Abstract
Water transport by the Na+ −K+ −2Cl− cotransporter (NKCC1) was studied in confluent cultures of pigmented epithelial (PE) cells from the ciliary body of the fetal human eye. Interdependence among water, Na+ and Cl− fluxes mediated by NKCC1 was inferred from changes in cell water volume, monitored by intracellular self-quenching of the fluorescent dye calcein. Isosmotic removal of external Cl− or Na+ caused a rapid efflux of water from the cells, which was inhibited by bumetanide (10 μm). When returned to the control solution there was a rapid water influx that required the simultaneous presence of external Na+ and Cl−. The water influx could proceed uphill, against a transmembrane osmotic gradient, suggesting that energy contained in the ion fluxes can be transferred to the water flux. The influx of water induced by changes in external [Cl−] saturated in a sigmoidal fashion with a Km of 60 mm, while that induced by changes in external [Na+] followed first order kinetics with a Km of about 40 mm. These parameters are consistent with ion transport mediated by NKCC1. Our findings support a previous investigation, in which we showed water transport by NKCC1 to be a result of a balance between ionic and osmotic gradients. The coupling between salt and water transport in NKCC1 represents a novel aspect of cellular water homeostasis where cells can change their volume independently of the direction of an osmotic gradient across the membrane. This has relevance for both epithelial and symmetrical cells.
Introduction
There is general agreement that water transport across cell membranes occurs not only through aquaporins and the lipid bilayer, but also via some cotransporters and uniporters (Agre et al. 2004; King et al. 2004). In cotransporters and uniporters water transport has a number of features that distinguishes it from osmotic water flow through aquaporins and lipid bilayers. For example, water fluxes are coupled to the substrate fluxes in a given ratio, and energy for the water transport can be derived from that of the substrate transport. Accordingly, water transport by cotransporters and uniporters can occur in the absence of, and even against, transmembrane osmotic gradients, i.e. the water transport can proceed thermodynamically uphill, energized by a downhill substrate flux (for a review, see Zeuthen, 2010). The precise molecular mechanism of the coupling between water and solute fluxes is unknown. Conventional unstirred layer effects in the external solutions and/or the cytosol can be ruled out because the diffusion coefficients in these compartments are too high to support significant concentration gradients (Zeuthen et al. 2002; Charron et al. 2006; Naftalin, 2008). Alternatively, it has been suggested that the coupling between water and substrates is an intrinsic property of some carrier molecules (Zeuthen & Stein, 1994; Naftalin, 2008).
Among cotransporter proteins, Na+ −K+ −2Cl− cotransporters (NKCCs) are known to play pivotal roles in cell water volume control in symmetrical and polarized cells (Russell, 2000; Alvarez-Leefmans, 2009). They are also important in the regulation of fluid secretion in epithelial cells (Russell, 2000; Zeuthen, 2010). In a previous study we investigated water transport mediated by isoform 1 of the Na+ −K+ −2Cl− cotransporters (NKCC1), in response to osmotic gradients (Hamann et al. 2005). The osmotic water transport had several properties that linked it to possible conformational changes in the protein: it saturated at osmotic gradients higher than 100 mosmol l−1, it exhibited high Arrhenius activation energy (21 kcal mol−1), it depended on the cotransport of Na+ and Cl−, and it was inhibited by bumetanide (10 μm). In the present study we address the mechanisms of water transport mediated by NKCC1 and test the hypothesis that water transport can be induced by ion fluxes in the absence of transmembrane osmotic gradients or in the presence of adverse osmotic gradients that would require uphill water transport. Specifically, we used cultured pigmented epithelial (PE) cells from the ciliary body of the fetal human eye to address the question of whether Cl− (or Na+) transport mediated by NKCC1 leads to concomitant water transport. The results show that ion fluxes mediated by NKCC1 lead to water fluxes that can proceed even in the direction opposite to that expected for passive osmotic water flow. The evidence presented is incompatible with simple osmotic transport in an aqueous pore, where water transport depends entirely on the osmotic gradient, does not saturate, and has low activation energy.
Methods
The methods were essentially the same as those described in our previous works (Hamann et al. 2002, 2003, 2005). Briefly, frozen stocks of cultures of human PE cells (Von Brauchitsch & Crook, 1993) were thawed and plated in eight-well chambers having square cover-glasses, each having a surface area of 0.81 cm2. PE cells were grown to confluence in culture medium (37°C, 5% CO2) supplemented with 15% fetal calf serum. Confluent layers of PE cells in their fifth to eighth passages were used for experiments. In these cultures the basolateral membrane expressing NKCC1 faces upwards (Layne et al. 2001). The frozen cultures of PE cells were kindly donated by Dr. R. B. Crook, University of California, San Francisco, USA. All protocols followed the Declaration of Helsinki.
Chambers with confluent layers of PE cells were mounted on the stage of an inverted epifluorescence microscope (Nikon, Diaphot 300), equipped with a 40×, N.A. 1.3 oil immersion Fluor objective (Nikon). Only the solution bathing the upper surface of the cell layer was changed (Fig. 1). At maximal flow rates (230 μl s−1), the superfusion solution was exchanged 90% in 5 s at the level of the cells. Changes in epithelial cell volume were measured using the fluorescent dye calcein (Crowe et al. 1995; Hamann et al. 2002). To this end, cells were incubated at room temperature in control solutions containing 1–5 μm calcein-AM (Molecular Probes/Invitrogen). The latter ester is cleaved intracellularly by esterases yielding the poorly membrane-permeant fluorescent dye calcein (free acid) that accumulates intracellularly until reaching self-quenching concentrations, which in free solutions are ≥4 mm (Hamann et al. 2002). After 40–60 min, the loading solution was washed out for 60 min before starting measurements of cell water volume.
Figure 1. Basic experimental set-up used to measure water transport in cultured pigmented ciliary epithelial cells.
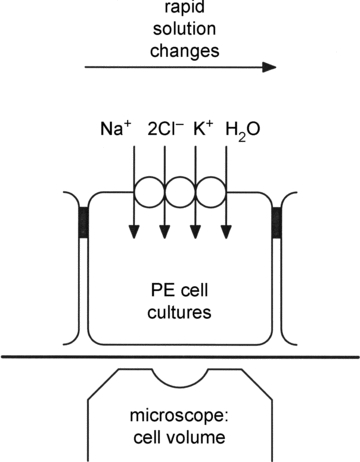
The basolateral membranes of cultured pigmented epithelial (PE) cells from the ciliary body of the eye face upwards, and express isoform 1 of the Na+ −K+ −2Cl− cotransporter (NKCC1). The water transport properties of the basolateral membranes are derived from the initial rates of change in cell water volume produced by rapid changes in ion concentrations of the bathing solution, at constant osmolarity, or by changing the external osmolarity (e.g. with mannitol). At the beginning of each experiment the cells are loaded with the fluorescent dye calcein; changes in cell volume are derived from self-quenching of this fluorophore monitored though the microscope's objective lens.
