We show that while Centrin2 is dispensable for centriole assembly, it is an Mps1 substrate that stimulates canonical and aberrant centriole assembly by two different Mps1-dependent mechanisms, HsSas-6–dependent and –independent. Centrin2 phosphorylation is also required for the ability of Mps1 to drive production of mature centrioles.
Abstract
The nondegradable Mps1Δ12/13 protein drives centriole overproduction, suggesting that Mps1 phosphorylates a subset of centrosomal proteins to drive the assembly of new centrioles. Here we identify three Mps1 phosphorylation sites within the centriolar protein Centrin 2 (Cetn2). Although centrioles can be assembled in the absence of Cetn2, centriole assembly is attenuated in the absence of Cetn2. While wild-type Cetn2 can compensate for this attenuation, a nonphosphorylatable version cannot. In addition, overexpressing Cetn2 causes Mps1-dependent centriole overproduction that requires each of the three Mps1 phosphorylation sites within Cetn2 and is greatly exacerbated by mimicking phosphorylation at any of these sites. Wild-type Cetn2 generates excess foci that are competent as mitotic spindle poles in HsSas-6–depleted cells, suggesting that Cetn2 can organize a subset of centriolar proteins independently of cartwheels. However, centriole overproduction caused by a phosphomimetic Cetn2 mutant requires HsSas-6, suggesting that Cetn2 phosphorylation stimulates the canonical centriole assembly pathway. Moreover, in the absence of Cetn2, Mps1Δ12/13 cannot drive the production of mature centrioles capable of recruiting γ-Tubulin, and a nonphosphorylatable Cetn2 mutant cannot compensate for this defect and exacerbates Cetn2 depletion. Together, our data suggest that Mps1-dependent phosphorylation of Cetn2 stimulates the canonical centriole assembly pathway.
INTRODUCTION
The mammalian centrosome is composed of a pair of centrioles that are surrounded by a pericentriolar matrix responsible for microtubule nucleation. During normal cell division, centrosomes act as poles of the mitotic spindle that mediates chromosome segregation (Doxsey, 2001). Because extra centrosomes and/or centrioles can form extra spindle poles, the faithful maintenance of genomic integrity requires that centrosomes be replicated just once each cell cycle. The canonical centrosome duplication pathway is initiated at the G1/S transition and results in the assembly of a single new centriole, called a procentriole, at a site adjacent to each existing centriole. These procentrioles are elongated during S and G2 but remain attached to the proximal end of the mother centriole until mitosis, when mother and daughter centrioles are physically disengaged.
RNA interference and time-lapse imaging in Caenorhabditis elegans have recently elaborated a pathway for canonical centriole assembly that requires Spd-2 (Kemp et al., 2004; Pelletier et al., 2004), Zyg-1 (O'Connell et al., 2001), Sas-4 (Leidel and Gonczy, 2003), Sas-5 (Delattre et al., 2004), and Sas-6 (Leidel et al., 2005). While there is no apparent human Sas-5 orthologue, the apparent Spd-2, Zyg-1, Sas-4, and Sas-6 orthologues [Cep192 (Zhu et al., 2008), PLK4 (Habedanck et al., 2005), CPAP/CENP-J (Cho et al., 2006), and Hs-Sas6 (Strnad et al., 2007), respectively] are required for centriole assembly in human cells. Reflecting the pathway described in worms, the recruitment of HsSas-6 to the site of centriole assembly requires Plk4 (Habedanck et al., 2005), and CPAP determines centriole length (Kohlmaier et al., 2009; Schmidt et al., 2009; Tang et al., 2009). However, additional factors not present in flies and/or worms have been implicated in centriole assembly in human cells, such as δ- and ε-Tubulin (Chang and Stearns, 2000; Chang et al., 2003), Mps1 (Fisk and Winey, 2001; Fisk et al., 2003; Kasbek et al., 2007), Centrin 2 (Cetn2) (Salisbury et al., 2002), the centrin binding protein hPoc5 (Azimzadeh et al., 2009), CP110 (Chen et al., 2002) that is required for the function of Plk4 (Habedanck et al., 2005) and cooperates with Cetn2 (Tsang et al., 2006), and Cep76 that binds to CP110 and represses excessive rounds of centriole assembly (Tsang et al., 2009).
The ability of cells to form daughter centrioles is restricted to S phase (Wong and Stearns, 2003), and the attachment between mother and daughter centrioles inhibits formation of additional procentrioles (Tsou and Stearns, 2006). However, the mother centriole can produce a second daughter centriole if its first daughter is removed [e.g., by laser ablation (Loncarek et al., 2008)]. The regulation of HsSas-6 protein levels is important for the proper formation of a single procentriole (Strnad et al., 2007), and overexpression of either Plk4 (Habedanck et al., 2005) or HsSas-6 (Strnad et al., 2007) drives the overproduction of centrioles. Furthermore, in some cells, such as the CHO and U2OS cell lines, a defective version of the canonical centriole duplication pathway can occur in which a maternal centriole can form more than one daughter centriole, either by the formation of multiple daughters at one time, or by stochastic daughter disengagement (Loncarek et al., 2008).
New centrioles can also be assembled de novo in the complete absence of parental centrioles, a phenomenon first described in the single celled green alga Chlamydomonas rheinhardtii (Marshall et al., 2001) and subsequently observed in flies (Peel et al., 2007; Rodrigues-Martins et al., 2007) and vertebrate cells (La Terra et al., 2005; Uetake et al., 2007; Loncarek et al., 2008). In vertebrate cells where centrosomes have been removed by laser ablation or microsurgery, de novo assembly begins with the formation of a random number of amorphous centrin-containing aggregates that become morphologically recognizable centrioles by mitosis, and the presence of existing centrioles appears to suppress de novo assembly (La Terra et al., 2005; Uetake et al., 2007), presumably to favor the production a precise number of centrioles via the canonical pathway.
Either centriole overduplication or de novo centriole assembly might lead to the production of abnormal spindles that produce aneuploid cells such as those found in tumors (Lingle and Salisbury, 2000). While the precise mechanisms that limit centriole assembly to one round of canonical duplication per cell cycle are not completely understood, our data suggest that these mechanisms include tight control over the centrosomal Mps1 protein kinase. While it is controversial whether Mps1 is essential for centriole assembly, both our work and that of others shows that increasing the centrosomal dosage of Mps1 promotes centriole overproduction. Overexpression of wild-type Mps1 causes centrosome reduplication in mouse cells (Fisk and Winey, 2001), and while it is not sufficient to cause centrosome reduplication in human cells, overexpression of wild-type Mps1 accelerates the onset of reduplication in human cell lines like U2OS (Fisk et al., 2003; Kanai et al., 2007; Kasbek et al., 2007) and 21NT (Kasbek et al., 2009). However, preventing the degradation of Mps1 at centrosomes is sufficient to cause centriole overduplication in all human cell types tested (Kasbek et al., 2007; Kasbek et al., 2009). Both mouse and human Mps1 proteins are Cdk2 substrates (Fisk and Winey, 2001; Kasbek et al., 2007), and Cyclin A–associated Cdk2 activity suppresses the degradation of centrosomal Mps1 in human cells by phosphorylating T468 within the Mps1 degradation signal (Kasbek et al., 2007). Overexpression of the Mps1T468D/E mutations that mimic Cdk2 phosphorylation is sufficient to cause centriole overduplication in all human cells tested (Kasbek et al., 2007), and the Mps1Δ12/13 mutation that removes the Mps1 degradation signal cause centriole overduplication at very modest expression levels (Kasbek et al., 2009). We hypothesize that excess centrosomal Mps1 causes centriole overproduction by promoting persistent phosphorylation of centriolar proteins, but the identity of such substrates is unknown.
Centrins are small calcium binding proteins that localize to centrosomes and have been implicated in centrosome duplication. Of the three human centrin genes Cetn1 is only expressed in male germ cells, while Cetn2 and Cetn3 are expressed in somatic cells (Lee and Huang, 1993; Errabolu et al., 1994; Middendorp et al., 1997). Cetn2 localizes to the distal lumen of centrioles (Paoletti et al., 1996), while Cetn3 has been reported to associate with both centrioles (Middendorp et al., 2000) and the pericentriolar matrix (Baron et al., 1992). While centrin is required for the assembly and stability of basal bodies in Tetrahymena thermophila (Stemm-Wolf et al., 2005) and was initially thought to be required for centriole duplication in human cells (Salisbury et al., 2002), it is not required for the recruitment of HsSas-6 to the site of centriole assembly (Strnad et al., 2007), and codepletion of Cetn2 and Cetn3 does not prevent Plk4-induced centriole overproduction (Kleylein-Sohn et al., 2007). Cetn2 is subject to extensive posttranslational modification. The nucleo-cytoplasmic shuttling of Cetn2 was recently shown to be regulated by SUMOylation (Klein and Nigg, 2009), and centrin is present in many isoforms that presumably reflect phosphorylation (Paoletti et al., 1996). Phosphorylation of Cetn2 at Serine 170 by protein kinase A promotes centriole separation (Lutz et al., 2001), and abnormal centrin phosphorylation is observed in human breast tumors that have aberrant centriole numbers (Lingle et al., 1998), suggesting that phosphorylation of centrin must be tightly regulated.
We have found that Cetn2 is an Mps1 substrate in vitro, and here we describe the identification of three Mps1 phosphorylation sites within Cetn2. While our evidence supports the suggestion that Cetn2 is not essential for centriole assembly, it is required for the overproduction of mature centrioles in cells expressing Mps1Δ12/13. We also describe phenotypes associated with both centriole overduplication and de novo centriole assembly that are caused by overexpression of GFP-Cetn2, require Mps1, and are exacerbated by phosphomimetic mutations within Cetn2.
