Abstract
There has been a high incidence of tumor recurrence after liver transplantation for primary hepatic malignancy. Nevertheless, a small but significant palliation has been possible with this approach, even in patients who eventually died of recurrence. Two patients with incidental malignancies in their excised livers apparently have been cured. Further gains will be possible only with extremely discriminating selection of prospective recipients.
When orthotopic liver transplantation was first done on a human in 1963, it was thought that primary liver malignancy which could not be treated with conventional techniques of subtotal liver resection would be an unequivocal indication for total hepatectomy and liver replacement (orthotopic liver transplantation) [1]. However, the high recurrence rate of the original liver malignancies partly invalidated this expectation [1–3]. A recent review of our 24 orthotopic liver transplantations performed in the presence of primary liver malignancy has provided a more definitive idea of what can be achieved with this approach.
Case Material
During the 18-year period from March, 1963 to September, 1980, a total of 184 patients underwent orthotopic liver transplantation. Twenty-four of the recipients had transplantation in the presence of a primary liver malignancy, and they were divided into 3 groups for analysis. Group I consisted of 3 patients who had liver replacement for what was thought to be end-stage non-neoplastic liver disease, but who were found to have an incidental primary liver malignancy. Group II consisted of 8 patients who received liver replacement because of primary liver malignancy, but who died within 30 days from early postoperative complications. Group III consisted of 13 patients who received liver replacement because of liver malignancy and who survived more than 2 months. In this group, it was possible to make meaningful observations about tumor recurrence.
Age, sex, diagnosis, survival, tumor recurrence, and main causes of death of all 3 groups of patients are listed in Tables 1, 2, and 3. The patients’ ages ranged from 19 months to 68 years (mean, 31 years). There were 12 males and 12 females. Fifteen of the 24 patients had hepatoma, 5 had carcinoma of intrahepatic bile ducts (Klatskin-type tumor), and one each had cholangiocarcinoma, hepatoblastoma, hemangioendothelial sarcoma, and sarcoma of undetermined cell type.
Table 1.
Fate of 3 patients who received liver replacement primarily for end-stage benign liver disease, but who were found to have primary liver malignancy as well (Group I).
| Patient/age (yr)/sex | Primary indication for transplant | Incidentally found liver malignancy | Survival | Tumor recurrence | Main cause of death |
|---|---|---|---|---|---|
| OT 33 3/F | Biliary atresia | Hepatoma | Alive more than 11 years | No | – |
| OT 80 7/F | Biliary atresia | Hepatoma | Postoperative death | – | Cardiac arrest |
| OT 142 5/F | Alpha-1 antitrypsin deficiency | Hepatoblastoma | Alive at 3 years | No | – |
Table 2.
Fate of 8 patients who received liver replacement for indication of primary liver malignancy, but did not survive long enough after operation to permit observation of the course of the malignancy (Group II).
| Patient/age (yr)/sex | Diagnosis | Survival | Metastases at autopsy and location | Main cause of death |
|---|---|---|---|---|
| OT 2 48/M | Hepatoma and cirrhosis | 21 days | No | Pulmonary emboli, sepsis |
| OT 3 68/M | Adenocarcinoma of bile duct (Klatskin tumor) | 8 days | No | Sepsis, pulmonary emboli, gastrointestinal bleeding |
| OT 4 52/M | Cholangiocarcinoma | 5 days | Bone, lung, kidney, lymph nodes | Pulmonary emboli, hepatic failure, pulmonary edema |
| OT 5 29/F | Hepatoma | 24 days | No | Sepsis, bile peritonitis, hepatic failure |
| OT 6 29/M | Hepatoma | 7 days | No | Hepatic failure, sepsis |
| OT 7 24/F | Hepatoma | 17 days | No | Pneumonitis |
| OT 25 45/M | Hepatoma | 29 days | No | Bile peritonitis, sepsis, hepatic failure |
| OT 79 60/M | Carcinoma of bile duct (Klatskin tumor) | 19 days | No | Hepatic failure due to biliary obstruction |
Table 3.
