.
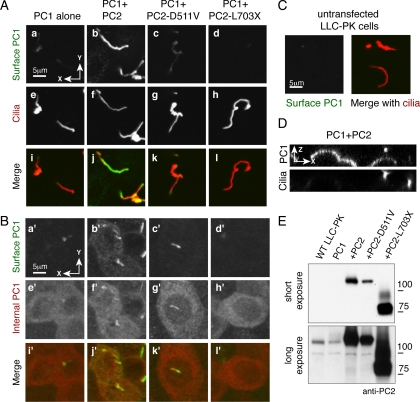
Localization in Ciliated Cells
The nonpolarized HEK293 cells provide a convenient and experimentally tractable system in which to observe the surface localization of polycystin proteins, but they yield no information about whether PC1 localizes to the cilia or to other polarized membrane domains. To investigate PC1 localization in polarized, ciliated cells we stably expressed PC1 alone or with PC2 constructs in LLC-PK cells. We grew cells on filters to allow complete polarization and then performed surface immunofluorescence against the N-terminal FLAG epitope to label PC1 at the plasma membrane. PC1 failed to substantially reach either the surface of the primary cilium or the cellular apical membrane when expressed alone (Figures 8, A and A′). Coexpression of PC1 with PC2 caused robust PC1 localization to the primary cilium (Figure 8B) and the apical plasma membrane (Figure 8B′). This localization was revealed even more clearly in the cross-sectional view showing surface PC1 in the apical and ciliary plasma membranes (Figure 8D). Coexpressing PC1 with PC2-D511V supported very weak ciliary accumulation of PC1, and no delivery of the protein to the apical membrane (Figure 8, C and 8C′). PC2-L703X failed to support any surface localization of PC1 (Figure 8, D and D′). All cell lines expressed comparable total amounts of PC1 protein (Figure 7, e′–h′). Variation in surface delivery was not due to PC2 expression, because cell lines transfected with PC2 had robust PC2 expression at the protein level (Figure 8E). Low levels of the endogenous PC2 protein were detected using the antibody against the N terminus (Figure 8E), suggesting that the presence of endogenous protein might explain the low level of ciliary PC1 observed in cell lines not stably transfected with PC2 cDNA.
Figure 8.
Coexpression with PC2 promotes PC1 localization to the apical and ciliary plasma membranes in polarized cells. LLC-PK cells stably expressing PC1 alone or together with PC2, PC2-D511V or PC2-L703X were grown to confluence and surface labeled with antibody directed against the N-terminal FLAG epitope (A, a–d and B, a′–d′). They were then permeabilized and labeled with an antibody directed against ciliary acetylated tubulin (A e-h), or the C-terminal HA epitope on PC1 (B, e′–h′). A small amount of PC1 was detectable on the primary cilium when PC1 was expressed alone (Aa). Expressing PC2 with PC1 dramatically increased the amount of PC1 at the ciliary membrane (Ab). Both PC2-D511V and PC2-L703X failed to stimulate PC1 delivery to the ciliary membrane (A, c and d). The anti-FLAG antibody gives no detectable ciliary background signal in untransfected cells (C). Images created by merging stacks of 20 successive Z plane confocal images reveal the extent to which PC1 is present at the apical and ciliary membranes (B, a′–d′) as well as the total amount of PC1 in the cells (B, e′–h′). Although all cell lines possess comparable amounts of total PC1 (B, e′–h′), PC1 is present at both the apical and ciliary membranes only when PC1 is expressed with PC2 (Bb′). A cross-section of the cells along the X-Z planes shows the presence of surface PC1 both at the apical membranes and in a pattern that colocalizes with that of the ciliary marker, an antibody directed against acetylated tubulin (E). A Western blot using an antibody directed against an N-terminal region of PC2 shows robust PC2 expression in the stable cell lines (E, top). A darker exposure shows low levels of endogenous PC2 in untransfected (WT) LLC-PK cells, and cells only transfected with PC1 (E, bottom).
