Abstract
Neuropathic pain is a common diabetic complication affecting 8–16% of diabetic patients. It is characterized by aberrant symptoms of spontaneous and stimulus-evoked pain including hyperalgesia and allodynia. Magnesium (Mg) deficiency has been proposed as a factor in the pathogenesis of diabetes-related complications, including neuropathy. In the central nervous system, Mg is also a voltage-dependant blocker of the N-methyl-d-aspartate receptor channels involved in abnormal processing of sensory information. We hypothesized that Mg deficiency might contribute to the development of neuropathic pain and the worsening of clinical and biological signs of diabetes and consequently, that Mg administration could prevent or improve its complications. We examined the effects of oral Mg supplementation (296 mg l−1 in drinking water for 3 weeks) on the development of neuropathic pain and on biological and clinical parameters of diabetes in streptozocin (STZ)-induced diabetic rats. STZ administration induced typical symptoms of type 1 diabetes. The diabetic rats also displayed mechanical hypersensitivity and tactile and thermal allodynia. The level of phosphorylated NMDA receptor NR1 subunit (pNR1) was higher in the spinal dorsal horn of diabetic hyperalgesic/allodynic rats. Magnesium supplementation failed to reduce hyperglycaemia, polyphagia and hypermagnesiuria, or to restore intracellular Mg levels and body growth, but increased insulinaemia and reduced polydipsia. Moreover, it abolished thermal and tactile allodynia, delayed the development of mechanical hypersensitivity, and prevented the increase in spinal cord dorsal horn pNR1. Thus, neuropathic pain symptoms can be attenuated by targeting the Mg-mediated blockade of NMDA receptors, offering new therapeutic opportunities for the management of chronic neuropathic pain.
Introduction
Diabetes mellitus is a common cause of neuropathy in industrialized countries (Fox et al. 1999; Booya et al. 2005; Said, 2007). The most frequent clinical manifestations of neuropathy include length-dependent diabetic polyneuropathy, focal diabetic neuropathies and chronic inflammatory demyelinating polyneuropathy (Barrett et al. 2007; Said, 2007). The point prevalence of diabetic patients with painful polyneuropathy varies from 8% (Wu et al. 2007) to 16% (Daousi et al. 2004). Characteristic features of neuropathic pain include spontaneous pain, alterations in pain perception, enhanced sensitivity to noxious stimuli (hyperalgesia) and abnormal pain sensitivity to previously non-painful stimuli (allodynia), all of which strongly impairs patient quality of life. Clinical management is complex and the analgesic response to first-line treatments, i.e. antidepressants and calcium channel α2-δ ligands (Attal et al. 2006), is unsatisfactory, with no more than 40–60% obtaining partial pain relief (Dworkin et al. 2007). There is therefore a high necessity for efficient additional therapies (Sang & Bennett, 2009).
Diabetes is also the most common pathological state in which secondary magnesium (Mg) deficiency occurs. Indeed, Mg deficiency has been described in 25–30% and 13.5–47.7% of type 1 and type 2 diabetic patients, respectively (Garland, 1992; Tossielo, 1996; Corsonello et al. 2000; Engelen et al. 2000; Rodriguez-Moran & Guerrero-Romero, 2003; Pham et al. 2007) and its incidence is correlated to diabetes complications (De Leeuw, 2001). Mg is an ATPase allosteric effector involved in inositol transport (Grafton et al. 1992) and the impaired Na+/K+-ATPase activity in peripheral nerves of diabetic animals (Garland, 1992) plays a role in the pathophysiology of diabetic neuropathy (Li et al. 2005).
In the central nervous system, Mg has voltage-dependent blocking properties that play an important role in pain processing at the N-methyl-d-aspartate (NMDA) receptor channel complex (Mayer et al. 1984; Xiao & Bennett, 1994; Begon et al. 2000). In vitro, this blockade operates at extracellular Mg concentrations of less than 1 mm (Mayer et al. 1984), i.e. within the ranges found in human and animal cerebrospinal fluid and plasma (Morris, 1992). The excess release of glutamate from central nociceptor terminals due to nerve damage releases Mg blockade and activates NMDA receptors known to trigger painful sensations (hyperalgesia, allodynia) and alter the sensitivity of postsynaptic cells, resulting in central sensitization (Bennett, 2000). This central sensitization involving the NMDA receptor can be induced in rats in vivo by Mg depletion (Alloui et al. 2003). Several studies suggest that phosphorylation of the NMDA receptor NR1 subunit is correlated to the presence of signs of neuropathy and to persistent pain following nerve injury (Gao et al. 2005; Ultenius et al. 2006; Gao et al. 2007; Roh et al. 2008). In support of the role of NMDA receptor in painful neuropathy, NMDA receptor antagonists D-CPP [R(-)-3-(2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid] and MK801 [(5R, 10S)-(+)-5-methyl-10, 11-dihydro-5H-dibenzo[a,d]-cyclohepten-5-10-imine hydrogen maleate] suppress mechanical hyperalgesia in diabetic rats (Malcangio & Tomlinson, 1998; Daulhac et al. 2006; Courteix et al. 2007). Given that the upregulation of NMDA currents by mechanisms like Mg blockade lifting and receptor phosphorylation is a crucial event for pain hypersensitivity, we hypothesized that a chronic decrease in Mg levels caused by diabetes might increase NMDA activity and contribute to neuropathic pain. We thus examined whether oral Mg supplementation can improve pain-related behaviour (mechanical hypersensitivity, tactile and thermal allodynia), and biological (blood glucose, plasma insulin, urine and erythrocyte Mg concentration) and clinical parameters (body weight, food and water intake) in streptozocin-induced diabetic (STZ-D) rats. Moreover, we quantified the phosphorylation of the NMDA receptor NR1 subunit, which is known to reflect the activity status of the NMDA receptor, in healthy rats, Mg-supplemented STZ-D rats and non-supplemented STZ-D rats.