MATERIALS AND METHODS
Plasmids
Previously described plasmids used for this study are as follows: pHF 7 (GFP) (Fisk and Winey, 2001; Fisk et al., 2003), pHF64 (GST-hMps1) (Fisk and Winey, 2001; Fisk et al., 2003), and pHF80 (GFP-Cetn2) (Fisk et al., 2003). Plasmids created for this study are as follows: Bacterial expression constructs in the pDEST17 vector (Invitrogen, Carlsbad, CA); pHF188 (6his-Cetn2), pHF190 (6his-Cetn2ATT), pHF191 (6his-Cetn2TAT), and pHF192 (6his-Cetn2TTA). Mammalian expression constructs in the previously described pECE-GFP vector (Fisk and Winey, 2001; Fisk et al., 2003; Kasbek et al., 2007); pHF206 (GFP-Cetn2TTA), pHF207 (GFP-Cetn2AAT), pHF213 (GFP-Cetn2TTD), pHF214 (GFP-Cetn2DDT), pHF233(GFP-sirCetn2), pHF236 (GFP-sirCetn2TTA), and pHF237 (GFP-sirCetn2AAT), pHF248 (GFP-Cetn2AAD), pHF249 (GFP-Cetn2TAD), pHF250 (GFP-Cetn2ATD), pHF251 (GFP-Cetn2DDD). Tet-inducible expression constructs in the pT-REx-DEST30 vector (Invitrogen); pHF218 (Tet-GFPCetn2), and pHF228 (Tet-GFPCetn2TTD). These plasmids were created as follows: Briefly, PCR products containing Cetn2 or GFP-Cetn2 were cloned into the pENTR/D/SD/TOPO entry vector, then transferred into pDEST17 and pT-REx-DEST30 using the Gateway® Recombination system (Invitrogen) to generate pHF188 and pHF218, respectively. All other plasmids were created by site-directed mutagenesis of pHF80, pHF118, and pHF218 using the GeneTailor kit (Invitrogen); sequences of primers used to mutate T45, T47, and T118 to alanine or aspartic acid (superscript nomenclature used above refers to the genotype at T45, T47, and T118), and to render the Cetn2 cDNA resistant to a Cetn2 Stealth siRNA (Invitrogen), are available upon request. The identity of all constructs was verified by sequence analysis.
Cells, Cell Culture
HeLa S3, HeLa T-REx (Invitrogen), and derived cell lines were cultured in DMEM supplemented with 10% FBS (Hyclone, Logan, UT), 20 U/ml penicillin G (Invitrogen), and 50 μg/ml streptomycin (Invitrogen). A tetracycline (Tet)-inducible GFP-Cetn2 cell line was generated by transfection of HeLa T-REx with pHF218 followed by selection with G418 (Invitrogen) for two weeks.
Transfections and siRNA
For experiments involving transient overexpression of GFP constructs, cells were transfected using Effectine reagent (Qiagen, Valencia, CA). siRNAs were transfected at 0.2 μM using Oligofectamine (Invitogen). siRNAs used were Mps1 (nucleotides 1360-1384) and Cetn2 (nucleotides 78-102) Stealth siRNAs (Invitrogen), HsSas-6 siGENOME SMARTpool, and siGLO LaminA/C siRNAs (Dharmacon RNA Technologies, Lafayette, CO). Efficiency of siRNA depletion was determined by immunoblot as described below.
Antibodies
To generate an antibody against Cetn2, 6His-Cetn2 was injected into rabbits (Lampire Biological Laboratories, Pipersville, PA). Serum from an immunized rabbit was affinity purified against 6His-Cetn2 coupled to Affi-gel 15 (Bio-Rad, Hercules, CA), dialyzed against Phosphate Buffered Saline (PBS), and stored at 4°C in PBS containing 0.05% sodium azide. Primary antibodies for indirect immunofluorescence (IIF) were as follows: GTU-88 mouse anti–γ-Tubulin, 1:200 (Sigma, St. Lois MO); rabbit anti–γ-Tubulin, 1:200 (T5192, Sigma); rabbit anti-Cetn2, 1:5000 (described above); mouse anti–Sas-6, 1:100 (sc-81431; Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-CP110, 1:500 (the generous gift of Dr. Brian Dynlacht, NYU School of Medicine); rabbit anti-Cep135, 1:1000 (Abcam, Cambridge, MA); GT335 mouse anti-polyglutamylated tubulin, 1:1000 (Enzo Life Science, Plymouth Meeting PA); rat anti-BrdU, 1:500 (Accurate Chemicals, Westbury, NY). Secondary antibodies for IIF were Alexa 350-conjugated goat anti-rat, 488-, 594-, or 750-conjugated donkey anti-rabbit and donkey anti-mouse (Invitrogen). Except in experiments where BrdU was the only nuclear stain, DNA was stained with Hochest 33342 (Sigma). Primary antibodies for Western blot were DM1A mouse anti-α-Tubulin, 1:10,000 (Sigma); mouse anti–Sas-6, 1:4000; N1 mouse anti-hMps1, 1:1000 (Invitrogen); rabbit anti-Cetn2, 1:500 (BioLegend, San Diego, CA). Secondary antibodies for immunoblot were Alexa 680–conjugated donkey anti-mouse and rabbit (Invitrogen), IRDye800-conjugated anti-mouse and rabbit (Rockland, Gilbertsville, PA) and horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG, 1:3000 (GE Healthcare, Piscataway, NJ).
Indirect Immunofluorescence (IIF) Analysis
For colocalization of GFP-Cetn2 constructs and γ-Tubulin, cells were fixed in PBS containing 4% formaldehyde (Ted Pella, Redding, CA), 1 mM MgCl2, and 0.2% Triton X-100 for 10 min at room temperature and processed for IIF as described previously (Fisk and Winey, 2001; Fisk et al., 2003; Kasbek et al., 2007). For staining Cetn2, HsSas-6, CP110, or Cep135 cells were processed as described (Strnad et al., 2007). Briefly, cells were fixed in −20°C methanol for 10 min at −20°C, washed four times with PBS, incubated in antibody dilution solution (1% bovine serum albumin, 0.05% Triton X-100 in PBS) for 20 min at room temperature then incubated with primary antibodies overnight at 4°C. Cells were then washed four times for 5 min in PBST (0.05% Triton X-100 in PBS), and stained with secondary antibodies and Hoechst 33258 as previously described for formaldehyde/triton fixation (Fisk and Winey, 2001; Fisk et al., 2003; Strnad et al., 2007). For BrdU staining, cells were fixed with formaldehyde in PBS (without detergent) for 10 min at RT, washed four times with 0.5 mM MgCl2 in PBS (PBS/Mg), extracted with 0.3% Triton X-100 in PBS for 15 min at RT, washed four more times with PBS/Mg, and then incubated with 0.36 Units of DNaseI (Invitrogen) in 150 μl of PBS with 4 mM MgCl2 for 30 min at RT. After washing once with PBS/Mg cells were incubated in blocking buffer (0.5% NP-40, 5 mg/ml BSA in PBS) for 30 min at RT, followed by rat-anti BrdU in blocking buffer for 45 min at 37°C. After washing three times with PBS/Mg cells were incubated with additional primary antibodies overnight at 4°C, washed four times with PBS/Mg, then incubated with secondary antibodies as described above. Centriole numbers in cells transfected with siRNA against HsSas-6 were compared by using Student's t test using Kalediagraph (Synergy Software, Reading PA).
Immunoblot Analysis
Efficiency of siRNA depletion for Mps1 and HsSas-6 was determined by quantitative dual color immunoblot using the Odyssey imaging system (LI-COR, Lincoln, NE) as previously described (Fisk et al., 2003; Kasbek et al., 2007; Kasbek et al., 2009). Efficiency of siRNA depletion for Cetn2 was determined using enzyme-linked chemiluminscence as follows. After incubation with rabbit anti-Cetn2 followed by HRP-Conjugated donkey anti-rabbit IgG, membranes were incubated with SuperSignal West Femto Maximum Sensitivity Substrate, 1:2 dilution (Thermo Fisher Scientific, Rockford, IL) for 5 min and exposed to Blue Basic Autoradiography Film (ISC Bioexpress, Kaysville, UT). Films were then digitized and analyzed using ImageJ software (http://rsbweb.nih.gov/ij/). The background corrected pixel intensity of each Cetn2 band from scanned images was normalized to the level of the loading control α-Tubulin, determined on a parallel blot using the Odyssey imaging system.
Kinase Assays
Kinase assay were performed as described previously (Fisk and Winey, 2001; Kasbek et al., 2007) using 10 μg of recombinant protein and 1 μg of GST-Mps1 protein. Kinase reactions were analyzed by SDS-PAGE followed by autoradiography of dried gels.
Cell Synchronization and BrdU Incorporation Assay
For S-phase arrest, HeLa cells were treated with 4 mM hydroxyurea (HU) 24 h after transfection. The beginning of S-phase arrest was considered to be 24 h after the addition of HU, and centriole number was assessed by Cetn2 staining 72 h after addition of HU (48 h S-phase arrest). For BrdU incorporation, HeLa cells were sequentially transfected with siGLO or Cetn2 siRNA and GFP or sirGFP Cetn2 constructs, each for 24 h. BrdU (40 uM) was added at 68 h post-siRNA transfection, and both BrdU incorporation and centriole number were assessed by IIF at 72 h post-siRNA transfection. For chasing BrdU-labeled cells, transfections, and BrdU labeling were as described above, but fresh medium lacking BrdU was added at 72 h post-siRNA transfection, and both BrdU incorporation and centriole number were assessed 4 h later at 76 h post-siRNA transfection.
Serial Section Electron Microscopy
For GFP-Cetn2TTT expression, HeLa T-REx GFP-Cetn2 cells were plated at a 1: 5 dilution onto 4-well Lab-Tek chamber slide (Fisher, Rochester, NY). Cells were induced to express GFP-Cetn2TTT by the addition of 1 μg/ml Doxycyline (Dox) and arrested in S phase for 48 h as described above. For GFP-Cetn2TTD, HeLa T-REx cells were plated at 1:10 and 1:5 dilutions onto 4-well Lab-Tek chamber slide, then transfected with GFP-Cetn2T118D and arrested in S phase as described above. Cells in chamber slides were fixed for 30 min at room temperature in 0.05 M cacodylate buffer pH 7.4 (CB) containing 2% glutaraldehyde. After fixation, cells were washed three times in CB then stained with 0.5% osmium tetroxide (OSO4) and 0.8% potassium ferrocyanide (K3Fe (CN)6) in CB for 15 min on ice, washed three times in CB before staining with 0.15% tannic acid in CB for 1 min at room temperature, washed twice in CB and twice in distilled water before being stained in 2% aqueous uranyl acetate at room temperature for 2 h, and washed once with distilled water (all washes were for 5 min). Cells were then dehydrated in 50%, 70%, 80%, 95%, 100%, and 100% ethanol 5 min for each, incubated in hydroxypropyl methacrylate for 15 min at room temperature, infiltrated with resin solution from a Poly-Bed 812 Resin Test Kit (Ted Pella, Redding, CA) for 2 h at room temperature, and embedded at 60°C overnight. After embedding, the cells were cut into 80-nm serial sections and imaged in a Tecnai G2 Spirit TEM.