The technique for measuring cell water volume changes using intracellular calcein self-quenching has also been described in detail in previous publications (Hamann et al. 2002, 2005). Drift in the fluorescence signals caused by cell volume-independent reduction in fluorescence intensity (e.g. dye leakage) was corrected in each experiment (Hamann et al. 2005). The relative background fluorescence caused by the fraction of intracellular calcein insensitive to changes in external osmolarity (Alvarez-Leefmans et al. 1995) was determined in each experiment using hyposmotic and hyperosmotic calibration solutions of known osmolarities (Hamann et al. 2005). The change in cell water volume was calculated using the following equation (Alvarez-Leefmans et al. 1995):
| (1) |
where Vt is the cell water volume at time t, V0 is Vt at t = 0, Ft is the drift-corrected fluorescence at time t, F0 is the drift-corrected steady-state fluorescence before and after a given experimental challenge, and fb is the background fluorescence, that is the relative intracellular calcein fluorescence that is insensitive to changes in external osmolarity. The value of fb was about 0.7. The net water flux per cm2 of epithelium was calculated from the following equation:
| (2) |
where dVt/Vo dt is the initial rate of change in cell water volume, estimated from the first 5–10 s of the transient elicited by changes in the composition of the external bathing solution; and ho is the volume to surface ratio, equivalent to the anatomical cell height at time zero, measured as 7.8 μm for PE cells using confocal depth scanning stacks obtained from cells loaded with calcein. The changes in cell water volume were linear during the first 10 s. Thus the initial rates of change in cell water volume were calculated by regression analysis during this period of time. To estimate the water permeability (Lp), cells were exposed to predefined osmotic gradients (Δπ), and Lp was derived from  .
.
The control solution contained (in mm): 118 Na+, 6 K+, 1.2 Mg2+, 2.6 Ca2+, 118 Cl−, 1 PO42−, 25 Hepes, 5.5 glucose, and 29 mannitol. The osmolarity of the control solution was about 300 mosmol l−1. Anisosmotic solutions used to determine Lp were prepared by mannitol removal or addition to the control solution. The isosmotic Cl−-free solution was made by replacing Cl− with gluconate in the control solution, on a mole-for-mole basis. The isosmotic Na+-free solution was prepared by mole-for-mole replacement of Na+ with N-methyl-d-glucamine (NMDG). The isosmotic K+-free solution was prepared by replacement of K+ with mannitol. These solutions are referred to as isosmotic because their osmolarity was the same as that in the control solution. All salts used were of analytical quality and the osmolarities were measured by means of a vapor pressure osmometer (model 5520, Wescor Biomedical Systems, Logan, UT, USA). All solutions were titrated to pH 7.40 with NaOH or, in the case of the Na+-free solutions, with HCl. In experiments using bumetanide, the cells were primed by superfusing with control solution containing 10 μm bumetanide for at least 1 min prior to testing other experimental solutions. Bumetanide was dissolved directly into the solutions. In experiments involving the use of calyculin-A, cells were primed for 5 min with control solution containing 10 nm calyculin-A before testing other experimental solutions.
Results are expressed as means ±s.e.m. Comparisons were made using Student's t test for unpaired data. Differences were considered significant when P < 0.05. Unless otherwise stated, numbers in parentheses correspond to the number of groups of cells tested, usually more than 4. Michaelis–Menten and sigmoid curves were fitted using Origin software (OriginLab Corp., Northampton, MA, USA).
Results
Relationship between fluxes of water and Cl−
To test for a direct interaction between Cl− and water fluxes in the basolateral membrane of PE cells, Cl− was removed abruptly from the external solution by isosmotic replacement with gluconate ions. The isosmotic removal of Cl− from the extracellular solution was accompanied by a rapid cell shrinkage that began within 1 s of the solution change, and was completed in less than 10 s (Fig. 2, left trace). The initial rate of cell shrinkage was 1.07 ± 0.07% s−1 (n = 53). This is equivalent to an efflux of water of 8.3 ± 0.5 nl cm−2 s−1 given a cell height of 7.8 μm. When Cl− was returned to the bathing solution, there was a rapid influx of water at an initial rate of 7.7 ± 0.5 nl cm−2 s−1 (n = 52). To test the effect of inhibiting the NKCC1 transporter on the water fluxes, the isosmotic removal of external Cl− was done in the continued presence of bumetanide (10 μm) in both the control and the test solutions. Addition of bumetanide to the control solution produced only small volume changes; over a 60 s period of exposure to bumetanide the steady-state cell volume changed by less than 3% (not shown). However, bumetanide had a marked effect on the cell volume changes induced by isosmotic removal and addition of Cl−; the initial water efflux induced by external Cl− removal was inhibited by 45 ± 6% (n = 10), as determined in paired experiments. When Cl− was returned to the bathing solution in the continued presence of bumetanide, the initial rate of water influx was inhibited by 40 ± 4% (n = 10). A representative experiment is shown in Fig. 2 (right trace), and data compiled for all experiments are shown in Fig. 5.
Figure 2. Removal of external Cl− in isosmotic media produced bumetanide-sensitive shrinkage of pigmented ciliary epithelial cells.

Isosmotic replacement of Cl− with gluconate caused rapid cell shrinkage. Vt/Vo denotes relative cell water volume. Black bars above each trace indicate the time of exposure to the isosmotic Cl−-free solution (0 Cl−). Dashed vertical lines indicate onset of exposure to 0 Cl−. On returning to the Cl−-containing control solution cells recovered their initial volume. Right trace: the initial rate of cell shrinkage was decreased in the presence of 10 μm bumetanide.
Figure 5. Ion requirements and sensitivity to bumetanide and calyculin-A of net water fluxes across the basolateral membrane.

Compiled data were obtained from experiments like those illustrated in Figs 2, 3 and 4. Net water fluxes were calculated from the initial rates of change in cell water volume and from measurements in cell height using eqn (2). The water efflux on exposure to the isosmotic Cl−-free solution (Cl−-removal) was reduced significantly (P < 0.001) by exposure to 10 μm bumetanide (Cl−-removal (bum)). The net water influx measured on returning Cl− to the bath (Cl−-addition) was not significantly different from the net water efflux measured on removal of Cl−, but was reduced significantly (P < 0.001) by bumetanide (Cl−-addition (bum)). Similarly, the net water efflux induced on exposure to the isosmotic Na+-free solution (Na+-removal) was significantly (P < 0.001) reduced in the presence of bumetanide (Na+-removal (bum)). The water influx measured on returning Na+ to the bath (Na+-addition) was not significantly different from that obtained on removal of Na+; the water influx, however, was significantly (P < 0.001) reduced by bumetanide (Na+-addition (bum)). The largest net water fluxes observed were those elicited on isosmotic removal of external Cl− in cells treated with 10 nm calyculin-A (Cl−-removal (caly)), a phosphatase inhibitor that stimulates NKCC1. Calyculin-A caused a twofold increase that was statistically significant (P < 0.001).
Calyculin-A, a phosphatase inhibitor, stimulates cotransport mediated by NKCC1 in PE cells (Layne et al. 2001). The water efflux induced by isosmotic Cl− removal in PE cells which had been incubated with 10 nm calyculin-A doubled to 17.2 ± 2.7 nl cm−2 s−1 (an increase of 107 ± 33%, n = 5) with respect to control cells (Figs 3 and 5). After the initial shrinkage induced by Cl− removal, the calyculin-A treated cells exhibited a slow volume recovery which made the effect of Cl− readmission hard to evaluate.
Figure 3. Effect of calyculin-A on pigmented ciliary epithelial cell shrinkage produced by isosmotic removal of external Cl−.

