The sodium pump interacts with AS160, a protein that regulates the trafficking of the GLUT4 glucose transporter. This interaction drives the internalization of the sodium pump from the cell surface, and this process is in turn controlled by the energy-sensing kinase adenosine monophosphate-stimulated protein kinase.
Abstract
The Na+,K+-ATPase is the major active transport protein found in the plasma membranes of most epithelial cell types. The regulation of Na+,K+-ATPase activity involves a variety of mechanisms, including regulated endocytosis and recycling. Our efforts to identify novel Na+,K+-ATPase binding partners revealed a direct association between the Na+,K+-ATPase and AS160, a Rab-GTPase-activating protein. In COS cells, coexpression of AS160 and Na+,K+-ATPase led to the intracellular retention of the sodium pump. We find that AS160 interacts with the large cytoplasmic NP domain of the α-subunit of the Na+,K+-ATPase. Inhibition of the activity of the adenosine monophosphate-stimulated protein kinase (AMPK) in Madin-Darby canine kidney cells through treatment with Compound C induces Na+,K+-ATPase endocytosis. This effect of Compound C is prevented through the short hairpin RNA-mediated knockdown of AS160, demonstrating that AMPK and AS160 participate in a common pathway to modulate the cell surface expression of the Na+,K+-ATPase.
INTRODUCTION
The Na+,K+-ATPase, also known as the sodium pump, is an ubiquitous transmembrane enzyme that uses the energy derived from the hydrolysis of one molecule of ATP to actively transport Na+ and K+ across the cell membrane (Sweadner, 1989; Kaplan, 2002). The sodium pump is composed of a heterodimeric complex consisting of one α-subunit and one β-subunit (Craig and Kyte, 1980). The α-subunit mediates the catalytic activity of the enzyme, whereas the glycosylated β-subunit is required for the pump's maturation, delivery, and insertion into the plasma membrane (Geering, 1990; Gottardi and Caplan, 1993). An additional γ-subunit manifests tissue-specific expression and can modulate the pump's activity, but it is not required for its functional expression (Geering, 2006). In polarized ion transporting epithelial cells, such as those that line renal tubules, the Na+,K+-ATPase is abundantly expressed and is usually restricted in its distribution to the basolateral surface of the plasma membrane (Jorgensen, 1980).
The Na+,K+-ATPase provides the principal driving force for ion, solute, and fluid transport in most tissues. At the level of individual cells, the Na+ export and K+ import that is catalyzed by the Na+,K+-ATPase is essential for cell volume homeostasis and for the maintenance of the electrochemical gradients that are exploited to drive the transport of a wide range of substances. In addition to the predominant plasma membrane-associated pool, it has been shown that Na+,K+-ATPase can reside in latent intracellular compartments (Barlet-Bas et al., 1990; Horisberger and Rossier, 1992; Liang et al., 2007). Some of the hormones that regulate sodium pump activity seem to do so by modulating its translocation to the plasma membrane or promoting its endocytosis (Chibalin et al., 1999; Summa et al., 2001; Ridge et al., 2002; Al-Khalili et al., 2003; Bertorello et al., 2003). In this sense, regulation of Na+,K+-ATPase function may resemble that of the GLUT4 glucose transporter. GLUT4 is concentrated in special intracellular compartments in muscle and fat cells and is translocated to the plasma membrane in response to insulin stimulation (Hashiramoto and James, 2000). In fact, insulin similarly induces the surface delivery of intracellular stores of sodium pump in muscle and fat (McGill, 1991; McGill and Guidotti, 1991; Hundal et al., 1992; Al-Khalili et al., 2003, 2004).
To identify novel partner proteins that interact with Na+,K+-ATPase and that may regulate its trafficking, we isolated Na+,K+-ATPase from cultured renal epithelial cells and performed mass spectrometry analysis. One of the proteins that we detected was AS160. AS160, or TBC1D4, is a Rab-GTPase-activating protein (GAP) that is substrate for phosphorylation by Akt and adenosine monophosphate-stimulated protein kinase (AMPK) and that plays a critical role in GLUT4 translocation to the plasma membrane in response to insulin stimulation or muscle contraction (Kane et al., 2002; Cartee and Wojtaszewski, 2007). In resting cells the Rab-GAP activity of AS160 prevents the Rab-dependent trafficking of GLUT4 to the cell surface. Consistent with this function, small interfering RNA (siRNA)-mediating silencing of AS160 perturbs intracellular retention of GLUT4 and produces insulin-independent translocation of GLUT4 to the plasma membrane (Eguez et al., 2005; Larance et al., 2005). After insulin stimulation, AS160 is phosphorylated by Akt, promoting the translocation of GLUT4 to the cell surface (Kane et al., 2002; Sano et al., 2003; Zeigerer et al., 2004).
We find that AS160 interacts directly with the Na+,K+-ATPase α-subunit. We also show that this interaction is important for regulating the cell surface expression of the pump and that AS160 binding is modulated by AMPK. These findings elucidate a novel mechanism for the regulation of Na+,K+-ATPase trafficking.
MATERIALS AND METHODS
Mass Spectrometry Analysis
The mass spectrometry assay was performed using the soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP)-tagged Na+,K+-ATPase α-subunit as a substrate (Juillerat et al., 2003; Keppler et al., 2003). Polarized Madin-Darby canine kidney (MDCK) cells stably expressing α1 Na+,K+-ATPase-SNAP-hemagglutinin (HA) (Farr et al., 2009) were scraped into 1 ml of buffer TN (100 mM NaCl, 50 mM Tris-HCl, pH 7.4, and 1 mM dithiothreitol) and protease inhibitors (Roche Diagnostics, Indianapolis, IN). Cells were lysed by sonication for 3 × 10-s bursts at the 40% power setting. Homogenates were incubated with or without 2 μM BG-biotin (New England Biolabs, Ipswich, MA) for 90 min at room temperature, and the unlabeled homogenate was used as a control. The reaction was stopped by adding 2 mM of EDTA and then subsequently centrifuged for 60 min at 100,000 × g. Pellets were resuspended in 500 μl of lysis buffer containing 100 mM NaCl, 50 mM Tris, pH 7.5, 1 mM EDTA, 1% Lubrol, and protease inhibitors. The lysates were incubated for 8 h at 4°C with streptavidin-Sepharose beads (Thermo Fisher Scientific, Waltham, MA). Proteins that coprecipitated with the bound Na+,K+-ATPase α-subunit were eluted in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer and separated by electrophoresis. After Coomassie Brilliant Blue staining (Supplemental Dataset 1), bands were excised and digested for 12 h with trypsin (12 ng/l) at 37°C, the samples were desalted using an Atlantis C18 column (Waters, Millford, MA) for nanospray liquid chromatography (LC)/tandem mass spectrometry analysis. The LC eluent was coupled to a nanospray source attached to a Micromass Q-Tof API mass spectrometer (Waters). The mass spectrometry analysis was performed using the services of Keck Facility (Yale University, New Haven, CT).