RESULTS
Mps1 Phosphorylates Centrin 2 in Vitro
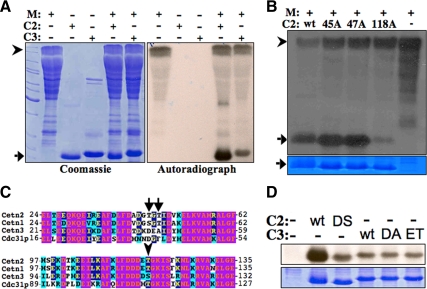
Although our data suggest that Mps1 is required for centrosome duplication in human cells (Fisk et al., 2003; Kasbek et al., 2007; Kasbek et al., 2009), the function of Mps1 in centrosome duplication has remained elusive and controversial (Stucke et al., 2002; Stucke et al., 2004). Because it is difficult to assign in vivo phosphorylation events to a particular kinase, we set out to test the hypothesis that Mps1 promotes centriole assembly and/or centriole overproduction by phosphorylating a subset of centriolar proteins using Mps1 kinase assays to assess the in vitro phosphorylation of various centriole proteins by Mps1. Concentrating our efforts on centrins for the simple reason that unlike other centrosomal proteins we could easily produce them in bacteria, we found that recombinant GST-Mps1 robustly phosphorylates recombinant 6his-Cetn2, and to a much lesser extent 6his-Cetn3 as well (Figure 1A). Using mass spectrometry we identified three Mps1 phosphorylation sites within Cetn2 (Supplemental Figure 1, A and B), T45 and T47 within the first EF hand, and T118 within the third EF hand of Cetn2 (Supplemental Figure 1C), but we were unable to identify phosphorylation sites within Cetn3. Mutation of T118 to the nonphosphorylatable residue alanine (T118A) significantly attenuated Cetn2 phosphorylation, while T45A or T47A had little affect, suggesting that at least in vitro T118 is the major site of phosphorylation (Figure 1B). While T45 and T47 are not well conserved among centrins, the region surrounding T118 is quite well conserved, and while this manuscript was under review the corresponding residue in Cdc31p was shown to be phosphorylated by Mps1p in S. cerevisiae (Araki et al., 2010). The only differences over an eleven amino acid stretch surrounding T118 are the residues corresponding to E117 and T118 in Cetn2, which are D114/S115 and H110/T111 in Cetn3 and Cdc31p, respectively (Figure 1C). Phosphorylation of a Cetn2 mutant protein containing the corresponding residues from Cetn3 (Cetn2E117D, T118S) was greatly attenuated (Figure 1D, “DS”). However, mutating Cetn3 S115 to alanine had no effect on Cetn3 phosphorylation (Figure 1D, “DA”), suggesting that despite the sequence conservation Mps1 does not phosphorylate Cetn3 at this region. Moreover, there was no increase in the phosphorylation of a Cetn3 mutant protein containing the corresponding residues from Cetn2 (Cetn3D114E, S115T) (Figure 1D, “ET”), suggesting that the ability of Mps1 to phosphorylate T118 is highly context-dependent, and that elements beyond the region immediately adjacent to T118 contribute to the ability of Mps1 to robustly phosphorylate Cetn2.
Figure 1.
Mps1 phsophorylates centrins in vitro. (A and B) Kinase assays consisting of purified recombinant GST-Mps1 (M), 6his-Cetn2 (C2), and 6his-Cetn3 (C3) were analyzed by SDS-PAGE. Shown are Coomasie-stained gels and corresponding 1-min autoradiographic exposures. Arrowheads indicate full-length GST-Mps1 and arrows indicate centrins. (A) GST-Mps1 phosphorylates 6his-Cetn2, and to a much lesser extent 6his-Cetn3. (B) T118 is the major in vitro phosphorylation site in Cetn2. Kinase assays containing GST-Mps1 and 6his-Cetn2 (wt), 6his-Cetn2ATT (45A), 6his-Cetn2TAT (47A), or 6his-Cetn2TTA (118A); top panel, autoradiographic image; bottom panel, coomassie staining of Cetn2 to indicate relative loading. (C) Alignment of human Cetn1, 2, and 3 with yeast Cdc31p. Arrows indicate T45 and T47, arrowhead indicates T118. (D) Phosphorylation is context-dependent. GST-Mps1 kinase assays with wild-type (wt) 6his-Cetn2 or 6his-Cetn3, 6his-Cetn2E117D, T118S (DS), 6his-Cetn3S115A (DA), or 6his-Cetn3D114E, S115T (ET); top panel, autoradiographic image; bottom panel, coomassie staining of Cetn2 and Cetn3 to indicate relative loading.
Cetn2 Depletion Delays the Incorporation of CP110 during Centriole Assembly
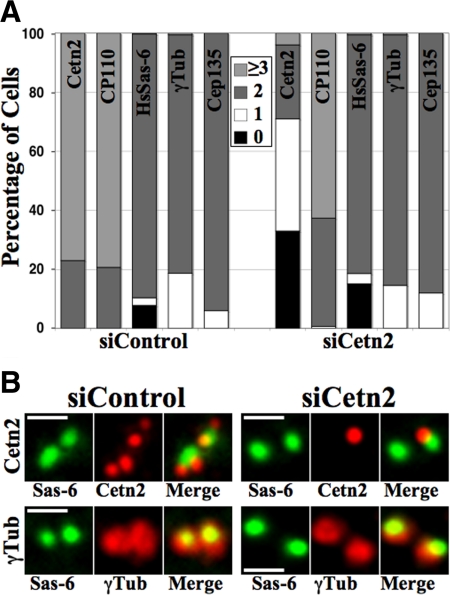
Before assessing the role of Cetn2 phosphorylation in centrosome duplication, we first sought to clarify the consequences of depleting Cetn2. First, using a previously reported Cetn2-specific siRNA sequence (Salisbury et al., 2002; Strnad et al., 2007) we faithfully recapitulated two apparently contradictory studies. Like Salisbury et al. (2002) we observed that the majority of Cetn2-depleted cells had one or zero Cetn2-positive centrioles (Figure 2A), suggesting that Cetn2 remains stably associated with existing centrioles and that Cetn2-positive centrioles are diluted by cell division. But like Strnad et al. (2007) we also found that Cetn2 depletion had little effect on HsSas-6 distribution (Figure 2, A and B). Despite the dilution of Cetn2-positive centrioles, there was little change in the percentage of cells with two γ-Tubulin foci (Figure 2A), and cells that had two γ-Tubulin foci and a single Cetn2-positive centriole (and had thus undergone at least one round of centriole assembly in the absence of Cetn2) typically had two HsSas-6 foci (Figure 2B). Moreover, we rarely observed cells with less than two CP110 foci (Figure 2A), which we would expect to observe if the number of Cetn2-positive centrioles corresponded to the total number of centrioles.
Figure 2.
Cetn2 is dispensable for centriole assembly. (A) Cetn2 depletion has little effect on recruitment of centriolar proteins. HeLa cells transfected with control (siControl) or Cetn2-specific (siCetn2) siRNAs were analyzed by IIF with antibodies against different centriolar markers at 72 h post-siRNA transfection. Bar graph shows the percentage of cells with zero (black bars), one (white bars), two (dark gray bars), or three or more (light gray bars) foci for the indicated markers. Values represent the mean for triplicate samples where at least 100 cells were counted per replicate. (B) There is little change in the distribution of HsSas-6. Shown are digitally magnified images of centrosomes of cells from the experiment described in A stained with HsSas-6 (green) and either Cetn2 (red, top panels) or γ-Tubulin (red, bottom panels); bar = 1 μm.
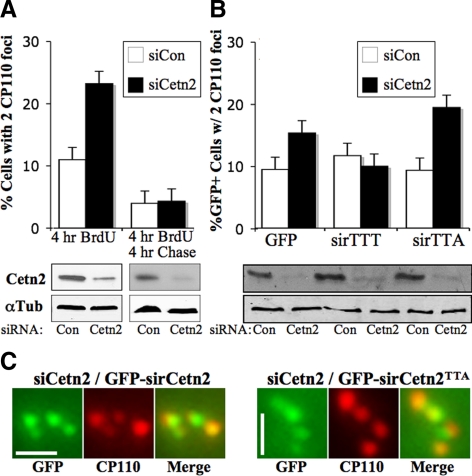
However, centriole assembly patterns were not completely normal in Cetn2-depleted cells, and we observed nearly twofold increases in the percentage of cells with no HsSas-6 foci or with two CP110 foci. Because a recent study demonstrated that depletion of the centrin binding protein hPOC5 caused a cell cycle delay (Azimzadeh et al., 2009), such cells might reflect a cell cycle delay rather than a defect in centriole assembly per se. Accordingly, we examined CP110 in cells that were in S-phase as judged by their ability to incorporate BrdU during a 4-h incubation. Although Cetn2 depletion had no effect on the percentage of BrdU-positive cells in this experiment, the percentage of BrdU-positive cells with two CP110 foci was twofold higher in Cetn2-depleted cells compared with control cells (Figure 3A), suggesting that in the absence of Cetn2 a significant fraction of S-phase cells exhibit either a delay or a block in the incorporation of CP110 during centriole assembly. When we included a 4-h chase period after BrdU labeling, the percentage of BrdU-positive Cetn2-depleted cells with two CP110 foci dropped to roughly 4% (Figure 3A), suggesting that the increase in cells with two CP110 foci in Cetn2-depleted cells represents a delay in CP110 incorporation rather than an outright block. A version of Cetn2 engineered to be siRNA-resistant (GFP-sirCetn2TTT, superscript nomenclature reflecting the genotype at the three Mps1 phosphorylation sites T45, T47, and T118) was capable of replacing endogenous Cetn2; while GFP alone had no effect, the percentage of cells with two CP110 foci in Cetn2-depleted cells expressing GFP-sirCetn2TTT was similar to that observed in control cells (Figure 3B). In contrast, GFP-sirCetn2TTA that cannot be phosphorylated at T118 failed to compensate for this delay, although it was readily incorporated into centrioles in the absence of endogenous Cetn2 (Figure 3C). In fact, the percentage of Cetn2-depleted cells expressing GFP-sirCetn2TTA with two CP110 foci was slightly higher than that in Cetn2-depleted cells expressing GFP alone (Figure 3), although this difference did not reach statistical significance. While our data show that structures that contain the centriole markers HsSas-6 and CP110, recruit γ-Tubulin, and incorporate the nonfunctional GFP-sirCetn2TTA can be assembled in the absence of Cetn2, these observations with CP110 demonstrate that in the absence of Cetn2 there is a delay in the incorporation of CP110 into newly assembled centrioles. To the extent that CP110 incorporation is part of centriole assembly, the delay in its incorporation that cannot be complemented by Cetn2TTA supports the hypothesis that Cetn2 increases the rate of centriole assembly in a phosphorylation-dependent manner. However, it will require extensive correlative light and electron microscopy (EM) to determine precisely what structures are formed in the absence of Cetn2.
