Abstract
The association of olfactory dysfunction with mortality was examined in 1162 older persons without dementia or Parkinson's disease. They completed a standard 12-item test of odor identification and then were followed for a mean of 4.2 years (standard deviation [SD] = 2.6, range: 0–9) during which 321 individuals died (27.6%). The relation of olfactory score to risk of death was assessed in a series of proportional hazards models adjusted for age, sex, education, and other covariates. Olfactory scores ranged from 0 to 12 correct (mean = 9.0, SD = 2.2). In an initial analysis, risk of death decreased by about 6% for each additional odor correctly identified (hazard ratio = 0.94; 95% confidence interval: 0.90, 0.98). Thus, mortality risk was about 36% higher with a low score (6, 10th percentile) compared with a high score (11, 90th percentile). The association persisted in subsequent analyses that controlled for naming ability, disability, cerebrovascular disease, characteristic patterns of leisure activity, depressive symptoms, and apolipoprotein E genotype. The results indicate that difficulty identifying familiar odors in old age is associated with increased risk of death.
Keywords: epidemiology, longitudinal studies, mortality, odor identification
Introduction
Both cross-sectional (Russell et al. 1993; Doty et al. 1984; Ship et al. 1996; Larsson and Finkel 2000) and longitudinal (Ship et al. 1996; Calhoun-Haney and Murphy 2005) studies suggest substantial loss of olfactory function in old age. However, there is wide variability in olfactory functioning among older individuals. Olfactory function in some differs little from younger persons; others show subtle deficits; and some are profoundly anosmic. Recent research has found that some impaired olfaction in old age is associated with postmortem evidence of neurodegenerative disease, particularly neurofibrillary tangles (Wilson, Arnold, et al. 2007; Wilson et al. 2009) and Lewy bodies (Ross et al. 2006). These associations, which are present even in the absence of dementia (Ross et al. 2006; Wilson, Arnold, et al. 2007; Wilson et al. 2009), may explain why olfactory impairment predicts important consequences of neurodegenerative conditions including cognitive decline (Graves et al. 1999; Swan and Carmelli 2002; Wilson et al. 2006), incidence of mild cognitive impairment and dementia (Devanand et al. 2000; Wilson, Schneider, et al. 2007), incidence of Parkinson's disease (Ponsen et al. 2004; Ross et al. 2008), worsening parkinsonian gait (Wilson, Arnold, Buchman, et al. 2008), and neuropsychiatric complications of Parkinson's disease (Stephenson et al. 2010).
In the present study, we test the hypothesis that impaired olfactory function predicts another consequence of neurodegenerative diseases: death. Participants are old persons from the Rush Memory and Aging Project, a longitudinal study of risk factors for common chronic conditions of old age. They did not have dementia or Parkinson's disease at study baseline when they completed a standard test of the ability to identify familiar odors. Vital status was monitored for a mean of approximately 4.2 years. In a series of proportional hazards models, we tested for the hypothesized association of olfactory performance with mortality and examined factors that might account for it.
Methods
Participants
Subjects are from the Rush Memory and Aging Project, a longitudinal study that involves annual clinical evaluations and brain donation at death (Bennett et al. 2005). Persons were recruited from various retirement communities and subsidized housing facilities in the Chicago metropolitan area. Following a presentation about the project, interested persons met with project staff who discussed the study further, answered questions, and obtained informed consent. The study began in 1997, expanded in 2001, and is continuing. It was approved by the Institutional Review Board of Rush University Medical Center.
At the time of these analyses, 1232 individuals had completed olfactory testing as part of their baseline clinical evaluation. We excluded 58 people who met criteria for dementia (McKhann et al. 1984) at baseline and another 12 people with a history of Parkinson's disease. This left 1162 individuals, and analyses are based on this group. They had a mean age at the time of olfactory testing of 79.7 years (standard deviation [SD] = 7.7), they had completed a mean of 14.4 years of education (SD = 3.3), and 74.5% were women.
Assessment of olfactory function
Olfaction was assessed with the Brief Smell Identification Test (Doty et al. 1989, 1996), a measure of the ability to identify 12 familiar odors. In each case, an odorant-containing microencapsulated patch was scratched with a pencil and placed under the nose of the subject who then chose 1 of 4 names for the odor. The score is the number of correct responses. Up to 2 missing responses were allowed and assigned an item score of 0.25. In previous research, short-term temporal stability has been adequate (Doty et al. 1989). In the subjects included in the present analyses, Cronbach's coefficient alpha was 0.68, suggesting sufficient internal consistency.