Isosmotic replacement of Cl− with gluconate caused rapid cell shrinkage (left trace). The initial rate of cell shrinkage increased significantly in cells treated with 10 nm calyculin-A, a phosphatase inhibitor known to stimulate NKCC1. Labels and symbols as in Fig. 2.
To study the relation between the initial rate of water influx and the Cl− concentration in the bathing solution, the cells were first equilibrated for about 60 s in the isosmotic Cl−-free solution until their volume reached a new (smaller) steady-state value. Then, the influxes of water elicited upon brief exposure to solutions of different Cl− concentrations were monitored. These isosmotic solutions were prepared by replacing a fraction of the gluconate with Cl−. The Cl− concentrations of these solutions were (in mm): 29.5, 59, 88.5 and 118. Following exposure to each of these test solutions, the cells were returned to the Cl−-free solution. Plotting the initial rate of water influx for each pulse as a function of the external Cl− concentration yielded a sigmoidal curve with a Hill coefficient of 2.4 ± 0.6 and a half-maximal activation of 59.9 ± 11.1 mm of Cl− (Fig. 6A).
Figure 6. Net water influx as a function of external Cl− and Na+ concentrations.

A, pigmented ciliary epithelial cells were equilibrated with isosmotic Cl−-free solution (0 Cl−). Then, they were exposed to single pulses of isosmotic solutions each of which had a different Cl− concentration (29.5, 59, 88.5, or 118 mm). At the end of each exposure the cells were returned to the Cl−-free solution. Net water influx ( ) for each pulse was estimated from the initial rates of cell swelling using eqn (2). Each data point represents the mean ±s.e.m. of 6 experiments. Data were fitted by a sigmoid curve with a Hill coefficient of about 2. B, cells were equilibrated with isosmotic Na+-free solution (0 Na+) and exposed to pulses of isosmotic solutions of increasing Na+ concentrations: 28.1, 56.2, 84.3, or 112.4 mm. At the end of each exposure the cells were returned to the Na+-free solution. Net water influx (
) for each pulse was estimated from the initial rates of cell swelling using eqn (2). Each data point represents the mean ±s.e.m. of 6 experiments. Data were fitted by a sigmoid curve with a Hill coefficient of about 2. B, cells were equilibrated with isosmotic Na+-free solution (0 Na+) and exposed to pulses of isosmotic solutions of increasing Na+ concentrations: 28.1, 56.2, 84.3, or 112.4 mm. At the end of each exposure the cells were returned to the Na+-free solution. Net water influx ( ) for each pulse was estimated from the initial rates of cell swelling using eqn (2). Each data point represents the mean ±s.e.m. of 5 experiments. Data were fitted by a Michaelis–Menten equation with an apparent Km value of about 40 mm.
) for each pulse was estimated from the initial rates of cell swelling using eqn (2). Each data point represents the mean ±s.e.m. of 5 experiments. Data were fitted by a Michaelis–Menten equation with an apparent Km value of about 40 mm.
Effect of step changes in extracellular Na+ and K+ on water influx
The interaction between Na+ and water fluxes in the basolateral membrane of PE cells was studied by exposing the cells to pulses of Na+-free isosmotic solutions in which Na+ was replaced with NMDG on a mole-for-mole basis (Fig. 4). Exposure to the Na+-free isosmotic solution produced an abrupt cell shrinkage that began within 1 s of the solution change and was completed in less than 10 s. The initial rate of cell shrinkage was 0.96 ± 0.15% s−1 (n = 24), which is equivalent to an efflux of water of 7.5 ± 1.2 nl cm−2 s−1. In the continued presence of bumetanide (10 μm) in the control and test solutions, the initial rate of cell shrinkage induced by isosmotic Na+ removal was inhibited by 36 ± 8% (n = 9), as determined in paired experiments. When the Na+-containing control solution was readmitted to the bath, there was a rapid recovery from shrinkage at an initial rate of 0.79 ± 0.11% s−1 (n = 24), equivalent to an influx of water of 6.2 ± 0.9 nl cm−2 s−1. When Na+ was returned in the presence of bumetanide, the initial rate of water influx underlying the recovery from shrinkage was inhibited by 28 ± 5% (n = 9) as determined also in paired experiments. The compiled data for all the experiments is shown in Fig. 5.
Figure 4. Isosmotic removal of external Na+ produced bumetanide-sensitive shrinkage of pigmented ciliary epithelial cells.

Isosmotic replacement of Na+ with N-methyl-d-glucamine caused rapid cell shrinkage. Black bars above each trace indicate the time of exposure to the isosmotic Na+-free solution (0 Na+). Dashed vertical lines indicate onset of exposure to 0 Na+. On returning to the Na+-containing control solution, cells recovered their initial volume. Right trace: the initial rate of cell shrinkage was decreased in the presence of 10 μm bumetanide. Labels and symbols as in Fig. 2.
To determine the relation between water influx and the Na+ concentration in the bathing solution, tissues were first equilibrated with the Na+-free isosmotic solution for about 60 s. Influx of water as a function of external Na+ concentration was elicited by applying pulses with isosmotic solutions having different Na+ concentrations (in mm): 28.1, 56.2, 84.3, or 112.4. These solutions were prepared by mole-for-mole replacement of NMDG with Na+. At the end of each test solution pulse the bathing solution was returned to the Na+-free solution. The dependence of the rate of water influx on external the Na+ concentration follows Michaelis–Menten kinetics with an apparent Km value of 43.8 ± 8.7 mm (Fig. 6B). This is probably an overestimate; if the bumetanide-insensitive component of the water influx is taken into account (Fig. 5), Km values of around 20 mm are obtained. A precise estimate of Km would require water fluxes elicited by small increases in the Na+ concentration, in the presence and in the absence of bumetanide. However, such experiments were not feasible due to limitations imposed by the signal to noise ratio of the method.
Attempts were made to determine the interaction between K+ and water fluxes. In two experiments, which had particularly low noise levels, isosmolar replacement of external K+ with mannitol resulted in an abrupt cell shrinkage that proceeded at initial rates of 0.17 and 0.22% s−1 equivalent to water effluxes of 1.3 and 1.7 nl cm−2 s−1, respectively. In most experiments of this type, however, cell volume changes induced by K+ removal were too small to be detected. This is probably due to the fact that an epithelial sheet like PE behaves as an infinite source of K+, and it is really impossible to remove it from the extracellular solution. This complicates an in-depth investigation of K+ dependence of water and ion transport via NKCC1 in epithelial preparations. Clarification of the role of K+ for water transport via NKCC1 will require a more suitable preparation.
Transmembrane water movements in pigmented ciliary epithelial cells require the simultaneous presence of external Na+ and Cl−
The results presented so far show that isosmotic removal of Na+ or Cl− from the bathing solution induced efflux of water from the PE cells, and that upon readmission of Na+ or Cl−, water influx ensued (Figs 2, 3 and 4). If the transport of water is strictly coupled to the transport of both Na+ and Cl− through NKCC1, as hypothesized in the present study, it should be possible to block the influx of water caused by readmission of Cl− by simultaneously removing external Na+. Likewise, the influx of water caused by the return of Na+ should be blocked by simultaneous removal of external Cl−. As illustrated in Fig. 7A, isosmotic removal of external Cl− caused rapid cell shrinkage that reversed upon readmission of the Cl−-containing control solution. In the absence of external Na+, however, the volume recovery from shrinkage was blocked (n = 4). This indicates that external Na+ is required for the inward movement of water induced on readmission of external Cl−. Analogously, isosmotic removal of external Na+ caused rapid cell shrinkage that recovered on returning to the Na+-containing control solution. However, in the absence of external Cl− the Na+-induced volume recovery from shrinkage was blocked (n = 6), as shown in Fig. 7B. These experiments are consistent with the hypothesis that NKCC1 cotransports water, Na+ and Cl−; water transport can only take place if all of the substrates of the cotransporter are present in the solution.