Figure 3.
Cetn2 increases the rate of centriole assembly in a phosphorylation-dependent manner. (A) Cetn2 depletion causes a delay in centriole assembly. HeLa cells transfected with control siRNA (siCon, white bars) or Cetn2-specific siRNA (siCetn2, black bars) were labeled with BrdU for 4 h, and centriole number was determined at 72 h post-siRNA transfection (4 h BrdU), or at 76 h post-siRNA transfection after a 4 h chase period (4 h BrdU 4 h Chase) using an antibody against CP110. Bar graph shows the percentage of BrdU-positive cells with two CP110 foci; values represent mean ± SD of triplicate samples, with at least 100 cells counted per replicate. (B) Cetn2TTA cannot replace the function of endogenous Cetn2. HeLa cells were sequentially transfected with the indicated siRNAs and constructs expressing GFP alone (GFP), siRNA-resistant GFP-sirCetn2 (sirTTT), or siRNA-resistant GFP-sirCetn2TTA (sirTTA), labeled with BrdU for 4 h, and centriole number was determined as in A. Bar graph shows the percentage of BrdU-positive cells that have two CP110 foci; bars and siRNA as in A; values represent mean ± SD of triplicate samples where at least 100 cells were counted per replicate. (C) GFP-Cetn2TTA is efficiently incorporated into centrioles in the absence of endogenous Cetn2. Digitally magnified images of centrosomes from representative BrdU positive Cetn2-depleted cells from the experiment in B; green, GFP; red, CP110. Bar = 1 μm. Central panels show immunoblots demonstrating the efficiency of Cetn2 depletion.
Cetn2 Overexpression Causes the Mps1-Dependent Production of Excess Cetn2-Containing Foci
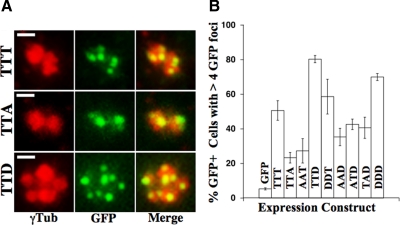
Interestingly, we found that after a prolonged S-phase arrest roughly 40% of HeLa cells overexpressing GFP-Cetn2TTT had five or more GFP foci rather than the expected four GFP foci (Figure 4A, B). The production of extra GFP foci was reduced in HeLa cells expressing nonphosphorylatable Cent2 proteins (Figure 4, A and B, TTA and AAT), suggesting that both EF hands 1 and 3 participate in their assembly. Interestingly, while GFP-Cetn2TTA was readily incorporated into centrioles in Cetn2-depleted cells (e.g., Figure 3C), it was difficult to detect at centrioles in the presence of endogenous Cetn2 (Figure 4A, TTA), suggesting that nonphosphorylatable Cetn2 cannot efficiently compete with endogenous Cetn2 for centriolar binding sites. In contrast to nonphosphorylatable mutations, mutation of these sites to aspartic acid to mimic phosphorylation caused the appearance of excess GFP foci in as many as 60–80% of cells (Figure 4, A and B; DDT, TTD, and DDD). Preventing phosphorylation within EF hand 1 attenuates the effect of mimicking phosphorylation within EF hand 3 (Figure 4B; AAD, ATD, and TAD), suggesting that EF hands 1 and 3 act in a cooperative manner.
Figure 4.
Cetn2 overexpression causes the production of excess Cetn2-containing foci. (A and B) HeLa cells transfected with GFP, GFP-Cetn2TTT (TTT), GFP-Cetn2TTA (TTA), GFP-Cetn2AAT (AAT), GFP-Cetn2TTD (TTD), GFP-Cetn2DDT (DDT), GFP-Cetn2AAD (AAD), GFP-Cetn2ATD (ATD), GFP-Cetn2TAD (TAD), or GFP-Cetn2DDD (DDD) were arrested in S-phase for 48 h and analyzed by IIF with an antibody against γ-Tubulin, and in the case of GFP alone an antibody against Cetn2. (A) T118 phosphorylation modulates the incorporation of GFP-Cetn2 into both existing centrioles and excess GFP foci. Shown are digitally magnified images of centrosomes from representative cells expressing GFP-Cetn2TTT, GFP-Cetn2TTA, or GFP-Cetn2TTD; red, γ-Tubulin; green, GFP; bar = 1 μm. (B) Mimicking Mps1 phosphorylation exacerbates centriole overproduction. Bar graph shows the percentage of cells transfected with the various expression constructs that have more than four GFP foci, or more than four Cetn2 foci for cells expressing GFP alone. Values represent mean ± SD of triplicate samples where at least 50 cells were counted per replicate.
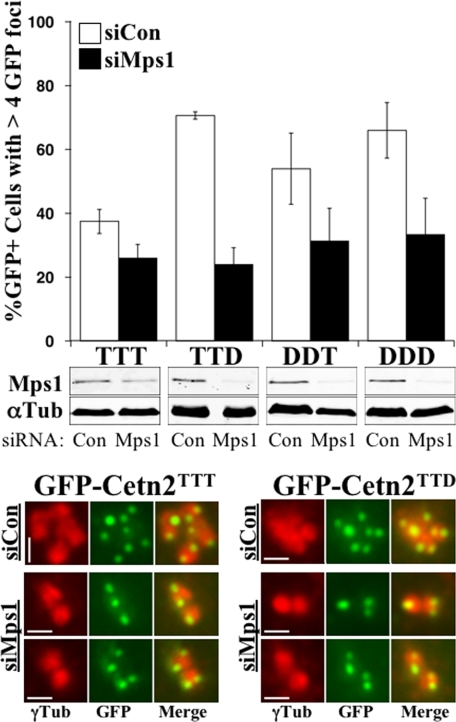
To determine whether the production of these excess GFP foci requires Mps1, we examined their production after the siRNA-mediated depletion of Mps1. Mps1 depletion attenuated the ability of wild-type and phosphomimetic Cetn2 constructs to support centriole overproduction (Figure 5). Interestingly, the triple phosphomimetic GFP-Cetn2DDD did not bypass the requirement for Mps1, suggesting that additional Mps1 phosphorylation sites remain to be identified, either within Cetn2 or other substrates. Regardless, the dependence of the formation of excess Cetn2-containing foci on Mps1 supports the hypothesis that Mps1 phosphorylates these residues in vivo.
Figure 5.
Cetn2-dependent centriole overproduction requires Mps1. HeLa cells were sequentially transfected with control (siCon, white bars) or Mps1-specific (siMps1, black bars) siRNAs and the indicated GFP-Cetn2 expression constructs, arrested in S-phase for 48 h, then analyzed by IIF. Top panel, bar graph showing the percentage of cells expressing the indicated constructs with more than 4 GFP foci; values represent mean ± SD of triplicate samples, with at least 50 cells per replicate. Mps1 was depleted by at least 70% as judged by parallel immunoblot analysis (middle panels) except in cells expressing GFP-Cetn2TTT where depletion was roughly 60%. Bottom panels, digitally magnified images of centrioles from representative cells expressing GFP-Cetn2TTT or GFP-Cetn2TTD; red, γ-Tubulin; green, GFP.
Because this effect of Cetn2 has not been previously reported, we performed several experiments to determine its specificity. We observed similar results whether we examined GFP foci in cells transfected with GFP-tagged constructs, or Cetn2 antibody staining in cells transfected with untagged constructs, suggesting that these effects are due to increased levels of Cetn2 and not the presence of the GFP tag (Supplemental Figure 2). Moreover, the effect appears to be specific to Cetn2, as the number of cells with excess GFP foci in cells overexpressing GFP-Cetn1 or GFP-Cetn3 was similar to that observed in cells transfected with GFP alone (Supplemental Figure 2). This effect of Cetn2 is not restricted to HeLa cells, because both GFP-Cetn2TTT and GFP-Cetn2TTD altered the pattern of centriole overproduction in CHO cells. The majority of CHO cells subjected to a prolonged S-phase arrest have extra centrosomes, making it difficult to observe an effect of Cetn2 on centrosome number in CHO cells. However, GFP-Cetn2TTT led to an increased percentage of cells that had one isolated Cetn2-containing focus, and GFP-Cetn2TTD led to an increase in the percentage of cells with two or more isolated Cetn2-containing foci (Supplemental Figure 2). In addition, GFP-Cetn2TTD could accelerate the onset of centrosome reduplication in U2OS cells, such that while centrosome reduplication is not normally apparent in U2OS cells until 48 h of S-phase arrest, excess GFP foci began to appear after just 24 h in U2OS cells expressing GFP-Cetn2TTD (Supplemental Figure 2). Interestingly, GFP-Cetn2TTT had no such effect in U2OS cells. Moreover, neither Cetn2 nor Cetn2TTD showed any effect in either MCF7 or RPE1 cells (data not shown). Together, these data demonstrate that the effect of Cetn2 overexpression is highly cell type specific. A similar cell type specificity for a centriole assembly factor was observed in a recent study of Cep76 (Tsang et al., 2009).