Assessment of covariates
Naming was assessed with a 15-item version (Morris et al. 1989) of the Boston Naming Test, which involves naming pictures of common objects. The Katz scale (Katz et al. 1963), a measure of the level of assistance required in basic activities of daily living (i.e., toileting, dressing) was used as an indicator of disability. The overall burden of cardiovascular disease was assessed with several variables (Wilson, Arnold, Beck, et al. 2008): number of 3 vascular risk factors present (i.e., smoking, hypertension, and diabetes mellitus), number of 4 vascular conditions present (i.e., myocardial infarction, congestive heart failure, stroke, and claudication), body mass index (i.e., weight divided by height squared [kilograms/meter squared]), and body mass index squared. We used 3 previously established measures of frequency of participation in leisure activities, with separate measures of cognitive activity, social activity, and physical activity (Wilson, Scherr, et al. 2007). Depressive symptoms were assessed with a 10-item version of the Center for Epidemiological Studies Depression Scale (Kohout et al. 1993). Apolipoprotein E genotyping was done by Agencourt Bioscience Corporation with high throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the apolipoprotein E gene on chromosome 19, as described elsewhere (Wilson, Beck, et al. 2007). Persons with at least one copy of the ϵ4 allele were compared with those without an ϵ4 allele.
Data analysis
The association of olfactory score with mortality was assessed with proportional hazards models (Cox 1972). All analyses included terms for age, sex, and education. The initial model included a term for olfactory test score. In separate subsequent analyses, we added terms for Boston Naming Test score; Katz score; numbers of cardiovascular risk factors, number of cardiovascular conditions, body mass index, and body mass index squared; cognitive activity frequency, social activity frequency, and physical activity frequency; and depressive symptoms. In 2 final analyses, we added a term for possession of the ϵ4 allele and then repeated the analysis with a term for the interaction of ϵ4 with olfactory score.
Results
During a mean of 4.2 years of observation (SD = 2.6, range: 0–9), 321 (27.6%) individuals died. As shown in Table 1, those who died were older, more likely to be male, and more cognitively impaired than survivors. They also had more health problems and were less active and more depressed.
Table 1.
Baseline information on participants who survived and those who died
| Characteristics | Survived (n = 841) | Died (n = 321) | P value |
| Age | 78.1 (7.7) | 83.8 (6.1) | <0.001 |
| Education | 14.4 (3.4) | 14.4 (2.9) | 0.805 |
| Women (%) | 78.4 | 64.5 | <0.001 |
| Boston Naming Test score | 13.9 (1.4) | 13.7 (1.3) | 0.035 |
| Katz score | 0.1 (0.5) | 0.3 (0.8) | <0.001 |
| Vascular risk factors | 1.1 (0.8) | 1.3 (0.8) | <0.001 |
| Vascular conditions | 0.3 (0.5) | 0.5 (0.7) | <0.001 |
| Body mass index | 27.6 (5.5) | 27.3 (5.3) | 0.487 |
| Cognitive activity | 3.2 (0.7) | 3.1 (0.7) | 0.015 |
| Social activity | 2.7 (0.6) | 2.5 (0.6) | <0.001 |
| Physical activity | 3.4 (3.8) | 2.7 (3.3) | <0.001 |
| Depressive symptoms | 1.2 (1.8) | 1.5 (1.8) | 0.002 |
| Apolipoprotein E ϵ4 (%) | 22.6 | 22.5 | 0.977 |
Data are presented as mean (SD) unless otherwise indicated.
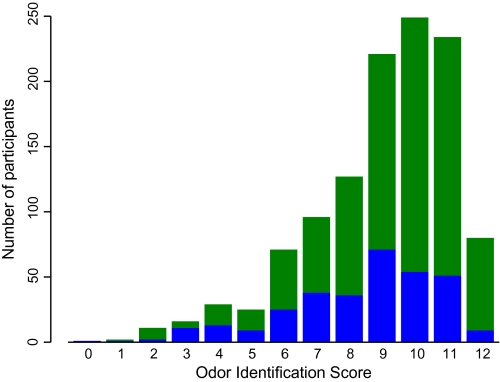
As shown in Figure 1, scores on the Brief Smell Identification Test in all subjects ranged from 0 to 12 correct (mean = 9.0, SD = 2.2) and were slightly skewed (skewness = −1.1). The figure also suggests that olfactory performance was somewhat higher in those who survived (green; mean = 9.2, SD = 2.1) compared with those who did not (blue; mean = 8.4, SD = 2.3).
Figure 1.
Distribution of odor identification scores in participants who survived (green) and those who died (blue).