Figure 7. Water transport in pigmented ciliary epithelial cells requires the simultaneous presence of external Cl− and Na+.

A, left trace: isosmotic removal of external Cl− (black bar, 0 Cl−) caused an immediate, rapid cell shrinkage that reversed on returning to the Cl−-containing bathing solution (vertical dashed line). Right trace: a second exposure to the 0 Cl− solution produced a similar cell shrinkage to that observed in the left trace. While the cells were shrunken, they were exposed to isosmotic Na+-free solution (black bar, 0 Na+) containing the control concentration of Cl−. In the absence of external Na+ there was no recovery from shrinkage (open arrow and dashed vertical line). Thus, on restoring the transmembrane Cl− gradient in the absence of external Na+ there is no cell water volume recovery. On returning to the control isosmotic solution the cells recovered their initial water volume. B, isosmotic removal of external Na+ (black bar, 0 Na+) caused immediate, rapid cell shrinkage that reversed on returning to the control isosmotic solution (left vertical dashed line). While the cells were shrunken, they were exposed to isosmotic Cl−-free solution (top black bar, 0 Cl−) containing the control Na+ concentration. In the absence of external Cl− there was no recovery from shrinkage even though the Na+ gradient had been restored (open arrow and dashed vertical line). On returning to the control isosmotic solution the cells recovered their initial water volume.
Cl− fluxes induce uphill water transport across the basolateral membrane of pigmented ciliary epithelial cells
When Cl− was returned to the external solution bathing PE cells that had been equilibrated in Cl−-free solutions, there was a rapid influx of water (e.g. Fig. 2, left trace, and arrow 1 in Fig. 8). Since the osmolarity of the bathing solution was kept constant, this water influx proceeds in the apparent absence of osmotic gradients across the membrane. To test if the Cl− induced influx of water flux could proceed against an osmotic gradient, mannitol (50 mm) was added simultaneously with Cl−. Under these conditions, the influx of water induced by Cl− competes with an adverse osmotic gradient generated by mannitol, which, by itself, would have induced an efflux of water (Fig. 8, arrows 2 and 3). However, when Cl− was re-admitted together with 50 mm mannitol, there was an influx of water of 4.5 ± 0.7 nl cm−2 s−1 (n = 6), which occurred against the transmembrane osmotic gradient (Fig. 8, arrow 3). This suggests that the free energy supplied by adding Cl− is transferred to power the uphill influx of water by NKCC1.
Figure 8. The Cl−-dependent influx of water in pigmented ciliary epithelial cells can proceed against an osmotic gradient.

Following equilibration in control solution, cells were exposed to isosmotic Cl−-free solution, which resulted in cell shrinkage. On readmission of the Cl−-containing control solution, cells recovered from shrinkage due to water influx (arrow 1 and dashed line). Addition of 50 mm mannitol to the control bathing solution (50 man), thereby rendering it about 17% hyperosmotic with respect to the control solution, resulted in osmotic shrinkage due to rapid cell water efflux (arrow 2 and vertical dashed line). On readmission of the control solution, cells recovered their initial water volume. However, when shrunken in isosmotic Cl−-free solution, and then exposed to the Cl−-containing control solution with 50 mm of mannitol added, cells increased their relative volume due to a rapid water influx (arrow 3 and dashed line). That is, water influx induced by concomitant Cl− influx proceeded uphill, against the osmotic gradient imposed by 50 mm mannitol. On returning to isosmotic Cl−-free solutions, cells went back to their initial (shrunken) volume, and on admission of the control solution, cells’ water volume recovered to its initial value.
The magnitude of the uphill influx of water is consistent with experiments where the additions of Cl− and mannitol are done separately. When Cl− was added the influx of water was 7.7 ± 0.5 nl cm−2 s−1 (n = 52), while the addition of 50 mm mannitol alone led to effluxes of 5.0 ± 0.6 nl cm−2 s−1 (n = 20); see below. The osmotic water efflux induced by mannitol can be divided about equally with 2.5 nl cm−2 s−1 via the NKCC1 pathway and 2.5 nl cm−2 s−1 via the parallel bumetanide-insensitive pathway (Fig. 9). Consequently, when 50 mm mannitol is added together with Cl−, there will be an osmotically induced water efflux of about 2.5 nl cm−2 s−1 via the bumetanide-insensitive pathway. This is close to the difference between the influx of 7.7 nl cm−2 s−1 observed when Cl− is added alone and the value of 4.5 nl cm−2 s−1 obtained when mannitol is added together with the Cl−.
Figure 9. Effect of bumetanide or changes in external ion composition on osmotic water permeability of the basolateral membrane of pigmented ciliary epithelial cells.

A, addition of mannitol to the control bathing solution (indicated by the vertical broken line and the black bar), produced cell shrinkage. The water permeability (LP) was derived from the initial rate of cell shrinkage measured within the first 5–10 s of the response, applying eqn (2). In the example shown, 50 mosmol l−1 of mannitol was added to the control bathing solutions during the time indicated by the black bar. Vt/V0 denotes relative cell water volume. B, effect of bumetanide or changes in ion composition on Lp measured by applying hyperosmotic mannitol challenges (open bars) or NaCl (hatched bars). Bumetanide (10 μm) reduced Lp to half its control value (Control + bum) in mannitol. In tissues equilibrated with isosmotic Na+-free solution (0 Na+), Lp was reduced to about one-third of the control value. A similar reduction in Lp was measured in tissues equilibrated with isosmotic Cl−-free solution (0 Cl−). If the transmembrane osmotic gradients were changed by hyperosmotic NaCl (hatched bars), the Lp values were about half those obtained using hyperosmotic mannitol challenges (Control, hatched bar). Bumetanide had no effect on Lp values determined using hyperosmotic NaCl (Control + bum, hatched bar). C, diagram illustrating the two water pathways proposed to be present in the basolateral membrane of PE cells.