Cetn2 Overexpression Causes Centriole Overproduction
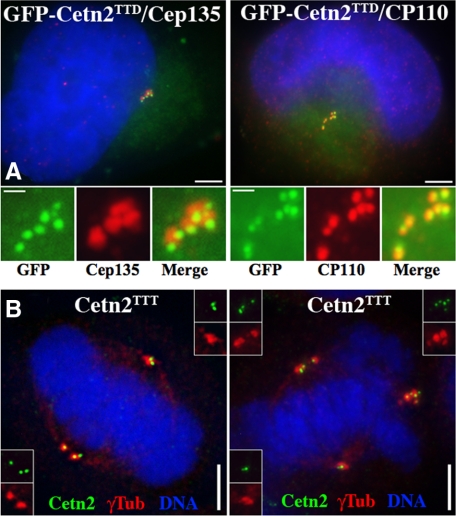
Because centrin overexpression has not previously been reported to increase centriole number, we sought to determine the nature of the excess GFP foci in cells overexpressing Cetn2. The excess GFP foci in S-phase–arrested cells overexpressing GFP-Cetn2TTD were associated with γ-Tubulin (e.g., Figure 4A), as well as the centriolar markers Cep135 and CP110 (Figure 6A). To assess whether the excess GFP foci represented functional centrioles, we tested their competence to participate in mitotic spindle assembly. Twenty-four hours after transfection with empty vector, roughly 9% of metaphase HeLa cells in an asynchronous population had either multipolar spindles or pseudobipolar spindles with more than four Cetn2-containing structures. In contrast, this number was nearly 30% for cells transfected with the same vector expressing untagged Cetn2. All Cetn2-containing structures in these cells were present at spindle poles and were associated with pericentriolar material, as judged by costaining with antibodies against γ-Tubulin and Cetn2 (Figure 6, B and C), supporting the suggestion that overexpression of Cetn2 leads to the production of mature centrioles. This approach actually underestimates the effect of Cetn2 overexpression; we used an antibody against Cetn2 to use the same method to identify Cetn2-positive structures in both untagged-Cetn2 and empty vector transfected cells, and because it was not possible to explicitly identify empty vector-transfected cells we counted randomly chosen metaphase cells without respect to transfection status.
Figure 6.
Centriole overduplication in GFP-Cetn2–expressing cells. (A) Extra Cetn2-containing foci contain several centriole markers. HeLa cells transfected GFP-Cetn2TTD and arrested in S-phase for 48 h were stained with antibodies against centriolar markers Cep135 and CP110. Shown are whole cell images (bar = 5 μm) and digitally magnified images of centrosomes (bar = 1 μm); green, GFP; red, Cep135 or CP110. (B) Extra Cetn2-containing foci recruit γ-Tubulin and participate in mitotic spindle assembly. HeLa cells were transfected with empty vector or untagged Cetn2 and analyzed with antibodies against Cetn2 and γ-Tubulin. Shown are cells with either pseudobipolar (left panel) or multipolar mitotic spindles from the Cetn2 transfection; green, Cetn2; red, γ-Tubulin. Bar = 5 μm. Insets show unmagnified images of spindle poles.
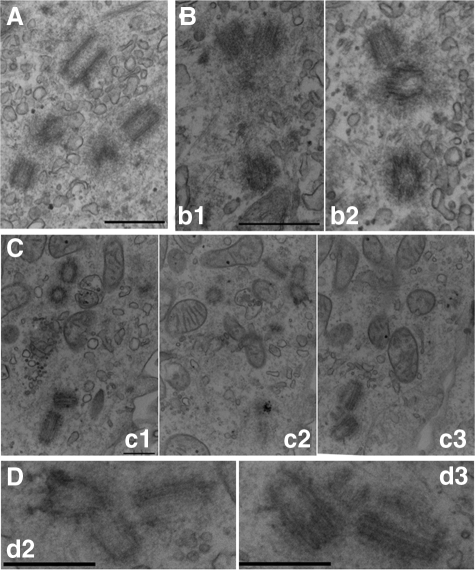
The observations that the extra Cetn2-containing foci in cells overexpressing Cetn2 can organize pericentriolar material and are competent to participate in mitotic spindle assembly suggest that they represent mature centrioles. To verify this assumption, we examined cells overexpressing either GFP-Cetn2TTT or GFP-Cetn2TTD by electron microscopy. We generated a stable, tetracycline-inducible GFP-Cetn2 cell line to facilitate electron microscopy. GFP-Cetn2TTT was detectable at centrioles in virtually all cells in the presence of Dox, and the percentage of cells that produced extra GFP foci was similar to that in the transient transfection experiments described above. Using this cell line, we processed cells that had been arrested in S-phase for serial section electron microscopy. Limiting our analysis to cells in which we could detect at least four centrioles, we observed defects in the both number and configuration of centrioles in 5 out of 14, or roughly 35% of cells. In three of these cells we detected more than four centrioles (e.g., Figure 7A), and in the remaining two we detected only four centrioles but observed the presence of a centriolar triplet indicative of the assembly of two procentrioles by a single parental centriole (Figure 7B). In all we observed three such centriolar triplets in cells expressing GFP-Cetn2TTT, the third being present in one of the three cells that had more than four centrioles. Therefore, while some of the extra GFP foci we observed by fluorescence microscopy may not reflect centrioles, we detected the bona fide production of extra centrioles in at least 35% of cells overexpressing GFP-Cetn2TTT, at least some of which arose via the production of multiple daughter centrioles by a single parental centriole.
Figure 7.
GFP-Cetn2TTT and GFP-Cetn2TTD cause the production of extra centrioles. (A and B) The HeLa GFP-Cetn2 cell line was induced to express GFP-Cetn2TTT by the addition of Dox during a 48 h S-phase arrest then analyzed using serial section electron microscopy; lowercase letters indicate serial sections. Section from two different HeLa GFP-Cetn2 cells showing (A) the presence of more than four centrioles and (B) a centriolar triplet. (C and D) GFP-Cetn2TTD causes centriole overproduction. HeLa cells transiently transfected with GFP-Cetn2TTD were arrested in S-phase for 48 h and analyzed by serial-section electron microscopy. (C) Nonconsecutive serial thin sections from a cell with ten centrioles and two centriolar triplets; bar = 500 nm. (D) Magnified images of the two centriolar triplets from the cell in (C); d2 and d3 correspond to the triplets from panels c2 and c3, respectively; bar = 100 nm.
We were unable to obtain a stable GFP-Cetn2TTD expression cell line using the T-REx inducible system. However, given that 80% of GFP-Cetn2TTD expressing cells have excess GFP foci, we were able to optimize transfection efficiency (obtaining as high as 70% transfected cells) sufficiently to make EM analysis of transiently transfected cells feasible. The phenotype observed in GFP-Cetn2TTD expressing cells was more robust than that in cells expressing GFP-Cetn2TTT, in that we observed both a much greater number of centrioles per cell and a much higher frequency of centriolar triplets. For example, Figure 7C shows serial sections from a cell with ten centrioles and two centriolar triplets (the triplets are magnified in Figure 7D). Of eleven cells expressing GFP-Cetn2TTD where we could detect at least four centrioles, seven had more than four centrioles.
Centrin 2 Overexpression Supports Assembly of Aberrant Centriole-Like Structures
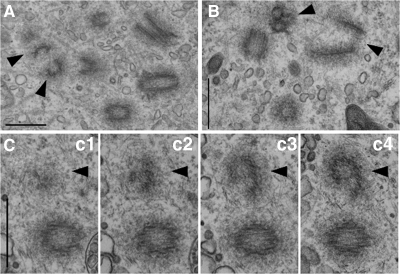
We also observed aberrant centriole-like structures in cells overexpressing GFP-Cetn2TTT (Figure 8) that were reminiscent of intermediates in the de novo pathway of centriole assembly that occurs after laser ablation of existing centrioles (La Terra et al., 2005; Uetake et al., 2007), except that they were formed in the presence of existing centrioles. We observed these structures in one of the GFP-Cetn2TTT expressing cells that contained more than four centrioles discussed above, but the other two GFP-Cetn2TTT expressing cells in which we observed these structures (Figure 8, B and C) were not scored as having extra centrioles because we were unable to observe at least four centrioles. Interestingly, we did not observe these structures in any of the GFP-Cetn2TTD-expressing cells that we examined by EM. Regardless, the presence of these structures suggests that in addition to causing centriole overduplication, GFP-Cetn2TTT might also promote some aberrant form of centriole assembly.
Figure 8.
GFP-Cetn2TTT overexpression produces aberrant centriole-like structures. Centriole-like structures reminiscent of de novo centrioles assembly intermediates in cells expressing GFP-Cetn2TTT. The HeLa GFP-Cetn2 cell line was analyzed by serial section electron microscopy as in Figure 6. (A) A single thin section showing several centrioles and two de novo-like structures (arrowheads). (B) A single thin section showing two centrioles and two de novo-like structures. (C) Consecutive serial thin sections showing a single centriole and a single de novo-like structure. (A–C) Bar = 500 nm.
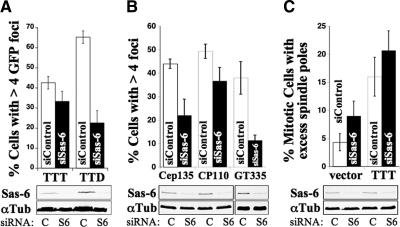
To further test this suggestion, we examined the role of the cartwheel protein HsSas-6 in the production of excess Cetn2-containing foci in cells expressing GFP-Cetn2 or GFP-Cetn2TTD. HsSas-6 is absent from G1 centrosomes because it is degraded in late G2 and mitosis (Strnad et al., 2007). During S-phase HsSas-6 is restricted to newly formed centrioles and is recruited to procentrioles before centrin (Strnad et al., 2007). Accordingly, if the excess foci arise through classical centriole overduplication their production should require HsSas-6. Despite depletion of the HsSas-6 protein by 80% as judged by immunoblotting, there was little difference in the percentage of GFP-Cetn2TTT expressing cells with more than four GFP foci between control and HsSas-6–depleted cells (Figure 9A). This difference was not statistically significant (P = 0.13 as judged by one way ANOVA followed by Tukey's HSD), suggesting that the excess foci in cells overexpressing wild-type centrin arise through a process that does not require HsSas-6. In contrast, the production of excess foci in cells expressing GFP-Cetn2TTD was greatly attenuated by HsSas-6-specific siRNAs (Figure 9A). In fact, the difference between HsSas-6–depleted cells expressing GFP-Cetn2TTT and GFP-Cetn2TTD was statistically significant (P < 0.01), suggesting that GFP-Cetn2TTT and GFP-Cetn2TTD act through different mechanisms.