Antibodies
Anti-Na+,K+-ATPase monoclonal antibody α5 is directed against the amino terminus of the rat Na+,K+-ATPase α1 subunit (Tamkun and Fambrough, 1986). The Anti-AS160 polyclonal antibody directed against the amino acids 1178-1189 of rat AS160 was obtained from Millipore (Billerica, MA). Polyclonal anti-FLAG, monoclonal mouse anti-HA antibody-conjugated agarose beads, and polyclonal rabbit anti-HA antibodies were purchased from Sigma-Aldrich (St. Louis, MO). The anti-calnexin antibody was obtained from Stressgen (Assay Designs, Ann Arbor, MI), and anti-β-actin was purchased from Abcam (Cambridge, MA). Anti-phospho-acetyl-CoA carboxylase (Ser79), anti-phospho-AMPKα (Thr172), and anti-glutathione transferase (GST) rabbit antibodies were purchased from Cell Signaling Technology (Danvers, MA).
Cell Culture and Transfection
COS and MDCK cells were cultured in a humidified incubator under 5% CO2 in α-minimal essential medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM l-glutamine. DNA transfection of COS cells was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. All COS cell-based assays were performed 30 h after transfection.
Plasmid Construction and Stable Cell Line Generation
The CMV-10 plasmids encoding the human AS160WT and the AS160-4P constructs with a triple FLAG-tag inserted at their amino termini were generated as described previously (Kane et al., 2002; Sano et al., 2003). The SNAP-tag sequence was amplified by polymerase chain reaction (PCR) from pSS26m (New England Biolabs). The primers included unique restriction sites and the resultant PCR product was inserted at the N terminus of an HA-tagged version of the rat Na+,K+-ATPase α1 subunit, generating Na+,K+-ATPase-SNAP-HA. This sequence was inserted into the pcDNA 3.1-Neo expression vector (Invitrogen). Details on the production of this construct and on the generation of an MDCK stable cell line that expresses it are presented previously (Farr et al., 2009).
Immunoprecipitation
Transfected or untransfected cells were incubated with 1 ml of lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1% Lubrol, and 5 mM EDTA and protease inhibitors for 30 min at 4°C. The insoluble fraction was eliminated through centrifugation at 10,000 × g for 30 min at 4°C. After the centrifugation, the lysates were incubated with the antibody of interest and protein A or G conjugated to Sepharose (Pierce Chemical, Rockford, IL) for 8 h at 4°C. To quantify the total amount of protein loaded, 20 μl of the lysates was saved. Beads were washed four times with lysis buffer. Proteins were eluted in SDS-PAGE sample buffer and separated by SDS-PAGE electrophoresis and analyzed by Western blotting. Blots were then probed with peroxidase-conjugated species appropriate secondary antibodies and visualized with the enhanced chemiluminescence reagent (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Short Hairpin RNA (shRNA) MDCK Cell Line
The sequence chosen for a shRNA construct targeting canine AS160 was 5′-GCAAGGGAGCATGGTATTA-3′ subcloned into pSUPER plasmid (Oligoengine, Seattle, WA). After sequencing, positively confirmed plasmids were transfected in to MDCK cell line by using Lipofectamine 2000. The selection and maintenance of stable MDCK cell clones were performed in α-MEM containing 5 mg/ml G418 (Invitrogen). Clones were screened for the reduced expression levels of AS160 by Western blot.
Immunofluorescence
COS cells were grown on coverslips, whereas MDCK cells were plated to 12-mm transwell filters (Corning Life Sciences, Lowell, MA) and allowed to polarize for 4 d. Cells were fixed with 4% paraformaldehyde and subsequently permeabilized with phosphate-buffered saline (PBS) (Sigma-Aldrich) with 1 mM MgCl2 and 100 μM CaCl2 (PBS2+) containing 1 mg/ml bovine serum albumin and 0.1% Triton X-100. Nonspecific binding was blocked using goat serum dilution buffer GSDB (33% goat serum, 40 mM NaPi, pH 7.4, 450 mM NaCl, and 0.6% Triton X-100). Primary and Alexa Fluor-conjugated secondary (Invitrogen) antibodies were diluted in GSDB. Cells were visualized on a confocal laser scanning microscope (model LSM 510; Carl Zeiss Microimaging, Thornwood, NY). Contrast and brightness settings were chosen so that all pixels were in the linear range. Images are the product of eightfold line averaging.
GST-Fusion Protein Assay
The A domain (residue 1–85) and NP domain (residues 137–280) of rat Na+,K+-ATPase α1 subunit were subcloned into the pGEX-4T-3 vector (GE Healthcare) as described previously (Zatti et al., 2005) to produce the cDNAs encoding GST-fusion proteins. The Escherichia coli strain BL21 (DE3) (Novagen/EMD Biosciences, San Diego, CA) was transformed with cDNAs encoding GST alone or GST-fusion proteins. A single colony was grown overnight in 50 ml of Luria-Bertani (LB) media supplemented with ampicillin (100 μg/ml). This culture was used to inoculate 500 ml of LB supplemented with ampicillin to an A600 of 0.1. The bacterial cells were grown at 37°C until an A600 of 0.6–0.8 was attained. Protein synthesis was induced with 0.1 mM isopropyl-1-thio-β-d-galactopyranoside for 4 h. Cells were centrifuged at 5000 × g for 15 min, and the pellets were resuspended in 10 ml of ice-cold PBS, pH 7.4 (150 mM NaCl and 15 mM NaH2PO4) supplemented with protease inhibitors. The cells were lysed by sonication, and after addition of 1% Lubrol, the mixture was incubated for 30 min at 4°C. Soluble proteins were separated from cellular debris by centrifugation (12,000 × g for 10 min at 4°C). The amount of GST-fusion protein in each preparation was determined by incubating 20 μl of glutathione-Sepharose 4 B (GE Healthcare) with a dilution series of the cleared bacterial lysates, and saturating amounts of protein were estimated by SDS-PAGE and visualization by Coomassie Brilliant Blue staining. For the pull-down assay, lysates from COS cells untransfected or transfected with the plasmid encoding AS160 were incubated overnight at 4°C with beads loaded with GST alone or with GST-fusion proteins. Samples were analyzed by Western blotting.