Methods
All experiments were performed in accordance with International Association for the Study of Pain (IASP) guidelines for animal experiments (Zimmermann, 1983) and approved by the local Ethics Committee (CEMEA, approval no. CE3-06).
Animals
Male Sprague–Dawley rats (Charles River, Cléon, France) (225–250 g) were housed three per cage under standard laboratory conditions and a 12 h–12 h light–dark cycle, and the litter was changed every day. Water and food were available ad libitum. The food formula used was Rat/Souris Entretien AO4 (Safe, Epinay-sur-Orge, France) containing Mg at 2000 mg kg−1. Animals were fed on this diet 1 week before the beginning of the experiment. Great care was taken, particularly with regard to housing conditions, in order to avoid or minimize animal discomfort.
Induction of diabetes and magnesium supplementation
Animals were rendered diabetic by an intraperitoneal (i.p.) injection of STZ (72 mg kg−1) (Sigma-Aldrich, St Quentin Fallavier, France) dissolved in distilled water. Diabetes was confirmed 1 week later by measuring tail vein blood glucose level on an ACCU-CHEK glucose-meter (Roche Diagnostics, Paris, France). Animals with blood glucose level ≥2 g l−1 were considered diabetic (D) and included in the study. Glycaemic control was systemically performed once a week.
Control (non-diabetic) rats were intraperitoneally injected with 5 ml kg−1 of distilled water and were used as controls for STZ-D rats.
One week after STZ or distilled water injection, the animals were assigned to the following three experimental groups:
MgSO4-supplemented STZ-D group: STZ-D rats receiving MgSO4 (296 mg l−1 of Mg) in drinking water for 3 weeks,
Non-supplemented STZ-D group: STZ-D rats given tap water,
Control non-diabetic group: rats given tap water.
Measurement of clinical, biological and metabolic parameters
Body weight and glycaemia were determined weekly. Food and water intake were estimated at the fourth week of the experiment. Rats were placed in individual metabolic cages with free access to food and water. Food and water intakes and urine excretion were recorded; urine was collected in tubes containing 200 μl of antiseptics (Amukine, 0.06%, Gifrer Barbezat, Décines, France).
Urine Mg concentration was measured after adequate dilution with 0.1% (w/v) LaCL3 (Feillet-Coudray et al. 2002). The mineral content was determined by atomic absorption spectrophotometry (using a Perkin-Elmer AA800 apparatus, Quebec, Canada) at a wavelength of 285 nm. Quality control and external calibration was included for each data series.
Plasma insulin concentration and erythrocyte Mg were estimated at the fourth week of the experiment. Blood was drawn from heart under volatile halothane (3.5%) anaesthesia, collected into heparinized tubes, and immediately centrifuged (3500 g for 10 min, 4°C). Plasma was separated and stored at −20°C until analysis. Plasma insulin concentration was measured using an enzyme-linked immunosorbent assay kit following the manufacturer's instructions (Insulin ELISA, Ultrasensitive Rat Insulin Elit, Mercodia, Uppsala, Sweden). As Mg is a predominantly intracellular ion, red blood cell magnesium was estimated. Red cells were washed with saline solution and haemolysed in water for total Mg determination. After appropriate dilution, magnesium was analysed as previously explained.
Pain-related behavioural parameters
Mechanical, tactile and thermal sensitivity were assessed before STZ-D or distilled water injection and then at weeks 2 and 4.
Measurement of mechanical sensitivity
Rats underwent the paw pressure test as described by Randall & Sellito (1957). Nociceptive thresholds, expressed in grams (g), were measured using an Ugo Basile analgesimeter (Bioseb, France) by applying increasing pressure to the left hind-paw until a squeak (vocalization) was elicited. The maximal pressure applied (cut-off) was 450 g. As this test involves animal handling, the experimenter got the rat used to being handled. The vocalization threshold was measured 3 or 4 times in order to obtain two consecutive values that differed no more than 10%, and respecting an interval of at least 10 min between two measures. The results are expressed as vocalization threshold (VT) variations using the formula: VT pre-STZ or distilled water – VT post-STZ or distilled water (g).
Measurement of tactile sensitivity
Rats were placed individually on an elevated plastic mesh (1 cm2 perforations) in a clear plastic cage and allowed at least 15 min to adapt to the testing environment. Von Frey hairs (Semmes-Weinstein monofilaments, Stoelting, IL, USA) with calibrated bending forces (from 1.479 g to 15.136 g) were used to deliver punctuate mechanical stimuli of varying intensity. Starting with the lowest filament force, von Frey hairs were applied from below the mesh floor to the plantar surface of the hind-paw with sufficient force to cause slight bending against the paw, and held for 1 s. Each stimulus was applied 5 times at interstimulus intervals of 4–5 s. Care was taken to stimulate random locations on the plantar surface. A positive response was recorded if the paw was robustly and immediately withdrawn. Paw withdrawal threshold was defined as the minimum pressure required to elicit a paw withdrawal reflex at least once out of the five trials. If no response was recorded in any trial, the process was repeated with the next-highest force hair. Mechanical allodynia was defined as a significant decrease in withdrawal thresholds to von Frey hair application. The 15.136 g hair was selected as the upper limit cut-off for testing.
Measurement of thermal sensitivity
The rat's tail was immersed in a water bath maintained at 42°C, i.e. a temperature normally innocuous in normal rats (Courteix et al. 1993), until tail withdrawal or signs of struggle were observed (cut-off time: 15 s). As this test involves handling of the animals, the experimenter got the rat used to being handled. The reaction latency (i.e. time before withdrawal of the tail from the bath) was measured two to three times in order to obtain two consecutive values that differed by no more than 10%, and respecting an interval of at least 15 min between two measures. The rat's tail was immediately dried with soft cellulose paper to avoid tail cooling between two measures. A shortened duration of immersion indicated allodynia.