DISCUSSION
We have shown that PC2 increases the autocatalytic cleavage of PC1 at its GPS site and that this cleavage correlates with movement of PC1 to the plasma membrane. Our results suggest that the increase in GPS cleavage is a prerequisite for surface delivery, rather than being a result of PC1 reaching the plasma membrane. In nonpolarized cells coexpressing any one of the four PC2 constructs tested, the amount of GPS cleavage correlates closely with the corresponding measurement of the intensity of the surface PC1 immunofluorescence signal. Our results also suggest that the cleavage occurs before PC1 arrives at the plasma membrane, because the population of PC1 that is detected at the cell surface is GPS cleaved. Furthermore, constructs in which this cleavage is prevented, including the PC1-L3040H and PC1-NTD constructs, are retained in the endoplasmic reticulum and do not reach the plasma membrane. These observations complement and are entirely consistent with the evidence gathered by Wei et al. (2007) showing that GPS cleavage takes place early in the secretory pathway.
The use of a surface immunofluorescence protocol allows us to measure quantitatively the PC1 surface population and to relate this localization to other aspects of the protein's processing. It has been clear from prior work that there is an effect of PC2 on PC1's localization, but the constructs and imaging protocols used had an important influence on the results obtained. In particular, in previous results from our own laboratory, Grimm et al. (2003) found a lack of PC1 at the plasma membrane in cells that coexpressed PC2 (2003). The cDNA encoding PC1 that was used in these experiments carried an inserted ClaI restriction site that was engineered into the protein's coding sequence to facilitate the construction of additional constructs. The missense sequence alteration that resulted from this insertion was located very close to the subsequently discovered GPS cleavage site. We have found that this alteration significantly reduces the GPS cleavage of the PC1 protein, which both explains the perplexing lack of PC1 surface localization observed in the Grimm et al. (2003) study and further underscores the importance of GPS cleavage for surface localization.
Several other studies have examined the parameters that govern the localization of both endogenous and transfected PC1 expressed in cultured cells. Imaging PC1 in Madin-Darby canine kidney (MDCK) cells has revealed PC1 at the lateral plasma membrane, in some cases colocalizing with E-cadherin (Peters et al., 1999; Boletta et al., 2001; Roitbak et al., 2004). Biotinylation experiments on stably transfected MDCK indicate the PC1 present at the lateral plasma membrane is full-length and not GPS cleaved (Boletta et al., 2001). Although Boletta et al. (2001) did not transfect PC2 with PC1, other studies have found a significant amount of endogenous PC2 in MDCK cells, so the requirement for PC2 coexpression may still hold in these cells (Scheffers et al., 2000). The presence of full-length protein at the lateral membranes of polarized cells, in contrast to the GPS-cleaved form of PC1 that we detect at the plasma membranes of nonpolarized cells, suggests that the different forms of PC1 may have functionally distinct roles, and strengthens the argument that regulating cleavage also may regulate localization.
In another study employing polarized cells, Roitbak et al. (2004) found normal levels of PC1 expression but reduced levels of PC1 at the plasma membrane in kidney cells cultured from tissue excised from ADPKD patients. They attributed this effect to excessive phosphorylation of the PC1 protein. This study did not determine the nature of disease-causing mutations in the gene encoding the PC1 protein. It would be interesting to determine whether these mutations affected GPS cleavage without altering protein expression levels.
In a study employing nonpolarized cells, Babich et al. (2004) found that PC1 colocalized with the plasma membrane marker CD4 when it was expressed in the presence of PC2 in CHO cells, similar to our results with HEK cells. They also concluded that expression of PC2-D511V was sufficient to induce accumulation of PC1 at the plasma membrane and that a PC1 construct lacking a portion of its N-terminal domain could reach the plasma membrane. It is important to note that these effects were quantified by measuring channel conductance induced by PC1 and PC2 expression and by assessing these proteins' presence at the cell surface by the extent of their colocalization with a plasma membrane marker (Babich et al., 2004). Their marker for PC1 at the surface membrane was its colocalization with CD4, but we could not detect any surface PC1 in CHO cells using our surface immunofluorescence protocol (data not shown). It is possible that the CHO cell line that they used may have expressed relatively high levels of endogenous wild-type PC2 compared with the cells used in our studies. It is also worth noting that their electrophysiological assay is likely to be able to detect very small surface populations of active PC1–PC2 channel complexes, whereas their fluorescence assay may not be able to distinguish among bulk populations of proteins associated with submembranous compartments located close to the cell surface from the much smaller contingents that have attained a bona fide surface localization (Babich et al., 2004).