SNAP-Tag Immunofluorescence
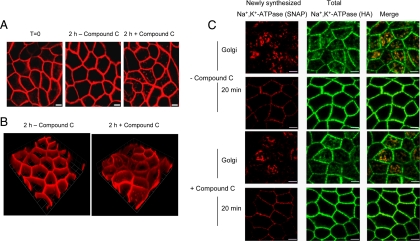
For live cell labeling, polarized, filter-grown MDCK cell cultures stably transfected with the SNAP-tagged Na+,K+-ATPase (Farr et al., 2009) were incubated with 0.5 μM tetramethylrhodamine-conjugated SNAP-substrate (TMR-STAR; New England Biolabs) for 30 min at 37°C. Cultures were washed three times and incubated with media for an additional 30 min at 37°C to wash out any unincorporated TMR-STAR and to allow for any labeled biosynthetic intermediates to reach the cell surface (see Figure 6A, T = 0). To ensure that any residual TMR-STAR is prevented from reacting with newly synthesized Na+,K+-ATPase cultures were incubated with 13.3 nM BTP (SNAP Block; New England Biolabs), as well as with either varying concentrations of Compound C (Calbiochem, San Diego, CA) or dimethyl sulfoxide (vehicle control) for 2 h at 37°C. After this incubation, samples were fixed in 4% paraformaldehyde and processed for microscopy. Fixed cell pulse-chase analysis was performed as described previously (Farr et al., 2009).
Figure 6.
Compound C leads to Na+,K+-ATPase internalization and does not affect Na+,K+-ATPase trafficking to the plasma membrane. (A) Cells were incubated with 0.5 μM TMR-STAR to “live label” the sodium pump (red). Essentially all of the Na+,K+-ATPase labeled in this manner is detected at the cell surface. Next, cells were washed and incubated for a further 30 min at 37°C to remove excess TMR-STAR (T = 0). Finally, cultures were incubated in the presence or absence of 40 μM Compound C for 2 h at 37°C. After the incubation, cells were fixed and prepared for imaging. Bar, 5 μm. Compound C treatment causes a fraction of the TMR-STAR–labeled pumps that were present at the cell surface immediately after labeling to be redistributed to intracellular compartments. (B) 3D image reconstructions of confocal z-stacks generated in A were performed with Volocity software 4.3 (Improvision, Coventry, United Kingdom). 1 U = 5.73 μm. (C) SNAP cells were incubated with BTP to block “old” pump, incubated at 37°C for 30 min to permit synthesis of new “unblocked” cohort of Na+,K+-ATPase and placed at 19°C for 2 h to accumulate the newly synthesized pump proteins in the Golgi. The 19°C incubation was performed in the presence or absence of 40 μM Compound C. Samples were then fixed immediately or warmed to 37°C to allow trafficking to the cell surface. TMR-STAR is depicted in red and anti-HA, which stains the total pool of Na+,K+-ATPase, is shown in green. Bar, 5 μm. Compound C treatment did not delay or reduce the delivery of the Golgi-accumulated newly synthesized Na+,K+-ATPase to the plasma membrane.
RESULTS
Coimmunoprecipitation of the Na+,K+-ATPase and AS160
MDCK cells stably expressing a fusion protein comprised of the SNAP-tag linked to the N terminus of the Na+,K+-ATPase α-subunit (Farr et al., 2009) were lysed and then labeled with BG-biotin. This treatment results in covalent addition of the biotin label exclusively to the SNAP-tagged Na+,K+-ATPase α-subunit. The biotin-labeled sodium pump was solubilized under nondenaturing conditions and recovered by streptavidin precipitation. The proteins that coprecipitated with the pump were identified by mass spectrometry. The Coomassie Brilliant Blue staining pattern of a SDS-PAGE gel that was prepared for analysis by mass spectrometry is presented in Supplemental Figure 1. One of the proteins detected in this analysis was AS160.
To confirm that Na+,K+-ATPase interacts with endogenous AS160, immunoprecipitation was performed using lysates prepared from polarized MDCK cells that stably express α1 Na+,K+-ATPase-SNAP-HA (Farr et al., 2009) or from untransfected wild-type (WT) cells. To demonstrate that the AS160 protein is endogenously expressed in the MDCK cell line, the lysates were blotted with anti-AS160. Na+,K+-ATPase-SNAP-HA was immunoprecipitated using mouse anti-HA beads and AS160 coprecipitating with Na+,K+-ATPase was detected by Western blotting with anti-AS160 (Figure 1). To demonstrate that the Na+,K+- ATPase-SNAP-HA protein was in fact recovered in the immunoprecipitates, the immunoprecipitates were blotted with anti-HA antibody. The results clearly demonstrated that endogenous AS160 was detectable in the anti-HA immunoprecipitates only from the MDCK cells expressing the HA-tagged α-subunit and not from wild-type control cells. Thus, AS160 forms a complex and coprecipitates with the Na+,K+-ATPase.
Figure 1.

Endogenous AS160 coimmunoprecipitates with Na+,K+-ATPase-SNAP-HA expressed by transfection in MDCK cells. Coimmunoprecipitation was performed using Sepharose beads conjugated to mouse anti-HA immunoglobulin G to pull down Na+,K+-ATPase-HA, and AS160 was detected by Western blotting with anti-AS160 antibody. Endogenous AS160 coimmunoprecipitates with Na+,K+-ATPase in lysates prepared from cells transfected with Na+,K+-ATPase-HA but not from untransfected cell lysates. As a control, the immunoprecipitates were blotted with rabbit anti-HA antibody to confirm that Na+,K+-ATPase-SNAP-HA was indeed recovered in the immunoprecipitates. Total membrane lysates from untransfected (WT) and transfected MDCK cells were blotted with antibodies directed against AS160 and HA to assess the levels of AS160 and Na+,K+-ATPase-HA expression. Typical results from one of three experiments are shown.