Western-blot: quantification of total NR1 and pNR1 expression
At week 4, the animals were killed by decapitation. Then, lumbar enlargements of the spinal cord dorsal horn were rapidly removed and homogenized on ice with lysis buffer (50 mm Hepes, pH 7.5, 150 mm NaCl, 10 mm EDTA, 10 mm Na4P2O7, 2 mm orthovanadate, 100 mm NaF, 1% Triton X-100, 0.5 mm phenylmethylsulfonyl fluoride, 20 μm leupeptin, 100 IU ml−1 aprotinin, Sigma, France) for 20 min at 4°C. The samples were then centrifuged for 15 min at 16,000 g at 4°C and the supernatants containing proteins were collected. Proteins (100 μg per condition) were electrophoresed in 7.5% SDS polyacrylamide gels and transferred to nitrocellulose membrane (BioTrace NT, France). Membranes were blocked with buffer containing 1% bovine serum albumin or 5% non-fat dried milk and incubated overnight at 4°C with the indicated antibodies, according to the manufacturer's recommendations. Antibodies were: total NR1 (anti-rabbit, 1:200, cat. no. 06–311, Upstate Biotechnology/Millipore, Saint-Quentin-en-Yvelines, France) and pNR1, which only recognizes NR1 phosphorylated on serine 896 (anti-rabbit, 1:500, cat. no. 06-640, Upstate Biotechnology). Proteins were visualized by autoradiography using horseradish peroxidase chemiluminescent reagent (SuperSignal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL, USA). The intensity of immunoreactive bands was quantified using Phoretix Advanced software (Non-Linear Dynamics Ltd, Newcastle, UK). Results were expressed as the percentage change from control, where control is the mean result from at least four spinal cords of non-diabetic rats.
Statistical analysis
Values are expressed as means ± standard error of the mean (s.e.m.). Differences among experimental groups were determined by one-way analysis of variance (ANOVA) followed by a Tukey–Kramer test or a chi-square test. Differences were considered significant at P < 0.05. The software used was SigmaStat 2.0 (Systat Software Inc., San Jose, CA, USA).
Results
Effect of diabetes and magnesium supplementation on biological and clinical parameters
As expected (Courteix et al. 1993), STZ-induced diabetes was associated with marked disturbances in biological and clinical parameters. By the first week after STZ treatment, 50% (20/40) of STZ-treated rats were hyperglycaemic, and blood glucose concentration had increased fivefold in non-supplemented (5.12 ± 0.32 g l−1) and future MgSO4-supplemented (5.46 ± 0.13 g l−1) STZ-D rats, compared with pre-STZ injection glycaemia values (Fig. 1). Despite a significant decrease in glucose levels in week 2 in MgSO4-supplemented STZ-D rats compared with non-supplemented STZ-D rats, hyperglycaemia was maintained throughout the experiment (5.97 ± 0.03 and 5.85 ± 0.07 g l−1 in non- and MgSO4-supplemented STZ-D rats, respectively, week 4). In non-diabetic rats, glucose concentrations measured before i.p. injection of distilled water (1.22 ± 0.02 g l−1) were maintained in a normal range throughout week 4 of the experiment (1.22 ± 0.03 g l−1) (Fig. 1).
Figure 1. Time course of glycaemia in non-diabetic (Non-D), non-supplemented STZ-diabetic (Non-suppl. STZ-D) and MgSO4-supplemented diabetic (MgSO4-suppl. STZ-D) rats.

Values are expressed as means ±s.e.m. in g l−1 (n = 10 for each group). Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test. ***P < 0.001 vs. non-D rats. #P < 0.05 vs. Non-suppl. STZ-D rats.
The non-diabetic rats showed a regular weight gain during the study (Fig. 2). Weight had become significantly lower in non-supplemented and MgSO4-supplemented STZ-D rats compared to non-diabetic rats by the first week, and had not changed significantly at the end of week 4. MgSO4 supplementation did not prevent the halt in weight gain in STZ-D rats. Since MgSO4 supplementation induced light diarrhoea, the body weights at weeks 2, 3 and 4 were significantly lower in MgSO4-supplemented STZ-D rats than in non-supplemented STZ-D rats.
Figure 2. Time course of body weight in non-diabetic (Non-D), non-supplemented STZ-diabetic (Non-suppl. STZ-D) and MgSO4-supplemented diabetic (MgSO4-suppl. STZ-D) rats.
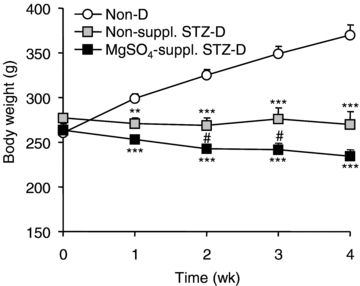
Values are expressed as means ±s.e.m. in g (n = 10 for each group). Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test. **P < 0.01, ***P < 0.001 vs. non-D rats; #P < 0.05 vs. Non-suppl. STZ-D rats.
Water intake was 10-fold and sixfold higher in non-supplemented and MgSO4-supplemented STZ-D rats, respectively, compared with non-diabetic rats. Water intake was significantly lower in MgSO4-supplemented STZ-D rats than non-supplemented STZ-D rats (Table 1). Consequently, urine excretion was 24-fold higher in non-supplemented STZ-D rats than non-diabetic rats. The MgSO4-supplemented STZ-D rats also developed polyuria corresponding to a 15-fold increase in urine excretion compared with non-diabetic rats, but which was nevertheless lower than the increase in non-supplemented STZ-D rats (Table 1). Polyuria in STZ-D rats was significantly correlated to water intake (P < 0.001).
Table 1.