Figure 9.
Sas-6 dependence of Cetn2-dependent centriole overduplication. (A) Cetn2TTT and Cetn2TTD differ in their dependence on HsSas-6. HeLa cells were sequentially transfected with either control (white bars) or HsSas-6–specific (black bars) siRNAs, followed by GFP-Cetn2TTT or GFP-Cetn2TTD, and the number of GFP foci was analyzed after 48 h of S-phase arrest. Bar graph shows the percentage of cells transfected with the indicated construct that have more than four GFP foci in Control or Sas6 siRNA. Values represent mean ± SD of triplicate samples where at least 50 cells were counted per replicate. (B) HsSas-6 is required for incorporation of glutamylated tubulin and Cep135, but not CP110 into excess GFP foci. Cells prepared as in A were analyzed by IIF with antibodies against polyglutamylated tubulin (GT335), Cep135, or CP110. Bar graph shows the percentage of cells with excess Cep135, CP110, or GT335 foci as indicated. Values represent mean ± SD of triplicate samples where at least 50 cells were counted per replicate. (C) HsSas-6 depletion does not prevent multipolar spindle formation in Cetn2 overexpressing cells. HeLa cells were sequentially transfected with either control or HsSas-6–specific siRNAs, followed by empty vector (vector) or untagged Cetn2 and analyzed with antibodies against Cetn2 and γ-Tubulin. Bar graph shows the percentage of mitotic cells that have either multipolar or pseudobipolar mitotic spindles. Values represent mean ± SD of triplicate samples where at least 100 mitotic cells were counted per replicate. (A–C) Below each bar graph are immunoblots with antibodies against α-Tubulin (aTub) and HsSas-6 (Sas6) showing HsSas-6 depletion for each experiment; C, control siRNA; S6, HsSas-6-specific siRNA.
Although EM analysis will be required to define the structure of the excess GFP foci formed in the absence of HsSas-6, three observations support the hypothesis that they are centriole-like structures. First, the majority of excess GFP foci in S-phase arrested HsSas-6-depleted cells expressing GFP-Cetn2 contained the centriole marker CP110 (Figure 9B), and CP110 staining recapitulated the HsSas-6-independence we observed for the GFP foci. Second, the excess GFP foci in S-phase arrested HsSas-6-depleted cells were competent to recruit γ-Tubulin (not shown). Third, HsSas-6 depletion in asynchronously growing cells overexpressing Cetn2 did not prevent the formation of excess Cetn2-containing foci that recruited γ-Tubulin, localized to mitotic spindle poles and promoted assembly of multipolar spindles (although HsSas-6 depletion caused a modest increase in spindle multipolarity on its own; Figure 9C). Interestingly, while HsSas-6 depletion did not prevent recruitment of CP110 or γ-Tubulin, HsSas-6 was required for the incorporation of Cep135 into the excess GFP foci in cells expressing GFP-Cetn2TTT (Figure 9B). The excess GFP foci in HsSas-6–depleted cells also did not stain with the GT335 antibody that recognizes polyglutamylated tubulin (Bobinnec et al., 1998), either in S-phase arrested cells (Figure 9B) or at mitotic spindle poles (not shown). While this suggests that they might not be mature centrioles, GT335 cannot always distinguish newly assembled centrioles (Tsang et al., 2009), and procentrioles formed in cells overexpressing Plk4 also lacked glutamylated tubulin (Kleylein-Sohn et al., 2007), although these ultimately became polyglutamylated by mitosis. Conversely, Cep76 depletion generated centriole intermediates that are GT335-positive despite failing to recruit PCM or participate in mitotic spindle assembly (Tsang et al., 2009). Accordingly, because the excess Cetn2-containing foci formed in cells lacking HsSas-6 are competent to function as mitotic spindle poles (Figure 9C), the precise significance of the results with GT335 is not entirely clear.
Based on our EM observations and the HsSas-6 dependence, we suggest that Cetn2TTD promotes centriole overproduction through the canonical centriole assembly pathway, whereas Cetn2TTT additionally stimulates an aberrant centriole assembly mechanism, at least some aspects of which can occur in the absence of HsSas-6. One hypothesis consistent with our observations is that increasing Cetn2 leads to the organization of distal centriole elements, which can then recruit proximal elements such as Cep135 in an HsSas-6-dependent manner. However, it will require correlative light and EM to determine the nature of the structures formed in the absence of HsSas-6.
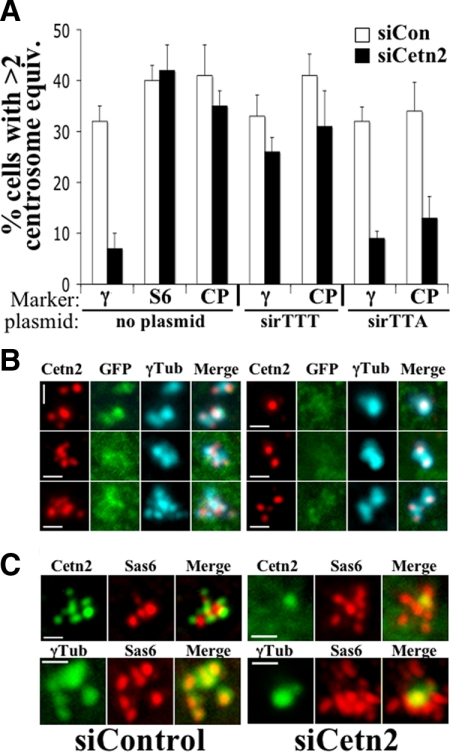
Mps1-Dependent Centriole Overproduction Requires Cetn2
While overexpression of wild-type Mps1 is not sufficient to cause centrosome reduplication in human cells, preventing the degradation of Mps1 at centrosomes is sufficient to do so, even at very modest expression levels (Kasbek et al., 2007; Kasbek et al., 2009). Reasoning that whether Cetn2 is a physiologically relevant Mps1 substrate it should be required for this Mps1-dependent centriole overproduction, we depleted Cetn2 in a HeLa-derived cell line that inducibly expresses a low level of the nondegradable GFP-Mps1Δ12/13 protein (Kasbek et al., 2009). The overproduction of mature centrioles by GFP-Mps1Δ12/13 was significantly reduced in Cetn2-depleted cells, as judged by a reduced percentage of cells with more than two γ-Tubulin foci (Figure 10, A and B). As expected for cells lacking Cetn2, there was no increase in the number of Cetn2-positive centrioles in Cetn2-depleted cells expressing GFP-Mps1Δ12/13; however, all Cetn2-positive centrioles were associated with γ-Tubulin in these cells (Figure 10B). In contrast, Cetn2 depletion had no effect on the percentage of cells with excess HsSas-6 or CP110 foci in GFP-Mps1Δ12/13-expressing cells (Figure 10, A and C), but unlike Cetn2 foci, the majority of HsSas-6 foci in Cetn2-depleted cells expressing GFP-Mps1Δ12/13 were not associated with γ-Tubulin (Figure 10C). This suggests that GFP-Mps1Δ12/13 cannot drive the production of mature centrioles that recruit pericentriolar material in the absence of Cetn2. Furthermore, the observation that GFP-Mps1Δ12/13 drives the assembly of excess HsSas-6 foci in the absence of Cetn2 supports the suggestion that Mps1 has other centriolar substrates. This suggestion is supported by our recent study showing that HsSas-6 is aberrantly incorporated into procentrioles upon inhibition of Mps1 activity (Kasbek et al., 2010) and provides an explanation for the inability of GFP-Cetn2DDD to bypass the requirement for Mps1 in centriole overproduction. sirGFP-Cetn2TTT was capable of replacing the function of endogenous Cetn2 in the overproduction of mature centrioles in response to GFP-Mps1Δ12/13 as judged by staining for γ-Tubulin and CP110 (Figure 10A). However, judged by the same criteria sirGFP-Cetn2TTA was unable to replace endogenous Cetn2 in the Mps1Δ12/13-dependent the assembly of mature centrioles. Moreover, while depletion of Cetn2 on its own had no affect on the number of CP110 foci in cells expressing GFP-Mps1Δ12/13, the percentage of cells with extra CP110 foci was decreased in Cetn2-depleted cells expressing sirGFP-Cetn2TTA compared with those expressing GFP alone. This suggests that while Cetn2 is dispensable for the formation of excess CP110-containing foci in cells expressing GFP-Mps1Δ12/13, the presence of the nonphosphorylatable Cetn2TTA actually interferes with this process.
Figure 10.
Cetn2 is required for the Mps1Δ12/13-dependent overproduction of mature centrioles. (A) Cetn2 depletion prevents the formation of excess γ-Tubulin-containing foci but not the formation of excess HsSas-6- and CP110-containing foci; Cetn2TTA cannot replace endogenous Cetn2 and exacerbates the effect of Cetn2 depletion with respect to CP110. HeLa Mps1Δ12/13 cells were either transfected with control siRNA (siCon, white bars) or Cetn2-specific siRNA (siCetn2, black bars) and no plasmid, or sequentially transfected with siRNAs and plasmids expressing siRNA-resistant GFP-sirCetn2TTT (sirTTT) or GFP-sirCetn2TTA (sirTTA), then analyzed by IIF after 48 h of S-phase arrest. Bar graphs show the percentage of cells with more than two centrosome equivalents; two foci in the case of γ-Tubulin (γ) or HsSas-6 (S6), or four foci in the case of CP110 (CP); values represent mean ± SD of triplicate samples, with at least 50 cells per replicate. (B and C) Representative images from the experiment in A showing (B) the reduction in the number of γ-Tubulin foci and the association of Cetn2-positive centrioles with γ-Tubulin (green, GFP; red, Cetn2; cyan, γ-Tubulin. Bar = 1 μm) or (C) the association of HsSas-6-containing foci with γ-Tubulin in control cells, and the lack of association in Cetn2 depleted cells (green, Cetn2 or γ-Tubulin as indicated; red, HsSas-6 (Sas-6). Bar = 1 μm).