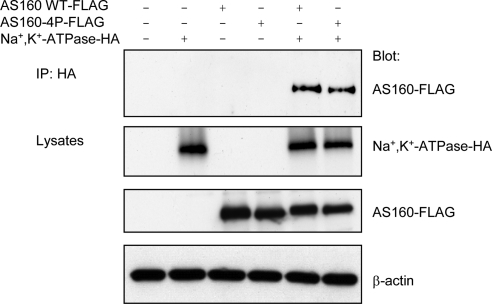
To explore whether the interaction between Na+,K+- ATPase and AS160 is influenced by phosphorylation of AS160 at sites that modulate the effects of AS160 on GLUT4 trafficking, we performed a coimmunoprecipitation of Na+,K+-ATPase and a dominant-negative mutant form of AS160, AS160-4P. In the AS160-4P construct, four potential phosphorylation sites are mutated to alanines. Expression of this construct inhibits GLUT4 translocation to the plasma membrane after insulin stimulation (Sano et al., 2003), consistent with the model in which insulin-induced phosphorylation of AS160 relieves the inhibition that AS160 activity imposes on GLUT4 trafficking. To assess whether the Na+,K+-ATPase and AS160 interaction is dependent on the four phosphorylation sites mutated in AS160-4P, COS cells were transiently transfected with cDNAs encoding the Na+,K+-ATPase (α1-HA and β1 subunits) and AS160WT-FLAG or AS160-4P-FLAG. Figure 2 shows the Western blot patterns obtained when untransfected or transfected COS cell lysates were subjected to immunoprecipitation with HA antibody that detects Na+,K+-ATPase-HA α-subunit, and blotted with anti-FLAG antibody, which recognizes AS160. To document the expression levels of the exogenous Na+,K+-ATPase, AS160WT and AS160-4P proteins, lysates were blotted with anti-HA and anti-FLAG, respectively. In addition, equal protein loading was confirmed by Western blotting the lysates with an antibody directed against β-actin. As expected, when Na+,K+-ATPase, AS160WT, or AS160-4P were transfected alone, no AS160 was observed in the anti-HA immunoprecipitation of Na+,K+-ATPase. However, AS160WT was immunoprecipitated in cells cotransfected with Na+,K+-ATPase and AS160WT, confirming the results produced with MDCK cells and presented in Figure 1. Interestingly, AS160-4P was also coimmunoprecipitated with Na+,K+-ATPase, suggesting that the interaction is independent of the availability for phosphorylation of the four residues mutated in the AS160-4P mutant.
Figure 2.
Elimination of four potential phosphorylation sites does not affect the interaction between Na+,K+-ATPase and AS160. COS cells were untransfected, singly transfected with Na+,K+-ATPase-HA, AS160WT-FLAG, or AS160-4P-FLAG or cotransfected with Na+,K+-ATPase-HA and AS160WT-FLAG or AS160-4P-FLAG. The immunoprecipitation was performed using mouse anti-HA immunoglobulin G-Sepharose beads (to capture the Na+,K+-ATPase-HA), and immunoprecipitates were probed with a rabbit anti-FLAG antibody to detect exogenous AS160 coimmunoprecipitating with Na+,K+-ATPase-HA. The immunoblot indicates that AS160 WT and AS160-4P mutant coimmunoprecipitated with Na+,K+-ATPase. As a control for protein loading and for the expression levels of AS160 and Na+,K+-ATPase, the lysates were blotted with mouse anti-β-actin, anti-FLAG, and anti-HA, respectively. Typical results from one of four experiments are shown.
Coexpression of AS160 and Na+,K+-ATPase Leads to Intracellular Retention of Na+,K+-ATPase in COS Cells
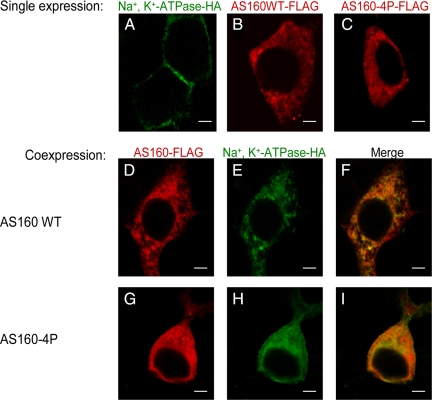
To analyze whether AS160 and Na+,K+-ATPase localize to the same subcellular compartment, an immunofluorescence assay was performed. COS cells were transfected with cDNAs encoding Na+,K+-ATPase and either AS160WT or AS160-4P. Antibodies directed against the HA and FLAG epitopes were used to detect exogenous Na+,K+-ATPase-HA and AS160WT/4P-FLAG, respectively. Figure 3 shows the resulting immunofluorescence images. When expressed singly, the Na+,K+-ATPase (3A), AS160WT (3B), and AS160-4P (3C) exhibited different subcellular localizations. As expected, the Na+,K+-ATPase was found predominantly at the plasma membrane when it was expressed in the absence of AS160WT or AS160-4P. In contrast, AS160WT or AS160-4P expressed alone in the COS cells localized to structures in the cytoplasm.
Figure 3.
Coexpression of Na+,K+-ATPase and AS160 in COS cells produces intracellular accumulation of the Na+,K+-ATPase. Immunofluorescence analysis was performed to localize exogenous Na+,K+-ATPase-HA, AS160WT-FLAG, and AS160-4P-FLAG. Cells were stained with anti-HA for Na+,K+-ATPase (A, E, and H) and anti-FLAG for AS160WT (B and D) or AS160-4P (C and G). Overlay patterns are shown in F and I. COS cells were singly transfected with Na+,K+-ATPase-HA (A), AS160WT-FLAG (B), and AS160-4P-FLAG (C) or cotransfected with Na+,K+-ATPase-HA and AS160WT-FLAG (D and E) and Na+,K+-ATPase-HA and AS1604P-FLAG (G–I). Na+,K+-ATPase is localized predominantly at the plasma membrane when it is singly expressed in COS cells. However, the Na+,K+-ATPase is located in intracellular structures when it is coexpressed with AS160WT or AS160-4P. Typical results from one of five experiments are presented. Bar, 5 μm.
The coexpression of Na+,K+-ATPase and AS160WT or AS160-4P led to the intracellular retention of the Na+,K+-ATPase (Figure 3, D–I). This finding reconfirms that an interaction between Na+,K+-ATPase and AS160WT or AS160-4P, demonstrated above by coimmunoprecipitation from lysed cells, can occur in cells in situ. It further suggests that this interaction can influence the subcellular distribution of the Na+,K+-ATPase.
AS160 Interacts In Vitro with Two Different Domains of Na+,K+-ATPase
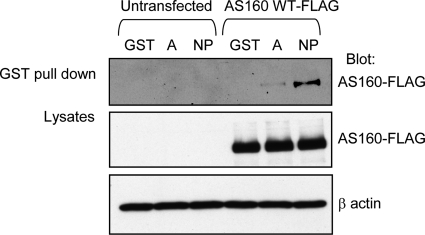
To begin to map where AS160 binds within the structure of the Na+,K+-ATPase α-subunit, we performed a GST pull-down assay. The cytosolic portion of the Na+,K+-ATPase α-subunit includes two large structurally autonomous domains (Morth et al., 2007; Shinoda et al., 2009). The A domain is composed of the N terminus and the loop between transmembrane domains 2 and 3. The NP domain is created by the loop between transmembrane domains 4 and 5. We used GST-fusion proteins incorporating the A domain and NP domain of the Na+,K+-ATPase α-subunit (Zatti et al., 2005). The GST-A domain fusion was prepared by creating a fusion protein in which the N-terminal sequence is attached through a flexible linker sequence (Gly-Gly-Gly-Gly-Ser-Gly-Gly-Gly-Gly-Ser) to the N terminus of the sequence of the 2–3 loop, which is in turn attached to the N terminus of GST. Both the GST-A domain and GST-NP domain proteins were produced in bacteria and immobilized on glutathione-Sepharose 4B beads. Lysates from untransfected COS cells and from COS cells expressing AS160WT-FLAG were incubated with the resultant fusion protein-charged beads. The precipitated product was analyzed by SDS-PAGE and then visualized by Western blot. The data presented in Supplemental Figure 2 demonstrate that roughly equal quantities of each of the GST protein products was used in each analysis. As shown in Figure 4, AS160 bound to both the A and NP domains of Na+,K+-ATPase α-subunit but not to GST alone. The extent of this binding, however, seems to be much greater with the NP domain fusion protein, suggesting that the NP domain constitutes the principle site of AS160 interaction with the pump.