Effect of streptozocin-induced diabetes (STZ-D) and MgSO4 supplementation to diabetic rats on water, food and Mg intakes and urine excretion at week 4 of the experiment
| Parameter | Non-diabetic | Non-suppl. STZ-D | MgSO4-suppl. STZ-D |
|---|---|---|---|
| Water intake (ml (24 h)−1) | 35.22 ± 2.36 | 376.6 ± 32.87*** | 214.4 ± 30.87***,### |
| Urine excretion (ml (24 h)−1) | 12.45 ± 1.51 | 300.1 ± 24.16*** | 184.4 ± 25.23***,## |
| Food intake (g (24 h)−1) | 30.7 ± 1.83 | 54.66 ± 3.67** | 42.06 ± 6.21 |
| Total Mg intake (mg (24 h)−1) | 61.40 ± 3.66 | 109.32 ± 7.34*** | 147.58 ± 1.58***,### |
Values are expressed as means ±s.e.m. (n = 10/group). Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test.
P < 0.01
P < 0.001 vs. Non-diabetic rats;
P < 0.01
P < 0.001 vs. Non-suppl. STZ-D rats.
Non-supplemented and MgSO4-supplemented STZ-D rats significantly increased food intake (+78 ± 12% and +37 ± 20%, respectively) compared with non-diabetic rats (Table 1). Hence, non-supplemented STZ-D rats presented a significantly higher Mg intake. MgSO4-supplemented STZ-D rats received an oral Mg supplementation of 63.5 ± 9.1 mg (24 h)−1 in drinking water and 84.1 ± 12.4 mg (24 h)−1 in food giving a total dose (food and water) of 147.6 ± 1.6 mg (24 h)−1. Mg intake by non-supplemented STZ-D and MgSO4-supplemented STZ-D rats was 236 ± 8% and 587 ± 20%, respectively, of the Mg intake of control rats (Table 1).
STZ provoked a marked hypoinsulinaemia (−52 ± 3% compared to controls). MgSO4 supplemention to STZ-D rats attenuated this hypoinsulinaemia (−35 ± 11% compared to controls) (Fig. 3).
Figure 3. Insulin plasma concentrations in non-diabetic (Non-D), non-supplemented STZ-diabetic (Non-suppl. STZ-D) and MgSO4-supplemented STZ-diabetic (MgSO4-suppl. STZ-D) rats at week 4 of the experiment.
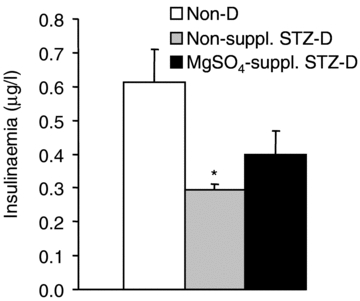
Values are expressed as means ±s.e.m. in μg l−1 (n = 6–8/group). Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test. *P < 0.05 vs. Non-D rats.
Erythrocyte Mg levels were −21 ± 5% and −32 ± 4% lower in non-supplemented and MgSO4-supplemented STZ-D rats, respectively, compared with non-diabetic rats (Table 2).
Table 2.
Effect of streptozocin-induced diabetes (STZ-D) and MgSO4 supplementation to diabetic rats on erythrocyte Mg concentration and urine Mg excretion at week 4 of the experiment
| Parameter | Non-diabetic | Non-suppl. STZ-D | MgSO4-suppl.STZ-D |
|---|---|---|---|
| Erythrocyte Mg (mg l−1) | 51.04 ± 2.19 (n = 6) | 40.59 ± 2.43* (n = 5) | 34.51 ± 1.70*** (n = 10) |
| Urine Mg (mg (24 h)−1) | 2.19 ± 0.24 (n = 7) | 8.99 ± 0.73** (n = 10) | 16.77 ± 1.94***, ### (n = 9) |
Values are expressed as means ±s.e.m. (n = 5–10/group). Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test.
P < 0.05
P < 0.01
P < 0.001 vs. Non-diabetic rats;
P < 0.001 vs. Non-suppl. STZ-D rats.
Urine Mg excretion was higher in non-supplemented and MgSO4-supplemented STZ-D rats (+341 ± 28% and +678 ± 90%, respectively) than in non-diabetic rats (Table 2).
Effect of diabetes and magnesium supplementation on mechanical thresholds
In non-diabetic rats, mechanical nociceptive thresholds did not vary during the 4 week study (Fig. 4). Non-supplemented STZ-D rats developed mechanical hyperalgesia within 2 weeks, characterized by a significant −125.1 ± 12.6 g reduction in paw pressure-induced vocalization thresholds that worsened in week 4 (−209.3 ± 13.4 g). The MgSO4 supplementation delayed the onset of mechanical hypersensitivity, which appeared over weeks 2–4 after the induction of diabetes; at week 4, mechanical hyperalgesia in MgSO4-supplemented STZ-D rats was characterized by a −98.4 ± 7.6 g threshold reduction, which was a significantly lower drop than in the non-supplemented STZ-D group.
Figure 4. Time course of mechanical sensitivity measured by paw pressure-induced vocalization threshold (VT) variations in non-diabetic (Non-D), non-supplemented STZ-diabetic (Non-suppl. STZ-D) and MgSO4-supplemented (MgSO4-suppl. STZ-D) rats.

Results are expressed as means ±s.e.m. in g (n = 10 for each group). Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test. **P < 0.01, ***P < 0.001 vs. Non-D rats; ###P < 0.001 vs. Non-suppl. STZ-D rats.
Effect of diabetes and magnesium supplementation on tactile allodynia
Before the administration of STZ, we recorded no withdrawal responses to the 15.136 g von Frey hair application in any of the three groups (Table 3). Four weeks after STZ injection, 60% (n = 6) of the non-supplemented STZ-D rats showed a painful reaction to the application of a non-noxious stimulation characterized by a paw-withdrawal threshold of 8.5 ± 2.3 g. Half of these allodynic rats (n = 3) already presented a painful reaction at 2 week after STZ injection (mean paw-withdrawal threshold: 11.8 ± 1.9 g). Mg supplementation totally prevented tactile allodynia in STZ-D rats, as demonstrated by the lack of any withdrawal responses to the strongest von Frey filament throughout the 4 week study.