The osmotic water permeability of the basolateral membrane of pigmented ciliary epithelial cells is inhibited by bumetanide and requires the simultaneous presence of external Na+ and Cl−
To determine the water permeability, Lp, of the basolateral membrane, PE cells were transiently exposed to a control solution to which mannitol (50 mosmol l−1) was added (Fig. 9). The Lp derived from the initial rate of the mannitol-induced shrinkage was (1.51 ± 0.12) × 10−4 cm s−1 (osmol l−1)−1 (n = 89). In the presence of bumetanide, at concentrations that selectively block NKCCs (10 μm), Lp was reduced to (0.81 ± 0.08) × 10−4 cm s−1 (osmol l−1)−1 (n = 8), which is about half its control value. To determine the Na+ dependence of Lp, the cells were primed for at least 1 min with solutions in which Na+ had been replaced with NMDG on a mole-for-mole basis. To measure Lp under these conditions, mannitol (50 mosmol l−1) was added to the Na+-free solution. In the virtual absence of external Na+, Lp was reduced to (0.55 ± 0.04) × 10−4 cm s−1 (osmol l−1)−1 (n = 6), a value that is about 3 times lower than that measured under control conditions. Analogously, to test the Cl− dependence of the Lp, cells were primed with solutions in which Cl− had been replaced with gluconate, and mannitol was added in the virtual absence of external Cl−. In the Cl−-free solution, Lp was (0.62 ± 0.04) × 10−4 cm s−1 (osmol l−1)−1 (n = 6), a value that is about two and a half times lower than that measured in control solutions. These results show that roughly half of the Lp of the basolateral membrane of PE cells is inhibited by bumetanide, and requires the simultaneous presence of Na+ and Cl−, suggesting that NKCC1 transports water in response to the transmembrane osmotic gradient imposed by mannitol. The effects of ion substitutions on Lp were more evident than the effect of bumetanide. The results suggest that not all the hydraulic permeability of the basolateral membrane is mediated by NKCC1 and that 10 μm bumetanide might not be a sufficiently high concentration to completely inhibit NKCC1. The latter is in agreement with what has been observed in PE cells (Hamann et al. 2005) and other preparations (Russell, 2000; Rocha-González et al. 2008). We conclude that the Lp of the basolateral membrane of PE cells results from two permeabilities of similar magnitude working in parallel, one via NKCC1 and the other via the lipid bilayer and/or proteins other than NKCCs (Fig. 9C).
Interestingly, NaCl was less effective than mannitol in inducing osmotic water movements across the basolateral membrane of PE cells. The Lp derived from the initial rate of shrinkage upon exposure to hyperosmolar NaCl (prepared by adding 37.5 or 50 mm NaCl to the control solution, equivalent to osmotic gradients of 75 and 100 mosmol l−1) was (0.83 ± 0.09) × 10−4 cm s−1 (osmol l−1)−1 (n = 26). This Lp is half that obtained with mannitol but similar to that observed with mannitol in the presence of bumetanide (Fig. 9B). Further, the Lp determined by hyperosmotic pulses of NaCl was not affected by bumetanide; in the presence of 10 μm bumetanide Lp was (0.78 ± 0.06) × 10−4 cm s−1 (osmol l−1)−1 (n = 9). This suggests that hyperosmolar NaCl does not give rise to net water transport mediated by NKCC1, but only via a parallel bumetanide-insensitive pathway (Fig. 9C). The low capacity of NaCl to drive water via NKCC1 suggests a balance between osmotic and chemical driving forces in this protein; the osmotic driving force for water efflux caused by the addition of NaCl is matched by the inward driving force arising from the coupling between Na+, Cl− and water through NKCC1.
Discussion
The cotransporter NKCC1 is expressed in the basolateral membrane of PE cells from the ciliary body of the human eye. A previous study aimed at characterizing the transport properties of NKCC1 in cultured PE cells showed that NKCC1 transported water in response to osmotic gradients (Hamann et al. 2005). The water transport mediated by NKCC1 exhibits several properties that are different from those of conventional channel-mediated, osmotic transport: (i) water transport saturates at osmotic gradients of 200 mosmol l−1, being half-maximal at around 100 mosmol l−1; (ii) the activation energy is higher than that for aqueous pores (21 kcal mol−1versus about 5 kcal mol−1), and (iii) the osmotic water transport is inhibited by bumetanide at concentrations compatible with specificity for Na+ −K+ −2Cl− cotransporters (10 μm). In the present study we confirm and extend our previous results by demonstrating a tight coupling between water and ion fluxes mediated by NKCC1. We show that water transport mediated by NKCC1 occurs in isosmotic media, i.e. in the absence of transmembrane osmotic gradients, and that the downhill flux of ions can energize uphill transport of water. Further, combining the previous with the new data we can estimate how many water molecules per turnover cycle are cotransported by NKCC1 with 1 Na+, 1 K+, and 2 Cl− ions, as discussed below.
Coupling of water and ion fluxes in NKCC1
Rapid changes in external Cl− or Na+ concentrations at constant osmolarity gave rise to immediate water fluxes. The rapid onset of the isosmotic water fluxes suggests a direct link between the transport of water and that of Na+ and Cl− through a transport mechanism sharing kinetic properties and ion requirements characteristic of transport mediated by NKCC1. Further, experiments with pharmacological inhibitors and stimulators of NKCC1 support the notion that this transport molecule cotransports ions and water. The evidence backing up this hypothesis is discussed below.
First, there was a close temporal link between water fluxes and Cl− fluxes. Moreover, the Cl−-induced water fluxes were inhibited by 10 μm bumetanide (Figs 2 and 5) and enhanced by calyculin-A (Figs 3 and 5), a phosphatase 1 inhibitor that promotes phosphorylation and activation of NKCC1 (Lytle & Forbush, 1992). This is consistent with other observations showing that calyculin-A nearly doubles the activity of NKCC1 in PE cells (Layne et al. 2001). Further, the recovery from shrinkage due to water influx that ensued on restoring the external Cl− concentration was blocked in the absence of external Na+ (Fig. 7A), a strong indication of a direct link between Cl−, water and Na+ movements. The relation between water fluxes and external Cl− concentration had a sigmoid shape (Fig. 6A); the water flux saturated at Cl− concentrations of about 120 mm and had a Hill coefficient of about 2. This sigmoid relation is the one expected if water is transported together with two Cl− ions per NKCC1 turnover cycle. Further, the Km for Cl− was about 60 mm, a value that falls within the range found for NKCC1 mediated ion transport (Isenring et al. 1998; Diecke et al. 2005). It is unlikely that our measurements were influenced by other Cl− uptake transporters such as Na+-independent anion exchangers; the experiments were performed with HCO3−-free solutions buffered with Hepes.
The effects of changing the external Na+ concentration on water fluxes also point to a direct link between water and ion transport mediated by NKCC1; step changes in external Na+ concentrations gave rise to immediate water fluxes, which were inhibited by bumetanide (Figs 4 and 5). Moreover, the recovery from shrinkage due to the water influx that ensued on restoring the external Na+ concentration did not occur in the absence of external Cl− (Fig. 7B). Likewise, the magnitude of the water fluxes saturated with increasing external Na+ concentration and exhibited first order Michaelis–Menten kinetics (Fig. 6B). This suggests that water is transported together with 1 Na+ per turnover cycle of NKCC1. The apparent Km for the Na+-dependent water transport was about 40 mm, which falls within the range determined for Na+ transport mediated by NKCC1 (Isenring et al. 1998; Diecke et al. 2005).
In sum, water influx underlying volume recovery from isosmotic shrinkage requires the simultaneous presence of external Na+ and Cl−, and is inhibited by bumetanide, indicating that the water flux is tightly linked to NKCC1 transport activity.