Figure 4.
AS160 directly associates with the Na+,K+-ATPase α-subunit. In vitro fusion protein pull-down assay was performed to map the site on the Na+,K+-ATPase that interacts with AS160. COS cells lysates were incubated with glutathione-Sepharose 4B beads carrying GST alone (GST), GST fused to the A-domain (A), or the NP domain of the Na+,K+-ATPase (NP) α-subunit. Precipitated AS160 was detected by immunoblotting with anti-FLAG. As a control for nonspecific binding, lysates from untransfected COS cells also were incubated with the GST-fusion proteins (lanes 1–3). When GST-fusion proteins were incubated with lysates from COS cells overexpressing AS160WT-FLAG, AS160 was precipitated with both A domain and NP domain of Na+,K+-ATPase α-subunit, although the extent of the interaction with the NP domain was considerably greater than that with the A domain. Lysates were blotted with anti-FLAG to detect the total expression of AS160WT-FLAG and with anti-β-actin to control for the total amount of protein loaded. The total GST-fusion protein input is shown in Supplemental Figure 2. Typical results of one of three experiments are shown.
An AMPK Inhibitor Induces Na+,K+-ATPase Endocytosis and Does Not Affect Sodium Pump Biosynthetic Trafficking
AMPK activation results in GLUT4 translocation to the plasma membrane (Kurth-Kraczek et al., 1999). AMPK may achieve this effect by phosphorylating AS160 and inhibiting its GAP activity, thus permitting Rab-guanosine triphosphate (GTP)-dependent translocation of GLUT4 to the cell surface (Treebak et al., 2010). We wondered whether manipulating the activity of AMPK would alter the distribution of the Na+,K+-ATPase, which normally resides primarily at the basolateral cell surface in polarized epithelial cells. To test this possibility, we made use of Compound C, which is a well characterized inhibitor of AMPK (Zhou et al., 2001). It has been shown that in order to be active as a kinase, AMPK must be phosphorylated on residue T172 of its α-subunit (Hawley et al., 1995). To examine AMPK phosphorylation as a consequence of treatment with varying concentrations of Compound C, lysates prepared from MDCK cells treated with vehicle alone (0) or with 1, 10, 20, 30, and 40 μM Compound C were blotted with an antibody specifically recognizing AMPK phosphorylated at Thr172. The level of AMPK phosphorylation decreased gradually with increasing Compound C concentration (Figure 5A). We next examined whether AMPK kinase activity is reduced by Compound C treatment. Acetyl CoA carboxylase (ACC) is a well-documented substrate for AMPK, and the extent of its phosphorylation reflects the in situ level of AMPK activity (Hardie et al., 2002). We observed a gradual decrease of ACC phosphorylation as a result of treatment with increasing concentrations of Compound C (Figure 5A).
Figure 5.
AMPK inhibition by Compound C induces Na+,K+-ATPase internalization in MDCK cells. MDCK cells were treated with vehicle (0) or with Compound C for 2 h at different concentrations (1, 10, 20, 30, or 40 μM). (A) Lysates of MDCK cells were blotted with phosphorylated (p)-AMPK Thr172 and p-ACC Ser79 to assess the levels of AMPK activity, and the β-actin antibody immunoblot reports on the total amount of protein loaded. Typical results of one of three experiments are shown. (B) Immunofluorescence analysis of endogenous Na+,K+-ATPase. MDCK cells were stained with an antibody directed against Na+,K+-ATPase (α5). An increase of the amount of Na+,K+-ATPase internalization was observed with increasing concentrations of Compound C (0 (vehicle), 1 (1 μM), 10 (10 μM), 20 (20 μM), 30 (30 μM), and 40 (40 μM)). Typical results of one of three experiments are shown. Bar, 5 μm.
To determine, whether Compound C in this dose range alters the distribution of the Na+,K+-ATPase, MDCK cells were once again treated with Compound C. Figure 5B shows the distribution of the Na+,K+-ATPase, determined by immunofluorescence, in MDCK cells treated with vehicle or with 0, 1, 10, 20, 30, and 40 μM Compound C for 2 h. The results indicated that Compound C treatment induces a dramatic intracellular accumulation of the Na+,K+-ATPase. Furthermore, the extent of internalization correlates with the degree of inhibition of AMPK by Compound C. Together, these results support the interpretation that Compound C inhibition of AMPK activity is responsible for the Na+,K+-ATPase internalization that is observed in response to Compound C treatment.
The intracellular accumulation of the Na+,K+-ATPase observed after Compound C treatment may arise from endocytosis of the plasma membrane resident sodium pumps. However, it is also possible that Compound C treatment alters the trafficking of newly synthesized Na+,K+-ATPase, resulting in its vectorial delivery directly to these intracellular compartments. To test these possibilities, we used the SNAP-tag system to specifically label temporally defined cohorts of sodium pump and followed their trafficking after Compound C treatment. As depicted in Figure 6A(T = 0), The SNAP-tagged sodium pump resident in stably transfected MDCK cell cultures is readily labeled by incubation of live cultures with cell-permeable TMR-STAR. After TMR-STAR labeling, cultures were further incubated in the presence or absence of 40 μM Compound C for 2 h at 37°C. As can be seen in Figure 6A, at time 0 as well as after 2 h of vehicle treatment, the localization of the Na+,K+-ATPase is limited almost exclusively to the plasma membrane. However, in samples treated with Compound C there was significant internalization of the pool of sodium pump that had been present at the cell surface at time 0, immediately after SNAP labeling (Figure 6A). The results are consistent with the interpretation that inhibition of AMPK led to an internalization of surface Na+,K+-ATPase. This impression is further strengthened through an examination of three-dimensional (3D) reconstructions of confocal image stacks (Figure 6B).