Fate of 13 patients who received liver replacement for indication of primary liver malignancy and who lived long enough to permit observations about the course of the malignancy (Group III).
| Patient/age (yr)/sex | Diagnosis | Recurrence first detected | Location of first recurrence | Metastases of homograft | Treatment of recurrent tumor | Organs ultimately involved | Time of death | Main causes of death |
|---|---|---|---|---|---|---|---|---|
| OT 8 1 7/12/F | Hepatoma | 3 mos | Lungs | Yes | Chemotherapy, radiation, debulking of abdominal mass | Brains, lungs, liver, other abdominal organs | 14 mos | Carcinomatosis |
| OT 14 16/F | Hepatoma | 13 mos | Diaphragm, liver, retroperitoneal space | Yes | Diaphragm, retroperitoneal space, liver, pancreas | 15 mos | Carcinomatosis: infection and duodenal fistula after retransplantation | |
| OT 15 43/M | Hepatoma, cirrhosis | 2 mos | Lungs | Yes | Lungs, liver, diaphragm | 12 mos | Carcinomatosis | |
| OT 23 15/M | Hepatoma | 1 mos | Lungs | Yes | Brain, lungs, liver, retroperitoneal space | 5 mos | Carcinomatosis | |
| OT 26 11/F | Biliary atresia, hepatoma | 3 mos | Lungs | No | Lung | 3 mos | Gastrointestinal hemorrhage, intra-abdominal sepsis | |
| OT 45 53/M | Hemangioendothelial sarcoma | 2½ mos | Lungs, liver | Yes | Brain, lungs, liver, spleen, pericardium, peritoneum, stomach, pancreas, kidney | 3 mos | Sarcomatosis | |
| OT 78 48/M | Adenocarcinoma of bile duct (Klatskin tumor) | 21 mos | Liver, bile duct | Yes | Liver, bile duct at reoperation. No autopsy | 25 mos | Tumor recurrence | |
| OT 90 41/M | Cholangiocarcinoma of bile duct (Klatskin tumor) | 42 mos | Bile duct | Yes | Radiation | Bile duct, liver, duodenum at reoperation. No autopsy | 54 mos | Tumor recurrence |
| OT 102 51/F | Cholangiocarcinoma of bile duct (Klatskin tumor) | No | No | No | None | 3 mos | Systemic candidiasis with brain abscess | |
| OT 111 9/F | Congenital tyrosinemia, hepatoma, cirrhosis | No | No | No | Microscopic metastasis in the lung and para-aortic lymph nodes at autopsy | 3 mos | Portal vein thrombosis, pyelophlebitis, cardiac arrest during attempted 2nd transplant | |
| OT 114 27/F | Sarcoma (undetermined cell type) of liver invading diaphragm, metastasis to right lung and peritoneum | Residual tumor at transplant | No | Grossly fine intra-abdominal and pulmonary metastases at operation which have been quiescent greater than 4 years | Alive 52 mos | |||
| OT 121 32/F | Hepatoma | No | No | No | None | 6 mos | Brain injury after falling from bed, inanition, and pneumonia | |
| OT 172 24/M | Hepatoma | No | No | No | None | Alive 10 mos |
Results
Group I
Two (OT 33, OT 142) of the 3 recipients who had concomitant incidental primary liver malignancy are still alive and well without any evidence of tumor recurrence, one (OT 142) after 3 years and the other (OT 33) after more than 11 years. The third patient (OT 80) died from cardiac arrest just after the transplant operation; careful postmortem search for residual tumor failed to show any spread of tumor outside the liver (Table 1).