Table 3.
Effect of streptozocin-induced diabetes (STZ-D) and MgSO4 supplementation of diabetic rats on the occurrence of tactile (von Frey hair application) and thermal (tail-immersion in a 42°C water bath) hypersensitivity at week 2 and 4 of the experiment
| Parameter | Non-diabetic | Non-suppl. STZ-D | MgSO4-suppl. STZ-D |
|---|---|---|---|
| Tactile hypersensitivity | |||
| Week 2 | 0/10 | 3/10 | 0/10# |
| Week 4 | 0/10 | 6/10* | 0/10# |
| Thermal hypersensitivity | |||
| Week 2 | 0/10 | 6/10* | 0/10# |
| Week 4 | 0/10 | 6/10* | 0/10# |
Values are expressed as number of responder animals/number of rats in the studied group. Statistical analysis was performed by chi-square test.
P < 0.05 vs. Non-diabetic rats;
P < 0.05 vs. Non-suppl. STZ-D rats.
Effect of diabetes and magnesium supplementation on thermal allodynia
Before the injection of STZ or distilled water, we recorded no responses from any rats to tail immersion in a 42°C water bath. Reaction latency shortened in 60% (n = 6) of the non-supplemented STZ-D rats at week 2 of diabetes (7.3 ± 1.1 s) and then remained stable until the end of the study (8.1 ± 0.4 s, at week 4) (Table 3). Mg supplementation totally prevented thermal allodynia in MgSO4-supplemented STZ-D rats, as demonstrated by the lack of any responses to the thermal stimulus throughout the 4-week study (reaction latency >15 s at week 2 and 4).
Effect of diabetes and magnesium supplementation on NMDA NR1 subunit phosphorylation
In order to establish a correlation between neuropathic pain symptoms and molecular changes following induced diabetes, we focused on the expression of neuropathic pain signs rather than on diabetic or non-diabetic status. Densitometric quantification of NMDA NR1 subunit phosphorylation performed in the spinal cord dorsal horn of hyperalgesic and allodynic non-supplemented STZ-D rats indicated a significant increase in pNR1 subunit compared with non-diabetic rats (Fig. 5A upper panel and B) whereas total NR1 expression was not altered (Fig. 5A lower panel and C). Oral Mg supplementation totally prevented the enhanced NR1 subunit phosphorylation in the spinal cord dorsal horn of MgSO4-supplemented STZ-D rats (Fig. 5A upper panel and B).
Figure 5. Expression of phosphorylated NR1 (pNR1) and total NR1 in spinal cord dorsal horn extracts from non-diabetic (Non-D), non-supplemented STZ-diabetic (Non-suppl. STZ-D) and MgSO4-supplemented (MgSO4-suppl. STZ-D) rats at week 4 of the experiment.

A, representative Western blots showing pNR1 (upper panel) or total NR1 (lower panel) expression; B and C, percentage changes of pNR1 and NR1 levels. Data are means ±s.e.m. expressed as a percentage of values measured in Non-D rats (% of controls), for 3 Non-D rats, 3 MgSO4-suppl. STZ-D rats and 3 Non-suppl. STZ-D rats. Statistical analysis was performed by one-way ANOVA followed by a Tukey–Kramer test. ***P < 0.001 vs. N rats; ##P < 0.01 vs. Non-suppl. STZ-D rats.
Discussion
This study clearly showed that Mg supplementation prevents tactile and thermal allodynia and attenuates and delays mechanical hyperalgesia in STZ-D rats. This effect was mediated, at least in part, by the prevention of NMDA receptor NR1 subunit phosphorylation in STZ-D rats. However, the study also showed that Mg supplementation did not improve most of the biological and clinical signs of diabetes despite restoration of normal insulin secretion.
The administration of STZ induced an experimental type-1 diabetes mellitus in the rats, with severe hyperglycaemia and hypoinsulinaemia. STZ-D rats showed a halt in weight gain, symptoms of polydipsia, polyuria and polyphagia, low Mg status, and hypermagnesiuria. Mg supplementation in STZ-D rats reduced the hypoinsulinaemia, polydipsia and polyuria but exacerbated hypermagnesiuria. This later is in agreement with higher Mg supply in these animals.
Despite a higher plasma insulin concentration in MgSO4-supplemented STZ-D rats than in non-supplemented STZ-D rats, the glycaemia was not improved. This could be due to insulin resistance and/or impaired insulin response. Our results are consistent with those of Chetan et al. (2003), who demonstrated that Mg supplementation improved insulinaemia but not glycaemia in alloxan-induced diabetic rats. Other studies using higher Mg concentrations have reported the preventive effect of Mg supplementation on hyperglycaemia in STZ-D rats (Soltani et al. 2005; Hasanein et al. 2006).
Despite hyperphagia resulting in a 1.5-fold increase in food and consequently Mg intake compared with non-diabetic rats, non-supplemented STZ-D rats showed a drop in intracellular Mg. This could be due to the increased delivery of Mg to the renal distal convoluted tubule (Davenport et al. 1990; Rude et al. 1999) resulting in excessive urinary excretion, as we found strong hypermagnesiuria characterized by a fourfold increase urinary excretion in non-supplemented STZ-D rats. Presumably for the same reasons (loss of renal function and polyuria), the low intracellular Mg level was not prevented by Mg supplementation, in contrast with other reports in diabetic rats (Soltani et al. 2005) and patients (Rodriguez-Moran & Guerrero-Romero, 2003; Lima Mde et al. 2005).
The halt in body growth, already described (Courteix et al. 1993; Fox et al. 1999; Ward et al. 2001), is related to insulin deficiency. Polydipsia, polyuria, polyphagia, the classical triad of diabetes, may in particular be induced by hyperglycaemia, increased glomerular flow rate and decreased fluid reabsorption (Ward et al. 2001). In our study, Mg supplementation only reduced polydipsia and polyuria.