Water transport mediated by NKCC1 can occur in the absence of osmotic gradients or even against the direction of an imposed osmotic gradient
As discussed above, isosmotic addition of external Cl− or Na+ to cells that had been depleted of these ions was associated with an immediate water influx. Importantly, these water fluxes took place in the absence of any apparent transmembrane osmotic driving forces; the osmolarities of the bathing solutions were identical and it is reasonable to assume that the intracellular osmolarity was similar to that of the extracellular solutions (Figs 2, 4, and 5). It is important to emphasize that the water fluxes were determined within the first 10 s after changes in external ions. Within this relatively short time period, the intracellular osmolarity can be assumed to be virtually unchanged, as discussed below. The influence of osmotic forces on water flow was addressed in another series of experiments demonstrating that net water influx induced by ions transported by NKCC1 occurred in the face of adverse osmotic gradients. Figure 8 shows an example of this uphill water flux; when Cl− was returned to the bathing solution together with mannitol (50 mosmol l−1), the influx of water induced by Cl− proceeded in the direction opposite to that of the osmotic gradient, indicating that water was actively transported. These observations suggest that energy stored in the sum of the chemical potential gradients of Na+, K+ and Cl− can be transferred to drive the uphill movement of water by a mechanism closely associated with the NKCC1 protein.
It is possible to give a crude quantitative estimate of the coupling between water and ions in NKCC1. The Lp assessed using NaCl was similar to that determined in cells in which NKCC1 was inhibited with bumetanide or by equilibration with Na+ or Cl−-free solutions (Fig. 9). In other words, hyperosmolar pulses of NaCl do not give rise to significant net water fluxes through NKCC1. This shows that for NKCC1 the outward osmotic driving force derived from the hyperosmolar pulse of NaCl is balanced by the inward driving force associated with the increased external Na+ and Cl− concentrations. From this balance it has been estimated that 570 water molecules are coupled to the transport of the 4 ions equivalent to about 145 water molecules per ion (Zeuthen, 2010). The magnitude of this coupling resembles that estimated for the K+–Cl− cotransporter isosform expressed in the choroid plexus from Necturus maculosus, where 500 water molecules are coupled to the transport of 1 K+ and 1 Cl− (Zeuthen, 1991a,b, 1994). Similar coupling ratios between water and substrate transport, in the range of 40 to 400 water molecules per turnover cycle, have been suggested for other secondary active transport mechanisms (Zeuthen, 2010).
NKCC1-dependent water transport does not arise from unstirred layer effects
It is unlikely that the coupling between Na+, K+, Cl− and water in NKCC1 arises from secondary effects such as solute accumulation in restricted areas adjacent to the cell membrane; the magnitudes of the ion fluxes are too small and the intracellular diffusion coefficients too high to give rise to significant changes in intracellular ion concentrations during the time course of the experiments. The rate of Na+, K+ and Cl− transport mediated by NKCC1 is estimated to be 8 × 10−12 mol cm−2 s−1 in PE cells (Hamann et al. 2005). Given a cell height of 8 μm and assuming a restricted intracellular free water volume of 30% (Pfeuffer et al. 1998), it can be calculated that the intracellular osmolarity changes by about 0.2 mosmol l−1 in 10 s. However, the initial rates of volume change observed on removal or re-addition of external Cl− or Na+ are about 1% s−1. To elicit volume changes of this order by osmosis given the measured Lp values would require a change in intracellular osmolarity of about 50 mosmol l−1 in less than 10 s. Clearly, the estimated changes in intracellular osmolarity are more than two orders of magnitude smaller than those that would be required to explain the observed initial rates of change in cell water volume. The osmotic effects of ion influx would be enhanced if the influx led to ion accumulation at areas adjacent to the inner face of the membrane, the so-called unstirred layer effects. However, the intracellular diffusion coefficients are too high to give rise to significant increases in osmolarity adjacent to the cytoplasmic side of the basolateral membrane during influx. It is generally accepted that intracellular diffusion coefficients are in the range of 20–50% of those in free solution, which are about 1.5 × 10−5 cm2 s−1 (Zeuthen et al. 2002, 2006, 2007; Charron et al. 2006; Naftalin, 2008). Given these values, it can be estimated that the osmolarity at the inner face of the basolateral membrane may increase by no more than 2 μm as a result of unstirred layer effects (Hamann et al. 2005).
Possible mechanism of water transport via cotransporters
The precise molecular mechanism by which water transport is mediated by cotransporters is not clear. Cotransporters adopt a number of different conformations during a transport cycle. A molecular model of water transport must link the state and distribution of water in each of these conformations with the overall water transport, both when this is driven by an applied osmotic gradient or when taking place in the absence of external osmotic driving forces.
Detailed measurements in the Na+-coupled cotransporters of glucose and of glutamate strongly suggest that each conformation is characterized by a distinct passive osmotic water permeability (for references see Zeuthen, 2010). Consequently, the passive water permeability of the cotransporter (Lp) during a transport cycle is the weighed average of the values for each conformation. For NKCC1, we found that the Lp in the absence of Na+ or Cl− was reduced to values similar to those found when the cotransporter was inhibited with bumetanide. This suggests that the absence of Na+ or Cl− locks the protein in a relatively water impermeable state.
The mechanism of water cotransport in the absence of external osmotic gradients remains to be elucidated, but it may be related to the way in which the cotransport proteins act as membrane-bound enzymes. Enzymes have aqueous cavities that enable substrates to reach the binding sites. During enzymatic activity the cavity closes and opens again to release the end-product. This lead to changes in both the size of the water-filled cavities and the amount of loosely bound surface water. The amount of water shifted between conformational changes lies in the range between 10 and 1320 molecules per turnover for both aqueous and membrane bound enzymes (Parsegian, 2002). Aqueous cavities with linear dimensions up to 50 Å have been demonstrated by high-resolution crystallography for several cotransporters and ATPases (reviewed in MacAulay et al. 2009 and Zeuthen, 2010). The number of water molecules in these cavities and that of the cotransported water molecules are comparable; the volume occupied by 500 water molecules is about 15,000 Å3, and given that the molecular weight of the NKCC1 core protein is about 135 kDa (Reshkin et al. 1993; Payne et al. 1995), this water would constitute less than 10% of the protein volume. Based on the above considerations, two models for mechanisms of water transport in cotransporters have been suggested. The hyperosmolar-cavity model is based on the osmotic forces that build inside the protein when the substrate leaves its binding site and moves freely in the cavity. If the separation between the outside solution and the cavity behaves as a semipermeable membrane, water will enter the cavity from the outside by osmosis and driven into the inside solution by the hydrostatic pressure in the cavity. The alternating-access model relates to the way in which the substrate gains access to a binding site in combination with occlusion of substrate and water (Zeuthen & Stein, 1994; Naftalin, 2008).
Physiological relevance of water cotransport mediated by NKCC1
The ability of NKCC1 to couple ion and water transport may play a direct role in salt and water secretion in epithelia. In secretory epithelia like that of glands, airway, and the cilliary body of the eye, NKCC1 and the Na+/K+-ATPase are localized at the basolateral membrane, i.e. the membrane across which water enters in these epithelial cells (Silva et al. 1977; Edelman et al. 1994; Crook et al. 2000; Gutiérrez et al. 2004; Walcott et al. 2005; Nakamoto et al. 2007). The low intracellular Na+ concentration maintained by the Na+/K+-ATPase generates favourable ion gradients for the inward cotransport of Na+, K+, 2Cl− and water by NKCC1. Thus, cotransport of salt and water by NKCC1 takes place in the same direction in which transepithelial secretion occurs (Fig. 10). In this model, 1 Na+, 1 K+ and 2 Cl− ions are cotransported into the cell together with 570 water molecules. Na+ is pumped back to the external fluid via the Na+/K+-ATPase, and K+ leaks out by electrodiffusion via channels. The result is the net entry of 2 Cl− and 570 water molecules across the basolateral membrane enabling water transport to proceed into the lumen by osmosis, across the apical membrane, which expresses aquaporins (Ma et al. 1999; Matsuzaki et al. 1999; Gresz et al. 2004; Gutiérrez et al. 2004). Water cotransport mediated by NKCC1 could account for as much as 80% of the water content in the final secretion (Zeuthen, 2010). Water transport by NKCC1 may also explain uphill water transport observed in secretory epithelia. In the present study we provide evidence that water transport mediated by NKCC1 can indeed occur against an osmotic gradient (Fig. 8). Given the estimated coupling ratio and typical values for intracellular and extracellular ion activities, it can be calculated that under physiological conditions NKCC1 is capable of transporting water against an osmotic gradient of the order of 100 mosmol l−1 (Zeuthen, 2010). This is consistent with the uphill water transport demonstrated in a variety of salivary glands (Ludwig, 1861; Imai et al. 1973; Nakahari et al. 1997; Murakami et al. 2006).