Group II
These 8 patients (liver replacement for known primary liver malignancy but with death during the first 30 postoperative days) were not suitable for assessment of tumor recurrence, but their postmortem examinations were used to determine residual tumor unknowingly left after total hepatectomy. Preoperatively, all 8 were thought to be free of extrahepatic tumors. At autopsy, only 1 patient (OT4) had residual neoplasm. This patient survived only 5 days after liver replacement and the postmortem examination revealed cholangiocarcinoma in the lung, vertebra, kidney, and some abdominal lymph nodes. The remaining 7 patients were tumor-free insofar as this could be determined from complete postmortem examination (Table 2). Thus, screening for candidacy had been grossly accurate in 87.5% of the cases with only 1 error out of 8 cases.
Group III
Thirteen patients had liver replacement for known primary liver malignancy and survived after transplantation for more than 2 months. Ten patients had recurrence of the original tumor and 7 of them died of the malignancy (Table 3). An eighth patient developed a single pulmonary metastasis, but her death after 3 months was from infection and gastrointestinal hemorrhage. A ninth patient who originally had congenital tyrosinemia and hepatoma died of portal vein thrombosis after 3 months. She had metastases in the lung and abdominal lymph nodes. The tenth patient represented an extraordinary example of tumor quiescence or even involution. The primary liver tumor was removed at transplantation, but there were fine miliary metastases on the pleura and peritoneum. She is still well after more than 4 years. The tumor was examined by pathologists at the University of Colorado, St. Mary’s Hospital and Medical School (London), the Armed Forces Institute of Pathology (District of Columbia), and the University of Southern California. All agreed that the tumor was malignant and the majority opinion was sarcoma of unknown subclassification. Minority opinions were sclerosing cholangiocarcinoma, sclerosing angiosarcoma, and mixed tumor of the liver.
The timing and location of the recurrences in these 10 patients are shown in Table 3. Usually, metastases were evident within a few months. The most commonly involved organ was the liver homograft (7 patients), followed by the lung and brain (3 patients each). Recurrences were seen with all of the tumor types. “Cures” were obtained only with hepatoma and hepatoblastoma, but long survival with known metastases was seen after treatment of bile duct carcinoma and a sarcoma.
Two of the 3 patients who did not develop metastases died after 3 and 6 months, respectively. Their follow-ups may have been too short for microscopic recurrences to become evident. The third patient is living after 10 months (Table 3).
The 9 patients who died from early postoperative complications within a month accounted for an overall operative mortality of 37.5%. One of these patients was from Group I and the other 8 from Group II. The 2 remaining patients from Group I are still alive after 3 and 11 years.
The 7 patients who died from metastases were all in Group III. Long survival (maximum, 52 months) was sporadically seen even after recurrence was first known.
The only patients thought to be definitely free of tumor are the 2 long survivors of Group I who had incidental tumors. It is as yet too early (10 months post-transplantation) for more than a hopeful prognosis in 1 Group III patient who had a hepatoma.
Actuarial survival curves were constructed of the total group of 24 recipients and of 15 patients who survived more than 1 month after transplantation (Fig. 1). Excluding the early deaths, the 15 who survived had 1-, 2-, and 3-year survival of 53%, 38%, and 29%, respectively.
Fig. 1.