We demonstrate that the effect of PC2 on PC1 localization is independent of a stable interaction between the two proteins. The PC2-D511V mutant stably binds PC1 but does not increase the amount of PC1 at the plasma membrane, whereas the truncated PC2-L703X cannot bind PC1 but still promotes its surface delivery. This suggests that the release of PC1 from the endoplasmic reticulum is not due to the release of PC1 from associations with chaperone proteins upon PC2 binding to form a heterodimeric complex with PC1, a mechanism that regulates the surface delivery of a other multimeric membrane protein complexes (Zerangue et al., 1999). The dramatically different degrees of enhancement of PC1 GPS cleavage produced by PC2-L703X and PC2-D511V also shows that stimulating GPS cleavage neither requires nor is guaranteed by a stable interaction with PC1.
The results obtained with the PC1-CTD construct reveal several significant details of PC2's effect on PC1 expression and cleavage. The most apparent is that, though the coiled-coil region of PC1 has been shown to bind PC2 in isolation from the rest of the PC1 protein (Qian et al., 1997), the fact that our PC1-CTD construct lacks the coiled-coil domain but still binds PC2 suggests that there are likely to be additional domains of in PC1 that are involved in the PC2 interaction. In regard to the effect of PC2 on PC1 stabilization and cleavage, coexpressing PC2 elevates PC1-CTD protein expression but does not increase the proportion of GPS-cleaved protein, suggesting that the stabilization and cleavage effects of PC2 on PC1 are mechanistically distinct.
A second implication arising from these studies is that an intact PC1 C-terminal tail is necessary for the enhancement of GPS cleavage. Our results suggest that the majority of the PC1 proteins that are present at the surface have intact C termini, because immunofluorescence analysis of PC2 coexpressing cells reveals surface patterns for the PC1 protein with both the anti-FLAG and anti-HA antibodies. Furthermore, biotinylated PC1 protein from the surface of cells expressing PC1 and PC2 is detectable with the anti-HA antibody on a Western blot. Together, these data suggest the possibility that PC1 protein with an intact C-terminal tail is the substrate for PC2-stimulated GPS cleavage, and this protein subsequently moves to the surface. Given that the C-terminal tail has multiple signaling roles once cleaved (Chauvet et al., 2004; Low et al., 2006), the prevalence of PC1 with an intact C-terminal tail at the plasma membrane suggests a spatial and possibly functional distinction between forms of PC1 that differ with respect to the states of their C termini.
The surprising incongruity in the effects of PC2-D511V and PC2-T721A on PC1 surface localization in nonpolarized cells underscores the significance of GPS cleavage in the promotion of trafficking PC1 to the plasma membrane. Both PC2-D511V and PC2-T721 are reported to manifest defective channel activity. Whereas PC2-D511V does not support PC1 GPS cleavage or surface delivery, both of these activities are accomplished by PC2-T721A. This is strong evidence that PC2's channel function is not the principle characteristic through which it mediates its effect on PC1 cleavage and distribution. We believe that, with respect to its effects on PC1 surface delivery, the significant point of difference between PC2-D511V and wild-type PC2 resides in the inability of the mutant PC2-D511V to promote GPS cleavage of PC1. This cannot be explained by a difference in the subcellular localizations of the wild type and D511V mutant PC2 proteins or by their capacity to interact physically with PC1 (Reynolds et al., 1999; Ma et al., 2005). We conclude that the regulation of GPS cleavage is dependent upon as yet unappreciated aspects of PC2 function and that further study will be necessary to understand how the D511V point mutation could cause such a dramatic shift in the functional link between PC1 and PC2.