DISCUSSION
While we ultimately conclude that centrioles can be assembled in the absence of Cetn2, our data nonetheless demonstrate that Cetn2 is required for the overproduction of mature centrioles in cells expressing Mps1Δ12/13, and the observation that Cetn2-depletion delays CP110 incorporation suggests that Cetn2 increases the rate of centriole assembly. Our data are also consistent with a role for Cetn2 in centriole stability, such as that observed in Tetrahymena where centrin is required both for assembly of new centrioles and stability of existing centrioles (Stemm-Wolf et al., 2005). The nonphosphorylatable mutant protein GFP-Cetn2TTA cannot support either function but is incorporated into centrioles of Cetn2-depleted cells, suggesting that phosphorylation is required for Cetn2 function as opposed to its targeting to centrioles. Moreover, GFP-Cetn2TTA exacerbates the phenotype of Cetn2-depleted cells, suggesting that centriole assembly is perturbed more by the presence of unphosphorylated Cetn2 than by the absence of Cetn2. Together our data support the hypothesis that Mps1 phosphorylation is required for a function of Cetn2 that is dispensable for centriole assembly. However, being dispensable is not equivalent to having no role. Many Cdk2 phosphorylation events have been implicated in centriole assembly (Loncarek and Khodjakov, 2009) and dominant-negative Cdk2 blocks centrosome duplication (Meraldi et al., 1999), yet Cdk2 is dispensable for centriole assembly (Duensing et al., 2006), presumably due to functional redundancy. A functionally redundant molecule for Cetn2 is not obvious, as our data shows that centrins are not functionally equivalent, and codepletion of Cetn2 and Cetn3 does not prevent Plk4-dependent centriole overproduction (Kleylein-Sohn et al., 2007).
We also found that overexpression of Cetn2 causes the assembly of excess centrioles and centriole-like structures in S-phase–arrested HeLa cells, an effect that is not due to the GFP tag and is not shared by Cetn1 or Cetn3. That at least some of these are centrioles is supported by fluorescence microscopy showing that they are associated with a variety of molecular markers expected of centrioles, recruit γ-Tubulin, and are competent to participate in mitotic spindle assembly, as well as by electron microscopy that revealed excess centriolar structures associated with electron dense material. However, we also observed aberrant centriole-like structures reminiscent of intermediates in centriole assembly after laser ablation of existing centrioles (La Terra et al., 2005). Interestingly, Cetn2TTT also promoted the assembly of excess foci that contain CP110, recruit γ-Tubulin, and participate in mitotic spindle assembly in the absence of HsSas-6, although without HsSas-6 these structures did not contain Cep135 or polyglutamylated tubulin. Although it will require detailed EM analysis to determine the structures associated with the foci formed in HsSas-6–depleted cells, our observations suggest the intriguing possibility that centriole overproduction in cells overexpressing Cetn2TTT might not occur through the canonical centriole assembly pathway.
One possible explanation for our observations is the suggestion that centriole assembly is a modular process, in analogy to phage assembly where assembly of heads and tails is coordinated to generate phage particles in an ordered manner (Wood, 1980). In the canonical centriole assembly pathway, Plk4 stimulates the assembly of cartwheels that serve as platforms for the assembly of additional centriole elements in a proximal to distal manner; overexpression of Mps1 (this study), Plk4 (Habedanck et al., 2005; Kleylein-Sohn et al., 2007), or Sas-6 (Leidel et al., 2005; Strnad et al., 2007) produces additional cartwheels that can recruit distal centriole modules. We suggest that perhaps Cetn2 can organize distal centriole elements in the absence of extra cartwheels, just as phage heads can be assembled in the absence of phage tails and vice versa (Wood, 1980). In support of this idea, depletion of HsSas-6 attenuates the incorporation of the proximal marker Cep135 into excess GFP-foci in S-phase arrested cells, but does not effect incorporation of the distal marker CP110, recruitment of γ-Tubulin, or the ability to function as mitotic spindle poles. Moreover, it was recently suggested that the centrin binding protein hPoc5 plays a similar role in organizing distal centriole elements (Azimzadeh et al., 2009).
We never observed these aberrant structures in GFP-Cetn2TTD expressing cells, and Cetn2TTD-dependent centriole overproduction requires HsSas-6. Accordingly, our data suggest that Cetn2 can support centriole assembly through multiple mechanisms. The observation that Cep76 can suppress centriole overproduction in S-phase–arrested cells, but not that caused by Plk4 overexpression (Tsang et al., 2009), supports the existence of multiple mechanisms for centriole overproduction. Moreover, depletion of Cep76 also generates excess centriole-like structures whose appearance requires HsSas-6, but unlike the structures we observe in cells overexpressing Cetn2TTT or Cetn2TTD they do not recruit γ-Tubulin or function as spindle poles (Tsang et al., 2009). Based on characteristics of HsSas-6 dependence and γ-Tubulin recruitment, GFP-Cetn2TTT, GFP-Cetn2TTD, and Cep76-depletion operate via subtly distinct mechanisms. Both Cetn2-dependent mechanisms require Mps1, but despite achieving 70–90% depletion of Mps1 in cells expressing GFP, GFP-Cetn2TTD, or GFP-Cetn2TTA, we could achieve no greater than 60% Mps1 depletion in cells expressing GFP-Cetn2TTT, and we observed a modest decrease in the fluorescence intensity of GFP-Mps1Δ12/13 in Cetn2-depleted cells (see Figure 10). While we were unable to detect a physical association between Mps1 and Cetn2, these observations suggest that Cetn2 can influence the stability and or/levels of Mps1, which is consistent with an interaction between the two proteins. Moreover, our data suggest not only that EF hands 1 and 3 act cooperatively, but also that the two Mps1 sites within EF hand 1 are not redundant, and that additional Mps1 substrates exist. A full understanding of these issues will require much additional follow-up work.
We generated an antibody (pT118) that recognizes a fusion protein containing the fifteen amino acids surrounding T118 only when it has been phosphorylated by Mps1 (Supplemental Figure 3A), and pT118 can stain centrosomes in an Mps1-dependent manner when these same fifteen amino acids are tethered to centrosomes via the PACT domain (Supplemental Figure 3B). T118 lies at the apex of a short loop connecting the two halves of an EF hand (as do T45 and T47; Supplemental Figure 1) (Thompson et al., 2006), a conformation unlikely to be mimicked by the peptide immunogen, and Cetn2 is remarkably resistant to denaturation (Paoletti et al., 1996). Unfortunately, and presumably due to these factors, pT118 fails to recognize full-length Mps1-phosphorylated Cetn2 (Supplemental Figure 3A), and we are therefore unable to use pT118 to study Cetn2 phosphorylation in vivo. We were also unable to detect phosphorylation at T45, T47, or T118 in immunoprecipitated Cetn2 by mass spectrometry. Perhaps this is not surprising given that the majority of Centrin is not found at centrioles (Paoletti et al., 1996), and these sites may be transiently phosphorylated in a small fraction of Cetn2 during a limited window of the cell cycle. However, we consequently do not know whether these sites are phosphorylated in vivo, and if so whether Mps1 is the kinase responsible. Regardless, taken together our data suggest that while Cetn2 is dispensable for centrosome duplication, the Mps1 phosphorylation sites we identified promote both normal and aberrant centriole assembly. However, the exact role of Cetn2 in centriole assembly remains unclear, and because Cetn2 is dispensable for centriole assembly elucidating its precise role will require extensive analysis of Cetn2 mutants such as that begun here.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs. Kari Greenchurch and Liwen Zhang of the Ohio State University Campus Chemical Instrument Center, who performed the mass spec, and Katherine Wolken and Richard Montione of the Ohio State University Campus Microscopy and Imaging Facility for sample preparation and help with electron microscopy. This work was supported by a National Institutes of Health grant (GM77311) and a seed grant from The Ohio Cancer Research Associates (to H.A.F.).
Abbreviations used:
- BrdU
5-Bromo deoxyuridine
- Dox
doxycycline
- HU
hydroxyurea
- sir
siRNA-resistant.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0298) on October 27, 2010.