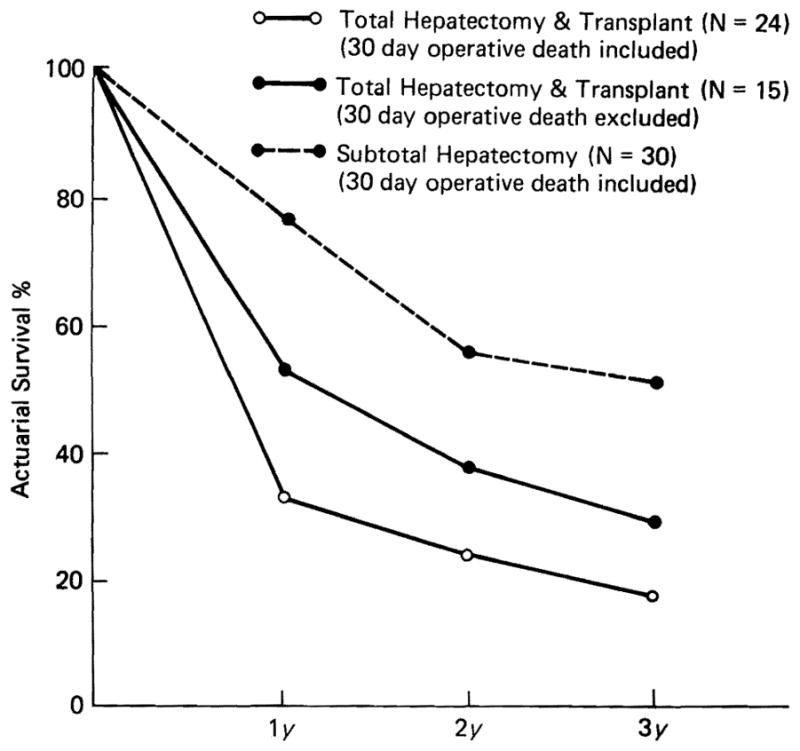
Actuarial survival of 24 patients with primary liver malignancy treated by total hepatectomy and transplant (open circle with solid line), and of 15 patients excluding operative deaths (closed circle with solid line) in comparison to the survival of 30 patients with primary liver malignancy treated by subtotal hepatectomy (closed circle with broken line).
Discussion
These results were compared to those of 30 patients with primary liver malignancy treated with subtotal hepatectomy (20 had right trisegmentectomy) during the same period. The 1-month mortality was 6.7% in the subtotal hepatectomy group, and the survival (uncorrected for operative mortality as was done in the transplant series) was 77%, 56%, and 52% at 1, 2 and 3 years, respectively (Fig. 1).
Thus, the salvage in the transplantation series was distinctly inferior to that in patients who had conventional subtotal hepatic resection, and the only definite cures were in the 2 patients with incidental hepatic malignancies. A third patient has had quiescent tumor for more than 4 years.
Nevertheless, an unduly pessimistic attitude about transplantation for malignancy is not fully warranted. A significant, although small, salvage was possible in those recipients who survived operation despite advanced stages of malignancy usually present. This was particularly striking in the handful of patients who lived for meaningful periods in spite of eventually lethal recurrences. Most of their postoperative lives were spent outside the hospital.
Calne and Williams have maintained an optimistic attitude for primary hepatic malignancy [4]. Their position is defensible, provided there is extremely discriminating case selection. To us there is no strong reason at present to consider any of the categories of liver tumors to be either especially advantageous or disadvantageous in the consideration of candidacy. A persuasive case can be made for treating intrahepatic duct cell carcinomas (Klatskin tumors) with alternative palliative techniques such as U-tube drainage and radiation [5].
Acknowledgments
Supported by research projects from the Veterans Administration; by U.S. Public Health Service Grant Nos. AM 17260 and AM-07772; and by Grant Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
References
- 1.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: W.B. Saunders Co; 1969. pp. 1–553. [Google Scholar]
- 2.Starzl TE, Porter KA, Putnum CW, Schroter GPJ, Halgrimson CG, Weil R, III, Hoelscher M, Reid HAS. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet. 1976;142:487. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schroter GPJ, Porter KA, Weil R., III Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375. [PMC free article] [PubMed] [Google Scholar]
- 4.Calne RY, Williams R. Liver transplantation. Curr Probl Surg. 1978;16:1. doi: 10.1016/s0011-3840(79)80004-0. [DOI] [PubMed] [Google Scholar]
- 5.Terblanche J, Koep LJ, Starzl TE. Liver transplantation. Med Clin North Am. 1979;63:507. doi: 10.1016/s0025-7125(16)31684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