Although our results strongly suggest that endocytosis plays a major part in the Compound C-mediated effect, it remains possible that biosynthetic trafficking is also altered by this treatment. To test this possibility, we took advantage of the pulse chase capability of the SNAP-tag system. In this experiment, cells were treated with unlabeled benzylguanine to block covalently the SNAP labeling sites on the pre-existing pool of SNAP-tagged sodium pumps. After a 30-min incubation at 37°C to permit the synthesis of a new “unblocked” cohort of Na+,K+-ATPase, the cells were transferred to 19°C for an additional 2 h to ensure that newly synthesized Na+,K+-ATPase was accumulated in the Golgi complex. Each of these incubations was performed in the presence or absence of Compound C. As expected, Compound C treatment had no detectable effect on protein synthesis or on the accumulation of the newly synthesized sodium pump at the Golgi complex during the 19°C incubation (Figure 6C, Golgi). To test the affect of treatment on post-Golgi trafficking, samples were warmed to 37°C for 20 min to release the Golgi block and to allow delivery of the sodium pump from the Golgi to the plasma membrane. When samples were warmed to 37°C, the time course and extent of Na+,K+-ATPase trafficking to the cell surface was not affected by Compound C treatment (Figure 6C, 20 min). Together, our results demonstrate that Compound C treatment results in the internalization of a plasma membrane localized pool of sodium pump and does not affect this protein's biosynthetic delivery.
Compound C Increases the Interaction between AS160 and Na+,K+-ATPase
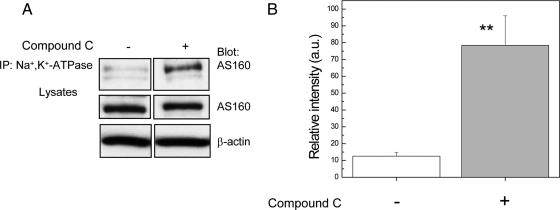
If AMPK inhibition causes pump internalization by preventing AMPK from inducing the dissociation of AS160 from the Na+,K+-ATPase, then we would expect that this treatment would lead to an increase in the quantity of AS160 that coimmunoprecipitates with the sodium pump. To determine whether the direct interaction between Na+,K+-ATPase and AS160 increases after Compound C treatment, coimmunoprecipitation from MDCK cells was performed. The Na+,K+-ATPase was recovered by immunoprecipitation and the associated AS160 that coprecipitated was detected by Western blotting with anti-AS160 (Figure 7). The results indicated that the inhibition of AMPK by Compound C clearly led to an increase in the extent of the interaction between the Na+,K+-ATPase and AS160. Figure 7B depicts the quantification of this coimmunoprecipitation, which indicates that the extent of the interaction increases by a factor of 7.
Figure 7.
AMPK inhibition by Compound C enhances the interaction between the Na+,K+-ATPase and AS160 proteins endogenously expressed in MDCK cells. Endogenous Na+,K+-ATPase was immunoprecipitated using a mouse antibody directed against the α-subunit (α5), and the resultant precipitates were blotted with anti-AS160 antibody. MDCK cells were incubated with vehicle (−) or with 40 μM Compound C (+), for 2 h. (A) Immunoblot of the coimmunoprecipitation. Lysates were blotted with anti-AS160 to detect the endogenous levels of AS160 and with anti-β-actin to control for the total amount of protein loaded. (B) Coimmunoprecipitation quantification (**p < 0.001, n = 3). The results demonstrated that the interaction between AS160 and Na+,K+-ATPase increases by a factor 7 after Compound C treatment.
shRNA-mediated Knockdown of AS160 in MDCK Cells Prevents the Na+,K+-ATPase Internalization Caused by Compound C Treatment
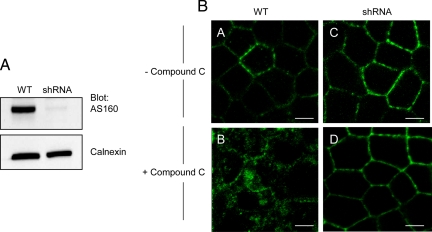
Finally, to confirm the role of AS160 in Compound C-induced Na+,K+-ATPase internalization in MDCK cells, we used shRNA to knock down AS160 expression. Figure 8A shows the Western blot pattern of lysates from AS160 shRNA knockdown and WT MDCK cells, demonstrating that the levels of AS160 protein expression were robustly reduced in a stably transfected clonal cell line. Figure 8B depicts the immunofluorescence patterns obtained from MDCK cells treated with vehicle (A) and with 40 μM Compound C (B), as a control. Figure 8B (C and D) shows the pattern of Na+,K+-ATPase distribution observed by immunofluorescence in AS160 knockdown MDCK cells treated with vehicle (C) or treated with 40 μM Compound C (D). Interestingly, the knockdown of AS160 prevents the Compound C-mediated internalization of the Na+,K+-ATPase. This result lends substantial support to the conclusion that AS160 mediates the Na+,K+-ATPase internalization caused by AMPK inhibition in MDCK cells.
Figure 8.
shRNA-mediated knockdown of AS160 inhibits the internalization of Na+,K+-ATPase induced by Compound C treatment in MDCK cells. (A) Lysates from MDCK cells: wild type cells (WT) and a clonal AS160 shRNA-expressing line (shRNA) were immunoblotted with an anti-AS160 antibody and with an anti-calnexin antibody to report on the total amount of protein loaded. The levels of AS160 expression were robustly decreased in the AS160 shRNA clone. Typical results of one of four experiments are shown. (B) Immunofluorescence analysis of endogenous Na+,K+-ATPase. MDCK cells were stained with antibody directed against Na+,K+- ATPase (α5). Wild-type (WT) and AS160 knockdown (shRNA) MDCK cells were treated with vehicle (A and C) or treated with 40 μM of Compound C (B and D) for 2 h. Compound C treatment induces Na+,K+-ATPase internalization in wild-type MDCK cells. This phenomenon is not observed in the AS160 shRNA knockdown cells. Typical results of one of three experiments are shown. Bar, 5 μm.
DISCUSSION
The Na+,K+-ATPase plays a critical role in driving fluid and electrolyte transport in a wide variety of tissues. It is not surprising, therefore, that its activity is governed by several physiological pathways and processes. There is substantial evidence that a portion of the cellular population of the Na+,K+-ATPase can be located in intracellular pools in many cell types, and physiological stimuli can promote its endocytosis or translocation to the plasma membrane (Barlet-Bas et al., 1990; Chibalin et al., 1999; Efendiev et al., 2007; Liang et al., 2007). However, the cellular and molecular mechanisms that modulate this intracellular retention and regulated trafficking of Na+,K+-ATPase are still unclear.