Two weeks of diabetes was sufficient to result in mechanical hypersensitivity and thermal allodynia in non-supplemented STZ-D rats, as shown previously (Courteix et al. 1993; Fox et al. 1999; Hasanein et al. 2006; Hoybergs & Meert, 2007). At the end of the study (week 4), mechanical hyperalgesia and allodynia (thermal and tactile) were observed in 100% and 60%, respectively, of the non-supplemented STZ-D rats. Only 20% of non-supplemented STZ-D rats were allodynic to both thermal and tactile stimulations. Mechanical hyperalgesia and tactile allodynia, but not thermal allodynia, got worse with the time course of the disease. Taking more time to develop than mechanical hyperalgesia or thermal allodynia, tactile allodynia affected progressively more animals: only 50% of allodynic rats presented an aversive reaction to a non-painful tactile stimulation at week 2 of diabetes. Thus, hyperalgesia to mechanical stimuli and allodynia to thermal stimuli, both of which are mediated by sensitization of transduction processes in small-diameter unmyelinated C-fibres and medium-diameter myelinated Aδ-fibres (polymodal nociceptors), occur more rapidly than tactile allodynia, which is sustained by large-diameter Aβ fibres. This is consistent with clinical neuropathological observations that show more severe and earlier loss of small fibres than large myelinated fibres (Behse et al. 1977; Bischoff, 1980; Said et al. 1983). These data are also consistent with behavioural and morphometric reports in STZ-D rats (Courteix et al. 1993; Kamiya et al. 2005).
In pain states associated with diabetic neuropathy, central sensitization is maintained, at least partially, by spinal NMDA receptors (Malcangio & Tomlinson, 1998). Indeed, when intrathecally injected, the competitive NMDA receptor antagonist D-CPP as well as the non-competitive antagonist MK801 both suppress mechanical hyperalgesia in STZ-D rats (Daulhac et al. 2006; Courteix et al. 2007). Further supporting the crucial role of NMDA receptors in diabetic neuropathic pain, the mRNA coding for the NMDA receptor NR2A and NR2B subunits (which have high affinity for glutamate) are reportedly increased in laminae III–V of D rats (Tomiyama et al. 2005). Besides the up-regulation of NMDA receptor subunits induced by diabetic neuropathy, the Mg deficiency could facilitate NMDA receptor activation and long-term sensitization of the nociceptive pathways. One expression of such sensitization is the increase in phosphorylated NR1 (pNR1) subunits, as previously shown in the ispsilateral spinal cord in response to nerve injury following sciatic nerve (Ultenius et al. 2006; Roh et al. 2008) or L5 spinal nerve (Gao et al. 2005, 2007) ligation in rats and, recently in diabetic hyperalgesic rats (Daulhac et al. 2010). Our results show high expression of pNR1 in the spinal dorsal horn of diabetic rats coinciding with pronounced signs of mechanical hyperalgesia and thermal and/or tactile allodynia. After Mg supplementation, MgSO4-supplemented diabetic rats showed no increase in pNR1 receptor expression but some slight mechanical hyperalgesia, and they lacked hypersensitivity to tactile and thermal innocuous stimuli. These data suggest that tactile and thermal allodynia, and to a lesser extent mechanical hyperalgesia, are related to an increase in the pNR1 pool in the spinal cord dorsal horn. Therefore, in the CNS, neuropathic pain is probably associated with Mg depletion, as already shown (Jeong et al. 2006), leading to a release of the voltage-dependant inhibition exerted by Mg on NMDA receptor activation. Presumably, the activated NMDA receptors lead to protein kinase C activation, which in turn phosphorylates serine residue 896 of the NR1 subunit (Tingley et al. 1997). NR1 phosphorylation at Ser 896 may promote the trafficking of NMDA receptors from intracellular stores to the cell membrane (Xia et al. 2001; Scott et al. 2003) and, in coordination with PKA phosphorylation of Ser 897, may also induce membrane insertion (Scott et al. 2003), enhancing synaptic activity and inducing central sensitization in chronic neuropathic pain. Several studies have reported that Mg deficiency induces a mechanical hyperalgesia that involves NMDA receptors (Begon et al. 2002; Alloui et al. 2003). In addition, we show for the first time that oral MgSO4 supplementation kept pNR1 expression to a normal level, prevented tactile and thermal allodynia, and attenuated and delayed the onset of hyperalgesia to mechanical stimuli similarly to a chronic treatment with the uncompetitive NMDA receptor antagonist MK801 (Daulhac et al. 2010). To date, several studies have demonstrated that Mg injected intraperitoneally (Begon et al. 2000, 2002), intrathecally (Kroin et al. 2000; Takano et al. 2000; Tsai et al. 2001) or intravenously (Crosby et al. 2000; Brill et al. 2002) relieves or prevents neuropathic pain in rats (Begon et al. 2000, 2002; Hasanein et al. 2006) and patients (Crosby et al. 2000; Brill et al. 2002; Wolf et al. 2008), or can at least potentiate morphine or fentanyl analgesia in rats (Kroin et al. 2000; Begon et al. 2002; Ulugol et al. 2002; Bujalska et al. 2008) and patients (Buvanendran et al. 2002). Only one study has reported that oral supplementation with Mg suppressed thermal hyperalgesia in diabetic rats, but the beneficial effect of Mg was not attributed solely to NMDA blockade but also to the normalization of glycaemia (Hasanein et al. 2006).
In conclusion, our data indicate that Mg supplementation improves mechanical hyperalgesia and thermal and tactile allodynia in painful diabetic neuropathy. The lack of effect of oral Mg supplementation on glycaemia (despite increased insulinaemia), weight growth or intracellular Mg concentrations suggests that this improvement is more likely to be due to the voltage-dependent blockade effect of Mg on NMDA receptors in the spinal cord than to systemic metabolic effects. Indeed, we also show that Mg supplementation prevents the neuropathic pain-induced overexpression of the phosphorylated NR1 subunit involved in central sensitization. Thus, oral Mg supplementation would be an original and valuable nutritional strategy effective and relatively free of major side effects to prevent the onset of diabetic neuropathic pain.