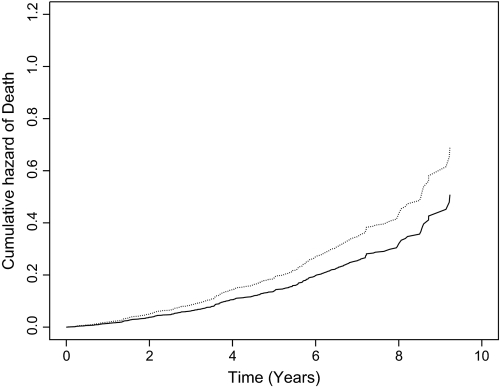
To test whether olfactory performance predicted mortality, we constructed a series of proportional hazards models. All analyses were adjusted for the potentially confounding effects of age (at study baseline), sex, and education. In the initial model, risk of death decreased by about 6% for each additional correct choice on the test (hazard ratio [HR] = 0.94, 95% confidence interval [CI]: 0.90, 0.98). Figure 2, which is based on this analysis, shows that a person with a score of 6 correct (solid line, 10th percentile) was about 36% more likely to die than a person with a score of 11 (dotted line, 90th percentile), which is an average score in young adults (Doty et al. 1989).
Figure 2.
Cumulative risk of death associated with a low (dotted line, 10th percentile) compared with high (solid line, 90th percentile) odor identification score, adjusted for age, sex, and education.
We first considered the possibility that difficulty with naming could account for the association of olfactory function with mortality. We repeated the analysis, therefore, with a term for level of naming ability on the Boston Naming Test, and results were unchanged (HR for olfactory score = 0.94; 95% CI: 0.90, 0.99).
We conducted additional analyses to see if health-related factors affected findings. In separate analyses, the association of olfactory performance with mortality persisted after controlling for disability on the Katz scale (HR = 0.95; 95% CI: 0.90, 0.99); cardiovascular risk factors and conditions (HR = 0.95; 95% CI: 0.90, 0.99); characteristic patterns of cognitive, social, and physical activity (HR = 0.95; 95% CI: 0.91, 0.99); and depressive symptoms (HR = 0.95; 95% CI: 0.91, 0.99).
Because apolipoprotein E genotype has been related to late life olfaction (Murphy et al. 1998) and its association with adverse health outcomes (Graves et al. 1999), we repeated the initial analysis with a term for possession of at least one ϵ4 allele (present in 22.6%). The relation of olfactory performance to mortality was unchanged (HR = 0.94; 95% CI: 0.90, 0.98), and in a subsequent analysis, there was no interaction between olfactory performance and the ϵ4 allele.
Discussion
We used a standard test to assess the ability to identify familiar odors in a group of more than 1000 community-dwelling older people. During a mean of about 4.2 years of observation, more than 300 individuals died. Those with impaired olfactory function were about 36% more likely to die than those with relative good olfactory function. The results indicate that olfactory dysfunction in old age predicts mortality.
We are not aware of previous research on the association of olfaction with risk of death. However, olfactory dysfunction in old age has been associated with neurodegenerative diseases that are known to contribute to mortality. Thus, olfaction is impaired in persons with Alzheimer's disease (AD) (Doty et al. 1987; Murphy et al. 1990) and its precursor, mild cognitive impairment (Wang et al. 2002; Devanand et al. 2010); olfactory dysfunction predicts incident mild cognitive impairment (Wilson, Schneider, et al. 2007) and AD (Devanand et al. 2000; Wilson, Schneider, et al. 2007); and level of olfactory performance is related to postmortem level of AD pathology, even in individuals who die without evidence of mild cognitive impairment or dementia (Wilson, Arnold, et al. 2007; Wilson et al. 2009). Olfactory function is also impaired in Lewy body dementia (Olichney et al. 2005; Williams et al. 2009) and associated with postmortem evidence of Lewy bodies in persons without dementia (Ross et al. 2006). In addition, olfactory impairment is an early sign of Parkinson's disease (Ponsen et al. 2004; Ross et al. 2008), most probably due to Lewy body inclusions in olfactory bulb and primary olfactory cortex (Hawkes et al. 1997; Braak et al. 2003; Hubbard et al. 2007). Therefore, it is likely that olfactory dysfunction predicts mortality mainly because it is a marker of common underlying neurodegenerative conditions.
The link between olfactory impairment and mortality in this study was evident even in the absence of other clinical signs of neurodegenerative disease. This implies that olfactory dysfunction is a relatively early sign of such conditions, which is consistent with prior clinical (Wang et al. 2002; Ponsen et al. 2004; Wilson, Schneider, et al. 2007; Ross et al. 2008; Devanand et al. 2010) and pathological (Ross et al. 2006; Wilson, Arnold, et al. 2007; Wilson et al. 2009) research. This suggests that olfactory testing may be indicated in older persons with suspected cognitive or motor impairment.