We have found that AS160 interacts directly with cytosolic NP loop domain of the α-subunit of the Na+,K+-ATPase. Our results demonstrate that endogenous AS160 interacts with Na+,K+-ATPase in MDCK cultured renal epithelial cells under basal conditions. Furthermore, we find that Na+,K+-ATPase interacts with AS160WT and AS160-4P, suggesting that the four phosphorylation sites that are changed to alanines in the AS160-4P mutant construct are not necessary for the binding of AS160 to Na+,K+-ATPase. Recent studies demonstrate the existence of many phosphorylation sites in the AS160 protein in addition to those that are mutated in the AS160-4P construct (Geraghty et al., 2007). In muscle isoforms of AS160, at least one novel site is phosphorylated in response to AMPK activation (Treebak et al., 2010). Future experiments are required to determine whether phosphorylation at any of these additional sites modulates the association of AS160 with the Na+,K+-ATPase and regulates the effects of this protein on sodium pump trafficking.
AS160 has been identified as a modulator of GLUT4 translocation to the plasma membrane in response to insulin (Sano et al., 2003), although an interaction between GLUT4 and AS160 has not been demonstrated. Knockdown of AS160 expression through siRNA techniques diminished the intracellular retention of GLUT4 and induced its translocation to the plasma membrane under basal conditions, demonstrating that AS160 participates in ensuring the intracellular storage of GLUT4 before insulin stimulation (Eguez et al., 2005; Larance et al., 2005). We have demonstrated that inhibition of AMPK by Compound C led to the internalization of Na+,K+-ATPase and an enhancement of the pump's interaction with endogenous AS160 in MDCK cells. In addition, shRNA-mediated knockdown of AS160 inhibits the internalization of the Na+,K+-ATPase in MDCK cells treated with Compound C, proving the central role of AS160 in mediating this effect of AMPK inhibition. Together, these findings suggest that the phenomenon of transport protein trafficking regulation by AS160 extends beyond GLUT4 and that an interaction between the Na+,K+-ATPase and AS160 regulates Na+,K+-ATPase trafficking in kidney cells.
Recently, Comellas et al. (2010) showed that insulin treatment induced Na+,K+-ATPase translocation to the plasma membrane in alveolar epithelial cells and that this process was mediated by Akt. These authors suggested that Rab10 may play an important role in sodium pump trafficking and that phosphorylation of AS160 by Akt may permit pump trafficking to the cell surface by inhibiting the Rab-GAP activity of AS160, thereby allowing the GTP-bound form of Rab10 to accumulate. Our data complement and extend these findings by demonstrating that AS160 associates directly with the Na+,K+-ATPase and by showing that AMPK as well as Akt can modulate AS160's effects on pump distribution. At first glance, it might seem somewhat surprising that the results presented in our study and those of Comellas et al. (2010) indicate that AS160 is involved in both the regulated endocytosis and exocytosis of the sodium pump, respectively. In fact, however, these findings are in no way inconsistent or contradictory. Our study shows that inhibiting AMPK results in the AS160-dependent intracellular accumulation of the Na+,K+-ATPase. Because AMPK can, like Akt, phosphorylate AS160 and thus inhibit its capacity to serve as a Rab-GAP, it seems likely that inhibition of AMPK by Compound C removes a tonic brake on AS160 activity. Under these circumstances, any recycling of endocytosed Na+,K+-ATPase back to the cell surface might be blocked if that recycling were dependent on the GTP-bound form of a Rab protein that is in turn a substrate for AS160. According to this model, the intracellular accumulation that we observe as a consequence of AMPK inhibition would be due to an inhibition of recycling rather than to a stimulation of endocytosis. It is worth noting that the studies of Comellas et al. (2010) were performed on pulmonary epithelial cells, whereas our experiments made use of a renal epithelial cell line. It is quite possible, therefore that the cellular machinery that influences or that is influenced by AS160 may differ in these different cell types. For example, the identity of the Rab whose function is influenced by the AS160 Rab-GAP activity may differ between pulmonary and renal epithelial cells. Further experiments will be required to determine whether different suites of Rab substrates are influenced by AS160 in different cell types.
It has been shown that Rab10 participates in regulated GLUT4 translocation in 3T3-L1 adipocytes and that this process is under the control of AS160 (Sano et al., 2007; Sano et al., 2008). Extensive evidence indicates that Rab proteins play important roles in the directed trafficking of membrane proteins from the trans-Golgi network to specific domains of the cell surface in polarized epithelial cells (Babbey et al., 2006; Schuck et al., 2007). It is interesting to note that in a previous study, we have shown that expression of a constitutively active form of the Rab10 protein does not perturb the initial biosynthetic delivery of the sodium pump from the trans-Golgi network to the basolateral cell surfaces of MDCK cells, although this manipulation did dramatically alter the surface delivery of the low-density lipoprotein receptor, another protein that normally accumulates at the MDCK cell basolateral plasma membrane (Farr et al., 2009). In addition, the SNAP-tag data presented here indicate that the intracellular pool of Na+,K+-ATPase that accumulates in response to AMPK inhibition derives from pumps resident at the plasma membrane rather than from newly synthesized pumps that were prevented from reaching the cell surface. Together, these findings suggest that if AS160 acts through Rab10 to alter the surface expression of the Na+,K+-ATPase in renal epithelial cells, then these effects are exerted not on the biosynthetic pool of Na+,K+-ATPase making its initial journey from the Golgi to the plasma membrane but rather on a pool of recycling pump that has been internalized by endocytosis. Further studies are required to elucidate fully the mechanisms through which Na+,K+-ATPase trafficking is controlled by its interaction with AS160 and by this polypeptide's capacity to alter the activation states of Rab proteins.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the members of the Caplan laboratory for helpful discussions and suggestions. This work was supported by Leducq Foundation Transatlantic Network for Hypertension and by National Institutes of Health grants DK-072612 and DK-17433.
Abbreviations used:
- ACC
acetyl CoA carboxylase
- AMPK
adenosine monophosphate-stimulated protein kinase
- Na+,K+-ATPase
sodium pump.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0507) on October 13, 2010.