Acknowledgments
A.E., A.-M.P., L.D., J.F. and C.C. were supported by the French Ministère de l'Enseignement Supérieur et de la Recherche, the Université d'Auvergne and INSERM.
Glossary
Abbreviations
- Mg
magnesium
- pNR1
phosphorylated NMDA receptor NR1 subunit
- STZ-D
streptozocin-diabetic
Author contributions
L.R., A.-M.P., L.D., N.D., A.M., J.F., A.E., C.C.: final approval of the version to be published. Conception and design of the experiments: L.R., A.E. and C.C.; collection, analysis and interpretation: L.R., A.-M.P., L.D., N.D. and C.C.: drafting the article: L.R., A.E., J.F., A.M. and C.C. All the experiments were undertaken in the Faculté de Pharmacie at the Université d'Auvergne except determination of Mg concentration conducted at the Centre de Recherche de l' INRA, Theix.
References
- Alloui A, Begon S, Chassaing C, Eschalier A, Gueux E, Rayssiguier Y, Dubray C. Does Mg2+ deficiency induce a long-term sensitization of the central nociceptive pathways? Eur J Pharmacol. 2003;469:65–69. doi: 10.1016/s0014-2999(03)01719-9. [DOI] [PubMed] [Google Scholar]
- Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8(Suppl 2):S50–62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- Begon S, Pickering G, Eschalier A, Dubray C. Magnesium and MK-801 have a similar effect in two experimental models of neuropathic pain. Brain Res. 2000;887:436–439. doi: 10.1016/s0006-8993(00)03028-6. [DOI] [PubMed] [Google Scholar]
- Begon S, Pickering G, Eschalier A, Dubray C. Magnesium increases morphine analgesic effect in different experimental models of pain. Anesthesiology. 2002;96:627–632. doi: 10.1097/00000542-200203000-00019. [DOI] [PubMed] [Google Scholar]
- Behse F, Buchthal F, Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977;40:1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ. Update on the neurophysiology of pain transmission and modulation: focus on the NMDA-receptor. J Pain Symptom Manage. 2000;19:S2–6. doi: 10.1016/s0885-3924(99)00120-7. [DOI] [PubMed] [Google Scholar]
- Bischoff A. Morphology of diabetic neuropathy. Horm Metab Res Suppl. 1980;9:18–28. [PubMed] [Google Scholar]
- Booya F, Bandarian F, Larijani B, Pajouhi M, Nooraei M, Lotfi J. Potential risk factors for diabetic neuropathy: a case control study. BMC Neurol. 2005;5:24. doi: 10.1186/1471-2377-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S, Sedgwick PM, Hamann W, Di Vaidi PP. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth. 2002;89:711–714. [PubMed] [Google Scholar]
- Bujalska M, Malinowska E, Makulska-Nowak H, Gumulka SW. Magnesium ions and opioid agonist activity in streptozotocin-induced hyperalgesia. Pharmacology. 2008;82:180–186. doi: 10.1159/000151346. [DOI] [PubMed] [Google Scholar]
- Buvanendran A, McCarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ. Intrathecal magnesium prolongs fentanyl analgesia: a prospective, randomized, controlled trial. Anesth Analg. 2002;95:661–666. doi: 10.1097/00000539-200209000-00031. table of contents. [DOI] [PubMed] [Google Scholar]
- Chetan P, Dharam P, Chaudhary, Devi D, Bansal M. Effect of magnesium supplementation on oxidative stress in alloxanic diabetic rats. Magnes Res. 2003;16:13–19. [PubMed] [Google Scholar]
- Corsonello A, Ientile R, Buemi M, Cucinotta D, Mauro VN, Macaione S, Corica F. Serum ionized magnesium levels in type 2 diabetic patients with microalbuminuria or clinical proteinuria. Am J Nephrol. 2000;20:187–192. doi: 10.1159/000013582. [DOI] [PubMed] [Google Scholar]
- Courteix C, Eschalier A, Lavarenne J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53:81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- Courteix C, Privat AM, Pelissier T, Hernandez A, Eschalier A, Fialip J. Agmatine induces antihyperalgesic effects in diabetic rats and a superadditive interaction with R(–)-3-(2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid, a N-methyl-d-aspartate-receptor antagonist. J Pharmacol Exp Ther. 2007;322:1237–1245. doi: 10.1124/jpet.107.123018. [DOI] [PubMed] [Google Scholar]
- Crosby V, Wilcock A, Corcoran R. The safety and efficacy of a single dose (500 mg or 1 g) of intravenous magnesium sulfate in neuropathic pain poorly responsive to strong opioid analgesics in patients with cancer. J Pain Symptom Manage. 2000;19:35–39. doi: 10.1016/s0885-3924(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21:976–982. doi: 10.1111/j.1464-5491.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- Daulhac L, Maffre V, Mallet C, Etienne M, Privat AM, Kowalski-Chauvel A, Seva C, Fialip J, Eschalier A. Phosphorylation of spinal N-methyl-d-aspartate receptor NR1 subunits by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-induced painful neuropathy. Eur J Pain. 2010 doi: 10.1016/j.ejpain.2010.06.003. (in press) [DOI] [PubMed] [Google Scholar]
- Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-d-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- Davenport GM, Boling JA, Gay N. Bioavailability of magnesium in beef cattle fed magnesium oxide or magnesium hydroxide. J Anim Sci. 1990;68:3765–3772. doi: 10.2527/1990.68113765x. [DOI] [PubMed] [Google Scholar]
- De Leeuw I. Rayssiguier Y, Mazur A, Durlach J, editors. Magnesium and diabetes: is magnesium depletion a risk factor for complications in type 1 diabetic patients? Adv Magnes Res. 2001:369–374. John Libbey & Company Ltd. [Google Scholar]
- Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Engelen W, Bouten A, De Leeuw I, De Block C. Are low magnesium levels in type 1 diabetes associated with electromyographical signs of polyneuropathy? Magnes Res. 2000;13:197–203. [PubMed] [Google Scholar]