The results obtained with polarized epithelial cells expand and provide important context for our understanding of PC2-enhanced PC1 plasma membrane delivery. In the kidney, the native polycystin proteins are expressed in the polarized epithelial cells of the renal tubules. Unlike the fibroblastic HEK293 cells, these cells possess plasma membranes divided into distinct apical and basolateral domains and contain sensory primary cilia. Thus, the surfaces of these cells provide a complex array of trafficking options to the machinery that controls the localization and physiological effects of PC1 and PC2. Given this distinction between polarized and nonpolarized cells, we thought it both important and potentially illuminating to expand findings from fibroblast cells into polarized cells. Our stable LLC-PK cell lines confirmed that expression of PC2 stimulates and is required for PC1 to achieve plasma membrane localization. This effect could be developmentally significant, given the nonsynchronous changes in PC1 and PC2 expression in the developing kidney: PC1 is most strongly expressed early in development, whereas PC2 expression is higher in mature tubules (Ward et al., 1996; Markowitz et al., 1999; Chauvet et al., 2002). Our results suggest that PC2 coexpression both increases PC1 protein levels and alters its localization. Hence, alterations in PC2 expression may coordinately affect PC1 levels and localization. Further study is needed to test this hypothesis in the developing tubule, as well as to determine whether there are the functional differences between the pools of PC1 molecules present at internal and plasma membrane locations.
We were quite surprised to find substantial differences in the capacity of mutant forms of PC2 to support PC1 cell surface delivery in polarized versus fibroblastic cells. The most notable of these differences was that PC2-L703X failed to support PC1 localization to either the apical plasma membrane or the primary cilium in LLC-PK cells, whereas PC2-L703X coexpression was sufficient to ensure robust PC1 surface expression in HEK293 cells. In addition, PC2-D511V expression allowed a very low level of PC1 delivery to the primary cilium in LLC-PK cells, whereas as no surface PC1 expression was detected in HEK293 cells in which PC1 was coexpressed with the PC2-D511V construct. Some of this surface PC1 localization may be explained by the presence of endogenous PC2, as detected by Western blot (Figure 8E). Endogenous PC2 also may explain the low level of ciliary membrane PC1 seen in cells not transfected with PC2. In sum, the incongruence between PC1 localization in polarized and fibroblastic cells suggests the unexpected possibility that the processes and requirements that govern the trafficking of PC1 in polarized, ciliated cells differ from those that operate in nonpolarized cells. Further studies are required to determine whether the postsynthetic pathways pursued by PC1 in polarized versus nonpolarized cells are governed by distinct itineraries, interactions, or check points. It will also be interesting to determine whether the presence of a cilium alters generates signals that influence or alter the trafficking options available to PC1.
Based upon our evidence, PC2 coexpression leads to the delivery of PC1 to the plasma membrane, and more specifically to the apical and ciliary membranes of polarized cells. PC2 acts on PC1 early in the secretory pathway to promote cleavage of PC1 at the GPS site, leading to delivery of PC1 to the plasma membrane, and this effect is independent of a stable physical interaction between the two proteins. Cleavage at the GPS site separates the intracellular pool of PC1 into two functionally distinct groups, as noted previously (Wei et al., 2007), and we find that the GPS-cleaved pool of PC1 is characterized at least in part by its ability to reach the membrane. A regulatory effect of PC2 on PC1 cleavage has already been shown for the cytoplasmic PC1 cleavage (Chauvet et al., 2004; Bertuccio et al., 2009), and results shown here suggest that PC2 enhances the GPS cleavage as well. Given that the majority of the surface population of PC1 retains its cytoplasmic HA tag, PC2's effects on C- and N-terminal PC1 cleavages may be exercised on distinct pools of PC1 protein that ultimately reside in different subcellular compartments and that serve functionally distinct roles. It remains to be determined whether there is any functional relationship between the GPS cleavage and the C-terminal tail cleavage, and whether it is the surface or ER pools of PC1 that are preferentially susceptible to C-terminal tail cleavage. Future studies will examine this relationship, and explore the molecular processes through which PC2 expression influences the GPS cleavage and cell surface delivery of PC1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Yiqiang Cai and Stefan Somlo for providing the B9 antibody and polycystin cDNA constructs. Drs. Stefan Somlo, Lloyd Cantley, and Carl Hashimoto and members of the Caplan laboratory provided helpful suggestions and comments. This work was supported by National Institutes of Health grant DK-57328 (to M.J.C.) and National Science Foundation graduate research fellowship 2005019941 (to H.C.C.).