REFERENCES
- Araki Y., Gombos L., Migueleti S. P., Sivashanmugam L., Antony C., Schiebel E. N-terminal regions of Mps1 kinase determine functional bifurcation. J. Cell Biol. 2010;189:41–56. doi: 10.1083/jcb.200910027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J., Hergert P., Delouvee A., Euteneuer U., Formstecher E., Khodjakov A., Bornens M. hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 2009;185:101–114. doi: 10.1083/jcb.200808082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A. T., Greenwood T. M., Bazinet C. W., Salisbury J. L. Centrin is a component of the pericentriolar lattice. Biol. Cell. 1992;76:383–388. doi: 10.1016/0248-4900(92)90442-4. [DOI] [PubMed] [Google Scholar]
- Bobinnec Y., Khodjakov A., Mir L. M., Rieder C. L., Edde B., Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P., Giddings T. H., Jr, Winey M., Stearns T. Epsilon-tubulin is required for centriole duplication and microtubule organization. Nat. Cell Biol. 2003;5:71–76. doi: 10.1038/ncb900. [DOI] [PubMed] [Google Scholar]
- Chang P., Stearns T. δ-Tubulin and ε-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nature: Cell Biology. 2000;2:30–35. doi: 10.1038/71350. [DOI] [PubMed] [Google Scholar]
- Chen Z., Indjeian V. B., McManus M., Wang L., Dynlacht B. D. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell. 2002;3:339–350. doi: 10.1016/s1534-5807(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Cho J. H., Chang C. J., Chen C. Y., Tang T. K. Depletion of CPAP by RNAi disrupts centrosome integrity and induces multipolar spindles. Biochem. Biophys. Res Commun. 2006;339:742–747. doi: 10.1016/j.bbrc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- Delattre M., Leidel S., Wani K., Baumer K., Bamat J., Schnabel H., Feichtinger R., Schnabel R., Gonczy P. Centriolar SAS-5 is required for centrosome duplication in C. elegans. Nat. Cell Biol. 2004;6:656–664. doi: 10.1038/ncb1146. [DOI] [PubMed] [Google Scholar]
- Doxsey S. J. Centrosomes as command centres for cellular control. Nat. Cell Biol. 2001;3:E105–108. doi: 10.1038/35074618. [DOI] [PubMed] [Google Scholar]
- Duensing A., Liu Y., Tseng M., Malumbres M., Barbacid M., Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25:2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errabolu R., Sanders M.A., Salisbury J.L. Cloning of a cDNA encoding human centrin, an EF-hand protein of centrosomes and mitotic spindle poles. J. Cell Sci. 1994;107:9–16. doi: 10.1242/jcs.107.1.9. [DOI] [PubMed] [Google Scholar]
- Fisk H. A., Mattison C. P., Winey M. Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc. Natl. Acad. Sci. USA. 2003;100:14875–14880. doi: 10.1073/pnas.2434156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk H. A., Winey M. The mouse mps1p-like kinase regulates centrosome duplication. Cell. 2001;106:95–104. doi: 10.1016/s0092-8674(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Habedanck R., Stierhof Y. D., Wilkinson C. J., Nigg E. A. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 2005;7:1140–1146. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- Kanai M., Ma Z., Izumi H., Kim S. H., Mattison C. P., Winey M., Fukasawa K. Physical and functional interaction between mortalin and Mps1 kinase. Genes Cells. 2007;12:797–810. doi: 10.1111/j.1365-2443.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Kasbek C., Yang C. H., Fisk H. A. Mps1 as a link between centrosomes and genomic instability. Environmental and Molecular Mutagenesis in press. 2009 doi: 10.1002/em.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasbek C., Yang C. H., Fisk H. A. Antizyme restrains centrosome amplification by regulating the accumulation of Mps1 at centrosomes. Mol. Biol. Cell. 2010 doi: 10.1091/mbc.E10-04-0281. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasbek C., Yang C. H., Yusof A. M., Chapman H. M., Winey M., Fisk H. A. Preventing the degradation of mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Mol. Biol. Cell. 2007;18:4457–4469. doi: 10.1091/mbc.E07-03-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C. A., Kopish K. R., Zipperlen P., Ahringer J., O'Connell K. F. Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell. 2004;6:511–523. doi: 10.1016/s1534-5807(04)00066-8. [DOI] [PubMed] [Google Scholar]
- Klein U. R., Nigg E. A. SUMO-dependent regulation of centrin-2. J. Cell Sci. 2009;122:3312–3321. doi: 10.1242/jcs.050245. [DOI] [PubMed] [Google Scholar]
- Kleylein-Sohn J., Westendorf J., Le Clech M., Habedanck R., Stierhof Y. D., Nigg E. A. Plk4-induced centriole biogenesis in human cells. Dev. Cell. 2007;13:190–202. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kohlmaier G., Loncarek J., Meng X., McEwen B. F., Mogensen M. M., Spektor A., Dynlacht B. D., Khodjakov A., Gonczy P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr. Biol. 2009;19:1012–1018. doi: 10.1016/j.cub.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Terra S., English C. N., Hergert P., McEwen B. F., Sluder G., Khodjakov A. The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J. Cell Biol. 2005;168:713–722. doi: 10.1083/jcb.200411126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. D., Huang B. Molecular cloning and centrosomal localization of human caltractin. Proc. Natl. Acad. Sci. USA. 1993;90:11039–11043. doi: 10.1073/pnas.90.23.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S., Delattre M., Cerutti L., Baumer K., Gonczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 2005;7:115–125. doi: 10.1038/ncb1220. [DOI] [PubMed] [Google Scholar]
- Leidel S., Gonczy P. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev. Cell. 2003;4:431–439. doi: 10.1016/s1534-5807(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Lingle W. L., Lutz W. H., Ingle J. N., Maihle N. J., Salisbury J. L. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle W. L., Salisbury J. L. The role of the centrosome in the development of malignant tumors. Curr. Top. Dev. Biol. 2000;49:313–329. doi: 10.1016/s0070-2153(99)49015-5. [DOI] [PubMed] [Google Scholar]
- Loncarek J., Hergert P., Magidson V., Khodjakov A. Control of daughter centriole formation by the pericentriolar material. Nat. Cell Biol. 2008;10:322–328. doi: 10.1038/ncb1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J., Khodjakov A. Ab ovo or de novo? Mechanisms of centriole duplication. Mol. Cells. 2009;27:135–142. doi: 10.1007/s10059-009-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W., Lingle W. L., McCormick D., Greenwood T. M., Salisbury J. L. Phosphorylation of centrin during the cell cycle and its role in centriole separation preceding centrosome duplication. J. Biol. Chem. 2001;276:20774–20780. doi: 10.1074/jbc.M101324200. [DOI] [PubMed] [Google Scholar]
- Marshall W. F., Vucica Y., Rosenbaum J. L. Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr. Biol. 2001;11:308–317. doi: 10.1016/s0960-9822(01)00094-x. [DOI] [PubMed] [Google Scholar]
- Meraldi P., Lukas J., Fry A. M., Bartek J., Nigg E. A. Centrosome duplication in mammalian somatic cells requires E2F and Cdk2- cyclin A. Nat. Cell Biol. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- Middendorp S., Kuntziger T., Abraham Y., Holmes S., Bordes N., Paintrand M., Paoletti A., Bornens M. A role for centrin 3 in centrosome reproduction. J. Cell Biol. 2000;148(3):405–415. doi: 10.1083/jcb.148.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middendorp S., Paoletti A., Schiebel E., Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiae CDC31 gene. Proc. Natl. Acad. Sci. USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell K. F., Caron C., Kopish K. R., Hurd D. D., Kemphues K. J., Li Y., White J. G. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell. 2001;105:547–558. doi: 10.1016/s0092-8674(01)00338-5. [DOI] [PubMed] [Google Scholar]
- Paoletti A., Moudjou M., Paintrand M., Salisbury J. L., Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Peel N., Stevens N. R., Basto R., Raff J. W. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 2007;17:834–843. doi: 10.1016/j.cub.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Ozlu N., Hannak E., Cowan C., Habermann B., Ruer M., Muller-Reichert T., Hyman A. A. The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Curr. Biol. 2004;14:863–873. doi: 10.1016/j.cub.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D. M., Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science. 2007;316:1046–1050. doi: 10.1126/science.1142950. [DOI] [PubMed] [Google Scholar]
- Salisbury J., Suino K., Busby R., Springett M. Centrin-2 is required for centriole duplication in Mammalian cells. Curr. Biol. 2002;12:1287. doi: 10.1016/s0960-9822(02)01019-9. [DOI] [PubMed] [Google Scholar]
- Schmidt T. I., Kleylein-Sohn J., Westendorf J., Le Clech M., Lavoie S. B., Stierhof Y. D., Nigg E. A. Control of centriole length by CPAP and CP110. Curr. Biol. 2009;19:1005–1011. doi: 10.1016/j.cub.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Stemm-Wolf A. J., Morgan G., Giddings T. H., Jr, White E. A., Marchione R., McDonald H. B., Winey M. Basal body duplication and maintenance require one member of the Tetrahymena thermophila centrin gene family. Mol. Biol. Cell. 2005;16:3606–3619. doi: 10.1091/mbc.E04-10-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad P., Leidel S., Vinogradova T., Euteneuer U., Khodjakov A., Gonczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke V. M., Baumann C., Nigg E. A. Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma. 2004;113:1–15. doi: 10.1007/s00412-004-0288-2. [DOI] [PubMed] [Google Scholar]
- Stucke V. M., Sillje H. H., Arnaud L., Nigg E. A. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 2002;21:1723–1732. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. J., Fu R. H., Wu K. S., Hsu W. B., Tang T. K. CPAP is a cell-cycle regulated protein that controls centriole length. Nat. Cell Biol. 2009;11:825–831. doi: 10.1038/ncb1889. [DOI] [PubMed] [Google Scholar]
- Thompson J. R., Ryan Z. C., Salisbury J. L., Kumar R. The structure of the human centrin 2-xeroderma pigmentosum group C protein complex. J. Biol. Chem. 2006;281:18746–18752. doi: 10.1074/jbc.M513667200. [DOI] [PubMed] [Google Scholar]
- Tsang W. Y., Spektor A., Luciano D. J., Indjeian V. B., Chen Z., Salisbury J. L., Sanchez I., Dynlacht B. D. CP110 cooperates with two calcium-binding proteins to regulate cytokinesis and genome stability. Mol. Biol. Cell. 2006;17:3423–3434. doi: 10.1091/mbc.E06-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang W. Y., Spektor A., Vijayakumar S., Bista B. R., Li J., Sanchez I., Duensing S., Dynlacht B. D. Cep76, a centrosomal protein that specifically restrains centriole reduplication. Dev. Cell. 2009;16:649–660. doi: 10.1016/j.devcel.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M. F., Stearns T. Controlling centrosome number: licenses and blocks. Curr. Opin. Cell Biol. 2006;18:74–78. doi: 10.1016/j.ceb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Uetake Y., Loncarek J., Nordberg J. J., English C. N., La Terra S., Khodjakov A., Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J. Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Bacteriophage T4 morphogenesis as a model for assembly of subcellular structure. Q. Rev. Biol. 1980;55:353–367. doi: 10.1086/411980. [DOI] [PubMed] [Google Scholar]
- Zhu F., Lawo S., Bird A., Pinchev D., Ralph A., Richter C., Muller-Reichert T., Kittler R., Hyman A. A., Pelletier L. The mammalian SPD-2 ortholog Cep192 regulates centrosome biogenesis. Curr. Biol. 2008;18:136–141. doi: 10.1016/j.cub.2007.12.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.