Figure 10. A molecular model of salt and water coupling in secretory epithelial cells.

In this model, the primary coupling between salt and water transport takes place in the NKCC1 protein located in the serosal (basolateral) membrane of the secretory epithelial cell. The secondary active import of Na+, K+, Cl− and H2O mediated by NKCC1 is energized by the Na+ gradient generated and maintained by the Na+/K+-ATPase. Na+ entering the cells via NKCC1 is pumped out by the Na+/K+-ATPase. K+ entering via NKCC1 leaks out through channels. Water and Cl− proceed through the cell. Water leaves passively across the luminal (apical) membrane, e.g. via aquaporins (AQP), whereas Cl− leave via channels (or transporters) in the apical membrane. Our data suggests that 570 water molecules and 2 Cl− are cotransported per cycle via NKCC1. Na+ ions are secreted paracellularly via leaky junctions. For simplicity other cellular or paracellular pathways are not shown.
Water transport mediated by NKCC1 may also be functionally relevant for dorsal root ganglion (DRG) neurons, symmetrical cells known to express NKCC1 abundantly (Alvarez-Leefmans et al. 1988; Sung et al. 2000; Funk et al. 2008). In the cell bodies of these sensory neurons, which are spherical and have no other process than a neurite, there is a tight link between Na+, K+, 2 Cl− cotransporter activity, water fluxes, steady-state intracellular Cl− concentration, and cell water volume (Rocha-González et al. 2008). Isosmotic removal of external Cl− resulted in intracellular Cl− depletion and immediate cell shrinkage. The extent of cell shrinkage could be entirely accounted for in terms of measured Cl− depletion. Upon readmission of the isosmotic control solution, water influx took place in isosmotic media, required the presence of external Na+ and K+, and was completely inhibited by 10 μm bumetanide. Interestingly, there was a component of the net Cl− influx that did not require external Na+, and that most likely occurs via channels. This Na+-independent component of the net Cl− influx did not result in changes in cell volume. Thus, all the net water flux was tightly linked to NKCC1 transport activity. It is tempting to think that the NKCC1-linked water fluxes in DRG neurons are mediated by water cotransport. However, the changes in cell volume upon removal and readmission of external Cl− were one order of magnitude slower than those occurring in PE cells (1% s−1versus 1% min−1), probably due to differences in kinetic regulation between the two cell types. In DRG neurons NKCC1 determines not only the higher than passive intracellular Cl− concentration characteristic of these sensory neurons but also the cell volume set point, through a negative feedback system in which intracellular Cl− regulates water and Cl− influx, thereby maintaining intracellular Cl− concentration and cell volume constant (Alvarez-Leefmans, 2009). Future experiments are needed to determine if the water fluxes in DRG cells and other neurons, characterized for having relatively small hydraulic permeability, are passive or carrier mediated.
Acknowledgments
T.Z. was funded by the Novo-Nordisk Foundation, Lundbeck Foundation, Statens Sundhedsvidenskabelige Forskningsråd, Loevens Foundation and Merck-Sharpe-Dome. S.H. was funded by grants from Oejenforeningen Vaern om Synet, Apotekerfonden af 1991 and Civilingenioer Lars Andersens legat. F.J.A.-L.'s research for this project was supported by the Instituto Nacional de Psiquiatría in Mexico. S. Christoffersen is thanked for artwork.
Author contributions
Conception and design of the experiments: S.H., T.Z. and F.J.A-L. Collection, analysis and interpretation of data: S.H., J.J.H-P, T.Z. and F.J.A-L. Drafting the manuscript and revising it critically for important intellectual content: S.H., T.Z. and F.J.A-L. The experiments were performed in the laboratory of F.J.A-L. at the Instituto Nacional de Psiquiatría in Mexico City, México. All authors approved the final version of the manuscript.
References
- Agre P, Nielsen S, Ottersen OP. Towards a molecular understanding of water homeostasis in the brain. Neuroscience. 2004;12:849–850. doi: 10.1016/j.neuroscience.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ. Chloride transporters in presynaptic inhibition, pain and neurogenic inflammation. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases. San Diego, CA: Elsevier-Academic Press; 2009. [Google Scholar]
- Alvarez-Leefmans FJ, Altamirano J, Crowe WE. Use of ion-selective microelectrodes and fluorescent probes to measure cell volume. Methods Neurosci. 1995;27:361–391. [Google Scholar]
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron FM, Blanchard MG, Lapointe J-Y. Intracellular hypertonicity is responcible for water flux associated with Na+/glucose cotransport. Biophys J. 2006;90:3546–3554. doi: 10.1529/biophysj.105.076745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook RB, Takahashi K, Mead A, Dunn JJ, Sears ML. The role of NaKCl cotransport in blood-to-aqueous chloride fluxes across rabbit ciliary epithelium. Invest Ophthalmol Vis Sci. 2000;41:2574–2583. [PubMed] [Google Scholar]
- Crowe WE, Altamirano J, Huerto L, Alvarez-Leefmans FJ. Volume changes in single N1E-115 neuroblastoma cells measured with a fluorescent probe. Neuroscience. 1995;1:283–296. doi: 10.1016/0306-4522(95)00219-9. [DOI] [PubMed] [Google Scholar]
- Diecke FP, Wen Q, Iserovich P, Li J, Kuang K, Fischbarg J. Regulation of Na-K-2Cl cotransport in cultured bovine corneal endothelial cells. Exp Eye Res. 2005;80:777–785. doi: 10.1016/j.exer.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Edelman JL, Sachs G, Adorante JS. Ion transport asymmetry and functional coupling in bovine pigmented and nonpigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 1994;266:C1210–C1221. doi: 10.1152/ajpcell.1994.266.5.C1210. [DOI] [PubMed] [Google Scholar]
- Funk K, Woitecki A, Franjic-Wurtz C, Gensch T, Mohrlen F, Frings S. Modulation of chloride homeostasis by inflammatory mediators in dorsal root ganglion neurons. Mol Pain. 2008;4:32. doi: 10.1186/1744-8069-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresz V, Kwon TH, Gong H, Agre P, Steward MC, King LS, Nielsen S. Immunolocalization of AQP-5 in rat parotid and submandibular salivary glands after stimulation or inhibition of secretion in vivo. Am J Physiol Gastrointest Liver Physiol. 2004;287:G151–G161. doi: 10.1152/ajpgi.00480.2003. [DOI] [PubMed] [Google Scholar]