REFERENCES
- Al-Khalili L., Kotova O., Tsuchida H., Ehren I., Feraille E., Krook A., Chibalin A. V. ERK1/2 mediates insulin stimulation of Na(+),K(+)-ATPase by phosphorylation of the alpha-subunit in human skeletal muscle cells. J. Biol. Chem. 2004;279:25211–25218. doi: 10.1074/jbc.M402152200. [DOI] [PubMed] [Google Scholar]
- Al-Khalili L., Yu M., Chibalin A. V. Na+,K+-ATPase trafficking in skeletal muscle: insulin stimulates translocation of both alpha 1- and alpha 2-subunit isoforms. FEBS Lett. 2003;536:198–202. doi: 10.1016/s0014-5793(03)00047-4. [DOI] [PubMed] [Google Scholar]
- Babbey C. M., Ahktar N., Wang E., Chen C. C., Grant B. D., Dunn K. W. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlet-Bas C., Khadouri C., Marsy S., Doucet A. Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J. Biol. Chem. 1990;265:7799–7803. [PubMed] [Google Scholar]
- Bertorello A. M., Komarova Y., Smith K., Leibiger I. B., Efendiev R., Pedemonte C. H., Borisy G., Sznajder J. I. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol. Biol. Cell. 2003;14:1149–1157. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee G. D., Wojtaszewski J. F. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metab. 2007;32:557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- Chibalin A. V., Ogimoto G., Pedemonte C. H., Pressley T. A., Katz A. I., Feraille E., Berggren P. O., Bertorello A. M. Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J. Biol. Chem. 1999;274:1920–1927. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- Comellas A. P., Kelly A. M., Trejo H. E., Briva A., Lee J., Sznajder J. I., Dada L. A. Insulin regulates alveolar epithelial function by inducing Na+/K+-ATPase translocation to the plasma membrane in a process mediated by the action of Akt. J. Cell Sci. 2010;123:1343–1351. doi: 10.1242/jcs.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. S., Kyte J. Stoichiometry and molecular weight of the minimum asymmetric unit of canine renal sodium and potassium ion-activated adenosine triphosphatase. J. Biol. Chem. 1980;255:6262–6269. [PubMed] [Google Scholar]
- Efendiev R., Das-Panja K., Cinelli A. R., Bertorello A. M., Pedemonte C. H. Localization of intracellular compartments that exchange Na,K-ATPase molecules with the plasma membrane in a hormone-dependent manner. Br. J. Pharmacol. 2007;151:1006–1013. doi: 10.1038/sj.bjp.0707304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguez L., Lee A., Chavez J. A., Miinea C. P., Kane S., Lienhard G. E., McGraw T. E. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Farr G. A., Hull M., Mellman I., Caplan M. J. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J. Cell Biol. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K. Subunit assembly and functional maturation of Na,K-ATPase. J. Membr. Biol. 1990;115:109–121. doi: 10.1007/BF01869450. [DOI] [PubMed] [Google Scholar]
- Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am. J. Physiol. Renal Physiol. 2006;290:F241–F250. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- Geraghty K. M., Chen S., Harthill J. E., Ibrahim A. F., Toth R., Morrice N. A., Vandermoere F., Moorhead G. B., Hardie D. G., MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem. J. 2007;407:231–241. doi: 10.1042/BJ20070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi C. J., Caplan M. J. Molecular requirements for the cell-surface expression of multisubunit ion-transporting ATPases. Identification of protein domains that participate in Na,K-ATPase and H,K-ATPase subunit assembly. J. Biol. Chem. 1993;268:14342–14347. [PubMed] [Google Scholar]
- Hardie D. G., Pan D. A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- Hashiramoto M., James D. E. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol. Cell. Biol. 2000;20:416–427. doi: 10.1128/mcb.20.1.416-427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S. A., Selbert M. A., Goldstein E. G., Edelman A. M., Carling D., Hardie D. G. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- Horisberger J. D., Rossier B. C. Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension. 1992;19:221–227. doi: 10.1161/01.hyp.19.3.221. [DOI] [PubMed] [Google Scholar]
- Hundal H. S., Marette A., Mitsumoto Y., Ramlal T., Blostein R., Klip A. Insulin induces translocation of the alpha 2 and beta 1 subunits of the Na+/K(+)-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J. Biol. Chem. 1992;267:5040–5043. [PubMed] [Google Scholar]
- Jorgensen P. L. Sodium and potassium ion pump in kidney tubules. Physiol. Rev. 1980;60:864–917. doi: 10.1152/physrev.1980.60.3.864. [DOI] [PubMed] [Google Scholar]
- Juillerat A., Gronemeyer T., Keppler A., Gendreizig S., Pick H., Vogel H., Johnsson K. Directed evolution of O6-alkylguanine-DNA alkyltransferase for efficient labeling of fusion proteins with small molecules in vivo. Chem. Biol. 2003;10:313–317. doi: 10.1016/s1074-5521(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Kane S., Sano H., Liu S. C., Asara J. M., Lane W. S., Garner C. C., Lienhard G. E. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Keppler A., Gendreizig S., Gronemeyer T., Pick H., Vogel H., Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 2003;21:86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek E. J., Hirshman M. F., Goodyear L. J., Winder W. W. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Larance M., et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- Liang M., Tian J., Liu L., Pierre S., Liu J., Shapiro J., Xie Z. J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- McGill D. L. Characterization of the adipocyte ghost (Na+,K+) pump. Insights into the insulin regulation of the adipocyte (Na+,K+) pump. J. Biol. Chem. 1991;266:15817–15823. [PubMed] [Google Scholar]
- McGill D. L., Guidotti G. Insulin stimulates both the alpha 1 and the alpha 2 isoforms of the rat adipocyte (Na+,K+) ATPase. Two mechanisms of stimulation. J. Biol. Chem. 1991;266:15824–15831. [PubMed] [Google Scholar]
- Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sorensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- Ridge K. M., Dada L., Lecuona E., Bertorello A. M., Katz A. I., Mochly-Rosen D., Sznajder J. I. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and -delta. Mol. Biol. Cell. 2002;13:1381–1389. doi: 10.1091/mbc.01-07-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Eguez L., Teruel M. N., Fukuda M., Chuang T. D., Chavez J. A., Lienhard G. E., McGraw T. E. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Sano H., Kane S., Sano E., Miinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Sano H., Roach W. G., Peck G. R., Fukuda M., Lienhard G. E. Rab10 in insulin-stimulated GLUT4 translocation. Biochem. J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- Schuck S., Gerl M. J., Ang A., Manninen A., Keller P., Mellman I., Simons K. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- Shinoda T., Ogawa H., Cornelius F., Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 2009;459:446–450. doi: 10.1038/nature07939. [DOI] [PubMed] [Google Scholar]
- Summa V., Mordasini D., Roger F., Bens M., Martin P. Y., Vandewalle A., Verrey F., Feraille E. Short term effect of aldosterone on Na,K-ATPase cell surface expression in kidney collecting duct cells. J. Biol. Chem. 2001;276:47087–47093. doi: 10.1074/jbc.M107165200. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Fambrough D. M. The (Na++ K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J. Biol. Chem. 1986;261:1009–1019. [PubMed] [Google Scholar]
- Treebak J. T., Taylor E. B., Witczak C. A., An D., Toyoda T., Koh H. J., Xie J., Feener E. P., Wojtaszewski J. F., Hirshman M. F., Goodyear L. J. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am. J. Physiol. Cell Physiol. 2010;298:C377–C385. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatti A., Chauvet V., Rajendran V., Kimura T., Pagel P., Caplan M. J. The C-terminal tail of the polycystin-1 protein interacts with the Na,K-ATPase alpha-subunit. Mol. Biol. Cell. 2005;16:5087–5093. doi: 10.1091/mbc.E05-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.