Confidence in these findings is strengthened by several factors. Odor identification was assessed with a standard test albeit brief. Because there were many participants, there was adequate power to detect an association between olfaction and mortality. The participants were clinically well characterized, making it possible to examine a range of factors with the potential to account for the association.
Funding
This research was supported by the National Institute on Aging (grants R01 AG17917 and R01 AG022018) and Illinois Department of Public Health.
Acknowledgments
We thank the many Illinois residents for participating in the Rush Memory and Aging Project; Traci Colvin and Karen Skish for coordinating the study; Woojeong Bang for statistical programming; and John Gibbons and Greg Klein for data management.
References
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K, Rub U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Calhoun-Haney R, Murphy C. Apolipoprotein ϵ4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain Cogn. 2005;58:178–182. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) J R Stat Soc Series B. 1972;74:187–220. [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. 1989;45:381–384. doi: 10.3758/bf03210709. [DOI] [PubMed] [Google Scholar]
- Doty RL, Marcus A, Lee WW. Development of the 12-item Cross Culture Smell Identification Test (CC-SIT) Laryngoscope. 1996;106(3, Pt 1):353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorksi L, Rosenbert L. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E ϵ4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Shephard BC, Daniel SE. Olfactory dysfunction in Parkinson's disease. J Neurol Neurosurg Psychiatr. 1997;62:436–446. doi: 10.1136/jnnp.62.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat. 2007;211:117–124. doi: 10.1111/j.1469-7580.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–923. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Larsson M, Finkel D, Pedersen NL. Odor identification: influences of age, gender, cognition, and personality. J Gerontol Psychol Sci. 2000;55B:P304–P310. doi: 10.1093/geronb/55.5.p304. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA work group under the auspices of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann N Y Acad Sci. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Murphy C, Hofstetter CR, Foster K, Hansen LA, Thal LJ, Katzman R. Anosmia is a very common in the Lewy body variant of Alzheimer's disease. J Neurol Neurosurg Psychiatr. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsen MM, Stoffers D, Booji J, van Eck-Smit BL, Wolters ECh, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol. 2004;56:173–181. doi: 10.1002/ana.20160. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Tanner CM, Davis DG, Nelson J, Markesbery WR, Hardman J, Masaki K, Launer L, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21:2062–2067. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Tanner CM, Popper J, Masaki K, Launer L, White LR. Association of olfactory dysfunction with future Parkinson's disease. Ann Neurol. 2008;63:167–173. doi: 10.1002/ana.21291. [DOI] [PubMed] [Google Scholar]
- Russell MJ, Cummings BJ, Profitt BF, Wysocki CJ, Gilbert AN, Cotman CW. Life span changes in the verbal categorization of odors. J Gerontol Psychol Sci. 1993;48:P49–P53. doi: 10.1093/geronj/48.2.p49. [DOI] [PubMed] [Google Scholar]
- Ship JA, Pearson JD, Cruise LJ, Brant LJ, Metter EJ. Longitudinal changes in smell identification. J Gerontol Med Sci. 1996;51A:M86–M91. doi: 10.1093/gerona/51a.2.m86. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Houghton D, Sundarararjan S, Doty RL, Stern M, Xie SX, Siderowf A. Odor identification deficits are associated with increased risk of neuropsychiatric complications in patients with Parkinson's disease. Mov Disord. 2010 Jul 28 doi: 10.1002/mds.23234. doi: 10.1002/mds.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21:58–67. doi: 10.1159/000048618. [DOI] [PubMed] [Google Scholar]
- Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN. Olfactory identification and apolipoprotein E epsilon 4 allele in mild cognitive impairment. Brain Res. 2002;951:77–81. doi: 10.1016/s0006-8993(02)03137-2. [DOI] [PubMed] [Google Scholar]
- Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. J Neurol Neurosurg Psychiatr. 2009;80:667–670. doi: 10.1136/jnnp.2008.155895. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65:439–446. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Buchman AS, Tang Y, Bennett DA. Odor identification and progression of parkinsonian signs in older persons. Exp Aging Res. 2008;34:173–187. doi: 10.1080/03610730802070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer's disease. Ann N Y Acad Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odor identification in old age. J Neurol Neurosurg Psychiatr. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychosom Med. 2007;69:131–137. doi: 10.1097/PSY.0b013e31803130ae. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in old age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]