- Gutiérrez AM, Hernandez CS, Whittembury G. A model for fluid secretion in Rhodnius upper Malpighian tubules (UMT) J Membr Biol. 2004;202:105–114. doi: 10.1007/s00232-004-0723-6. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herrera-Perez JJ, Bundgaard M, Alvarez-Leefmans FJ, Zeuthen T. Water permeability of Na+-K+-2Cl− cotransporters in mammalian epithelial cells. J Physiol. 2005;568:123–135. doi: 10.1113/jphysiol.2005.093526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Kiilgaard JF, la Cour M, Prause JU, Zeuthen T. Cotransport of H+, lactate and H2O in porcine retinal pigment epithelial cells. Exp Eye Res. 2003;76:1–12. doi: 10.1016/s0014-4835(02)00329-9. [DOI] [PubMed] [Google Scholar]
- Hamann S, Kiilgaard JF, Litman T, Alvarez-Leefmans FJ, Winther BR, Zeuthen T. Measurement of cell volume changes by fluorescence self-quenching. J Flouresc. 2002;12:139–145. [Google Scholar]
- Imai Y, Nishikawa H, Yoshizaki K, Watari H. Evidence for the osmotic flow across dog submaxillary gland epithelia as a cause of salivary secretion. Jpn J Physiol. 1973;23:635–644. doi: 10.2170/jjphysiol.23.635. [DOI] [PubMed] [Google Scholar]
- Isenring P, Jacoby SC, Payne JA, Forbush B. Comparison of Na-K-Cl Cotransporters. J Biol Chem. 1998;18:11295–11301. doi: 10.1074/jbc.273.18.11295. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: The evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Layne J, Yip S, Crook RB. Down-regulation of Na-K-Cl cotransport by protein kinase C is mediated by protein phosphatase 1 in pigmented ciliary epithelial cells. Exp Eye Res. 2001;72:371–379. doi: 10.1006/exer.2000.0966. [DOI] [PubMed] [Google Scholar]
- Ludwig C. Lehrbuch der Physiologie des Menschen. 2nd edn. Leipzig and Heidelberg: C.F. Wintersche Verlagshandlung; 1861. pp. 1–780. [Google Scholar]
- Lytle C, Forbush BI. The Na-K-Cl cotransport protein of shark rectal gland. J Biol Chem. 1992;267:25438–25443. [PubMed] [Google Scholar]
- Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- MacAulay N, Hamann S, Zeuthen T. Chloride transporters as water pumps: Elements in a new model of epithelial water transport. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases. San Diego, CA: Elsevier-Academic Press; 2009. [Google Scholar]
- Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;295:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- Murakami M, Murdiastuti K, Hosoi K, Hill AE. AQP and the control of fluid transport in a salivary gland. J Membr Biol. 2006;210:91–103. doi: 10.1007/s00232-005-0848-2. [DOI] [PubMed] [Google Scholar]
- Naftalin RJ. Osmotic water transport with glucose in GLUT2 and SGLT. Biophys J. 2008;94:3912–3923. doi: 10.1529/biophysj.107.122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahari T, Steward MC, Yoshida H, Imai Y. Osmotic flow transients during acetylcholine stimulation in the perfused rat submandibular gland. Exp Physiol. 1997;82:55–70. doi: 10.1113/expphysiol.1997.sp004015. [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J, Begenisich T, Melvin JE. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2380–R2390. doi: 10.1152/ajpregu.00591.2006. [DOI] [PubMed] [Google Scholar]
- Parsegian VA. Protein-water interactions. Int Rev Cytol. 2002;215:1–31. doi: 10.1016/s0074-7696(02)15003-0. [DOI] [PubMed] [Google Scholar]
- Payne JA, Xu JC, Haas M, Lytle CY, Ward D, Forbush B., III Primary structure, functional expression, and chromosomal localization of the bumetanide-sensitive Na-K-Cl cotransporter in human colon. J Biol Chem. 1995;270:17977–17985. doi: 10.1074/jbc.270.30.17977. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Flögel U, Dreher W, Leibfritz D. Restricted diffusion and exchange of intracellular water: theoretical modeling and diffusion time dependence of 1HNMR measurements on perfused glial cells. NMR Biomed. 1998;1:19–31. doi: 10.1002/(sici)1099-1492(199802)11:1<19::aid-nbm499>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Reshkin SJ, Lee SI, George JN, Turner RJ. Identification, characterization and purification of a 160 kD bumetanide-binding glycoprotein from the rabbit parotid. J Membr Biol. 1993;136:243–251. doi: 10.1007/BF02505766. [DOI] [PubMed] [Google Scholar]
- Rocha-González HI, Mao S, Alvarez-Leefmans FJ. Na+, K+, 2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. J Neurophysiol. 2008;100:169–184. doi: 10.1152/jn.01007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Silva P, Stoff J, Field M, Fine L, Forrest JN, Epstein FH. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol Renal Physiol. 1977;233:F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Brauchitsch DK, Crook RB. Protein kinase C regulation of a Na+,K+,Cl− cotransporter in fetal human pigmented ciliary epithelial cells. Exp Eye Res. 1993;57:699–708. doi: 10.1006/exer.1993.1178. [DOI] [PubMed] [Google Scholar]
- Walcott B, Birzgalis A, Moore LC, Brink PR. Fluid secretion and the Na+-K+-2Cl− cotransporter in mouse exorbital lacrimal gland. Am J Physiol Cell Physiol. 2005;289:C860–C867. doi: 10.1152/ajpcell.00526.2004. [DOI] [PubMed] [Google Scholar]
- Zeuthen T, Belhage B, Zeuthen E. Water transport by Na+-coupled cotransporters of glucose (SGLT1) and of iodide (NIS). The dependence of substratesize studied at high resolution. J Physiol. 2006;570:485–499. doi: 10.1113/jphysiol.2005.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Secondary active transport of water across ventricular cell membrane of choroid plexus epithelium of Necturus maculosus. J Physiol. 1991a;444:153–173. doi: 10.1113/jphysiol.1991.sp018871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Water permeability of ventricular cell membrane in choroid plexus epithelium from Necturus maculosus. J Physiol. 1991b;444:133–151. doi: 10.1113/jphysiol.1991.sp018870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Cotransport of K+, Cl− and H2O by membrane proteins from choroid plexus epithelium of Necturus maculosus. J Physiol. 1994;478:203–219. doi: 10.1113/jphysiol.1994.sp020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T. Water-transporting proteins. J Membr Biol. 2010;234:57–73. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- Zeuthen T, Stein WD. Co-transport of salt and water in membrane proteins: Membrane proteins as osmotic engines. J Membr Biol. 1994;137:179–195. doi: 10.1007/BF00232587. [DOI] [PubMed] [Google Scholar]
- Zeuthen T, Zeuthen E, Klaerke DA. Mobility of ions, sugar, and water in the cytoplasm of Xenopus oocytes expressing Na+- coupled sugar transporters (SGLT1) J Physiol. 2002;542:71–87. doi: 10.1113/jphysiol.2001.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen T, Zeuthen E, MacAulay N. Water transport by GLUT2 expressed in Xenopus laevis oocytes. J Physiol. 2007;579:345–361. doi: 10.1113/jphysiol.2006.123380. [DOI] [PMC free article] [PubMed] [Google Scholar]