Abbreviations used:
- ADPKD
autosomal dominant polycystic kidney disease
- CTD
C-terminal deletion
- CTF
polycystin-1 C-terminal fragment
- GPS
G protein-coupled receptor proteolytic site
- NTD
N-terminal deletion
- PC
polycystin.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-05-0407) on October 27, 2010.
REFERENCES
- Babich V., Zeng W. Z., Yeh B. I., Ibraghimov-Beskrovnaya O., Cai Y., Somlo S., Huang C. L. The N-terminal extracellular domain is required for polycystin-1-dependent channel activity. J. Biol. Chem. 2004;279:25582–25589. doi: 10.1074/jbc.M402829200. [DOI] [PubMed] [Google Scholar]
- Bertuccio C. A., Chapin H. C., Cai Y., Mistry K., Chauvet V., Somlo S., Caplan M. J. Polycystin-1 C-terminal cleavage is modulated by polycystin-2 expression. J. Biol. Chem. 2009;284:21011–21026. doi: 10.1074/jbc.M109.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A. K., Piontek K., Boletta A., Liu L., Qian F., Xu P. N., Germino F. J., Germino G. G. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Boletta A., et al. Biochemical characterization of bona fide polycystin-1 in vitro and in vivo. Am. J. Kidney Dis. 2001;38:1421–1429. doi: 10.1053/ajkd.2001.29282. [DOI] [PubMed] [Google Scholar]
- Cai Y., et al. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 2004;279:19987–19995. doi: 10.1074/jbc.M312031200. [DOI] [PubMed] [Google Scholar]
- Cai Y., Maeda Y., Cedzich A., Torres V. E., Wu G., Hayashi T., Mochizuki T., Park J. H., Witzgall R., Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J. Biol. Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- Casuscelli J., Schmidt S., Degray B., Petri E. T., Celic A., Folta-Stogniew E., Ehrlich B. E., Boggon T. J. Analysis of the cytoplasmic interaction between polycystin-1 and polycystin-2. Am. J. Physiol. Renal Physiol. 2009;297:F1310–F1315. doi: 10.1152/ajprenal.00412.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin H. C., Rajendran V., Capasso A., Caplan M. J. Detecting the surface localization and cytoplasmic cleavage of membrane-bound proteins. Methods Cell Biol. 2009;92:223–239. doi: 10.1016/S0091-679X(08)94011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet V., et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am. J. Pathol. 2002;160:973–983. doi: 10.1016/S0002-9440(10)64919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet V., et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P., Nauli S. M., Li X., Coste B., Osorio N., Crest M., Brown D. A., Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 2004;18:740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- Delmas P., et al. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J. Biol. Chem. 2002;277:11276–11283. doi: 10.1074/jbc.M110483200. [DOI] [PubMed] [Google Scholar]
- Gabow P. A. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- Gottardi C. J., Dunbar L. A., Caplan M. J. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am. J. Physiol. 1995;268:F285–F295. doi: 10.1152/ajprenal.1995.268.2.F285. [DOI] [PubMed] [Google Scholar]
- Grimm D. H., Cai Y., Chauvet V., Rajendran V., Zeltner R., Geng L., Avner E. D., Sweeney W., Somlo S., Caplan M. J. Polycystin-1 distribution is modulated by polycystin-2 expression in mammalian cells. J. Biol. Chem. 2003;278:36786–36793. doi: 10.1074/jbc.M306536200. [DOI] [PubMed] [Google Scholar]
- Hanaoka K., Qian F., Boletta A., Bhunia A. K., Piontek K., Tsiokas L., Sukhatme V. P., Guggino W. B., Germino G. G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Kleymenova E., Ibraghimov-Beskrovnaya O., Kugoh H., Everitt J., Xu H., Kiguchi K., Landes G., Harris P., Walker C. Tuberin-dependent membrane localization of polycystin-1, a functional link between polycystic kidney disease and the TSC2 tumor suppressor gene. Mol. Cell. 2001;7:823–832. doi: 10.1016/s1097-2765(01)00226-x. [DOI] [PubMed] [Google Scholar]
- Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B. E., Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Low S. H., Vasanth S., Larson C. H., Mukherjee S., Sharma N., Kinter M. T., Kane M. E., Obara T., Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev. Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Ma R., Li W. P., Rundle D., Kong J., Akbarali H. I., Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol. Cell. Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz G. S., Cai Y., Li L., Wu G., Ward L. C., Somlo S., D'Agati V. D. Polycystin-2 expression is developmentally regulated. Am. J. Physiol. 1999;277:F17–F25. doi: 10.1152/ajprenal.1999.277.1.F17. [DOI] [PubMed] [Google Scholar]
- Muth T. R., Caplan M. J. Transport protein trafficking in polarized cells. Annu. Rev. Cell Dev. Biol. 2003;19:333–366. doi: 10.1146/annurev.cellbio.19.110701.161425. [DOI] [PubMed] [Google Scholar]
- Peters D. J., van de Wal A., Spruit L., Saris J. J., Breuning M. H., Bruijn J. A., de Heer E. Cellular localization and tissue distribution of polycystin-1. J. Pathol. 1999;188:439–446. doi: 10.1002/(SICI)1096-9896(199908)188:4<439::AID-PATH367>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Qian F., Boletta A., Bhunia A. K., Xu H., Liu L., Ahrabi A. K., Watnick T. J., Zhou F., Germino G. G. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc. Natl. Acad. Sci. USA. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F., Germino F. J., Cai Y., Zhang X., Somlo S., Germino G. G. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat. Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- Qian F., Watnick T. J., Onuchic L. F., Germino G. G. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- Reynolds D. M., et al. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. J. Am. Soc. Nephrol. 1999;10:2342–2351. doi: 10.1681/ASN.V10112342. [DOI] [PubMed] [Google Scholar]
- Roitbak T., Ward C. J., Harris P. C., Bacallao R., Ness S. A., Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol. Biol. Cell. 2004;15:1334–1346. doi: 10.1091/mbc.E03-05-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R. J., Husson H., Joly D., Bukanov N. O., Patey N., Knebelmann B., Ibraghimov-Beskrovnaya O. Impaired formation of desmosomal junctions in ADPKD epithelia. Histochem. Cell Biol. 2005;124:487–497. doi: 10.1007/s00418-005-0055-3. [DOI] [PubMed] [Google Scholar]
- Scheffers M. S., van der Bent P., Prins F., Spruit L., Breuning M. H., Litvinov S. V., de Heer E., Peters D. J. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum. Mol. Genet. 2000;9:2743–2750. doi: 10.1093/hmg/9.18.2743. [DOI] [PubMed] [Google Scholar]
- Seabra M. C., Mules E. H., Hume A. N. Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- Tsiokas L., Kim E., Arnould T., Sukhatme V. P., Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl. Acad. Sci. USA. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veland I. R., Awan A., Pedersen L. B., Yoder B. K., Christensen S. T. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron. Physiol. 2009;111:39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. J., Turley H., Ong A. C., Comley M., Biddolph S., Chetty R., Ratcliffe P. J., Gattner K., Harris P. C. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc. Natl. Acad. Sci. USA. 1996;93:1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Hackmann K., Xu H., Germino G., Qian F. Characterization of cis-autoproteolysis of polycystin-1, the product of human polycystic kidney disease 1 gene. J. Biol. Chem. 2007;282:21729–21737. doi: 10.1074/jbc.M703218200. [DOI] [PubMed] [Google Scholar]
- Yu S., et al. Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc. Natl. Acad. Sci. USA. 2007;104:18688–18693. doi: 10.1073/pnas.0708217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N., Schwappach B., Jan Y. N., Jan L. Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.