- Feillet-Coudray C, Coudray C, Tressol JC, Pepin D, Mazur A, Abrams SA, Rayssiguier Y. Exchangeable magnesium pool masses in healthy women: effects of magnesium supplementation. Am J Clin Nutr. 2002;75:72–78. doi: 10.1093/ajcn/75.1.72. [DOI] [PubMed] [Google Scholar]
- Fox A, Eastwood C, Gentry C, Manning D, Urban L. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain. 1999;81:307–316. doi: 10.1016/S0304-3959(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland HO. New experimental data on the relationship between diabetes mellitus and magnesium. Magnes Res. 1992;5:193–202. [PubMed] [Google Scholar]
- Grafton G, Bunce CM, Sheppard MC, Brown G, Baxter MA. Effect of Mg2+ on Na+-dependent inositol transport. Role for Mg2+ in etiology of diabetic complications. Diabetes. 1992;41:35–39. doi: 10.2337/diab.41.1.35. [DOI] [PubMed] [Google Scholar]
- Hasanein P, Parviz M, Keshavarz M, Javanmardi K, Mansoori M, Soltani N. Oral magnesium administration prevents thermal hyperalgesia induced by diabetes in rats. Diabetes Res Clin Pract. 2006;73:17–22. doi: 10.1016/j.diabres.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Hoybergs YM, Meert TF. The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett. 2007;417:149–154. doi: 10.1016/j.neulet.2007.02.087. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Hahn KD, Shin JW, Leem JC, Lee C, Han SM. Changes in magnesium concentration in the serum and cerebrospinal fluid of neuropathic rats. Acta Anaesthesiol Scand. 2006;50:211–216. doi: 10.1111/j.1399-6576.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Murakawa Y, Zhang W, Sima AA. Unmyelinated fiber sensory neuropathy differs in type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2005;21:448–458. doi: 10.1002/dmrr.541. [DOI] [PubMed] [Google Scholar]
- Kroin JS, McCarthy RJ, Von Roenn N, Schwab B, Tuman KJ, Ivankovich AD. Magnesium sulfate potentiates morphine antinociception at the spinal level. Anesth Analg. 2000;90:913–917. doi: 10.1097/00000539-200004000-00025. [DOI] [PubMed] [Google Scholar]
- Li F, Obrosova IG, Abatan O, Tian D, Larkin D, Stuenkel EL, Stevens MJ. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 2005;288:E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- Lima Mde L, Pousada J, Barbosa C, Cruz T. [Magnesium deficiency and insulin resistance in patients with type 2 diabetes mellitus] Arq Bras Endocrinol Metabol. 2005;49:959–963. doi: 10.1590/s0004-27302005000600016. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain. 1998;76:151–157. doi: 10.1016/s0304-3959(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Morris ME. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes Res. 1992;5:303–313. [PubMed] [Google Scholar]
- Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- Randall LO, Sellito JJ. A method for measurement of analgesic activity on inflammed tissue. Arch Int Pharmacodyn. 1957;61:409–419. [PubMed] [Google Scholar]
- Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care. 2003;26:1147–1152. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-d-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain. 2008;12:552–563. doi: 10.1016/j.ejpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Rude RK, Kirchen ME, Gruber HE, Meyer MH, Luck JS, Crawford DL. Magnesium deficiency-induced osteoporosis in the rat: uncoupling of bone formation and bone resorption. Magnes Res. 1999;12:257–267. [PubMed] [Google Scholar]
- Said G. Diabetic neuropathy – a review. Nat Clin Pract Neurol. 2007;3:331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- Said G, Slama G, Selva J. Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy. Brain. 1983;106:791–807. doi: 10.1093/brain/106.4.791. [DOI] [PubMed] [Google Scholar]
- Sang CN, Bennett GJ. Novel therapies for the control and prevention of neuropathic pain. Neurotherapeutics. 2009;6:607–608. doi: 10.1016/j.nurt.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Soltani N, Keshavarz M, Sohanaki H, Dehpour AR, Zahedi Asl S. Oral magnesium administration prevents vascular complications in STZ-diabetic rats. Life Sci. 2005;76:1455–1464. doi: 10.1016/j.lfs.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Takano Y, Sato E, Kaneko T, Sato I. Antihyperalgesic effects of intrathecally administered magnesium sulfate in rats. Pain. 2000;84:175–179. doi: 10.1016/s0304-3959(99)00207-9. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res. 2005;136:275–281. doi: 10.1016/j.molbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Tossielo L. Hypomagnesemia and diabetes mellitus. Arch Intern Med. 1996;156:1143–1148. [PubMed] [Google Scholar]
- Tsai PS, Cheng JK, Marsala M, Lin CR, Wen GH, Yang LC. Intrathecal magnesium sulfate attenuates algogenic behavior and spinal amino acids release after kainic acid receptor activation in rats. Neurosci Lett. 2001;301:115–118. doi: 10.1016/s0304-3940(01)01604-4. [DOI] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Ulugol A, Aslantas A, Ipci Y, Tuncer A, Hakan Karadag C, Dokmeci I. Combined systemic administration of morphine and magnesium sulfate attenuates pain-related behavior in mononeuropathic rats. Brain Res. 2002;943:101–104. doi: 10.1016/s0006-8993(02)02618-5. [DOI] [PubMed] [Google Scholar]
- Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, Garland HO, Riccardi D. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol. 2001;12:779–790. doi: 10.1681/ASN.V124779. [DOI] [PubMed] [Google Scholar]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Wu EQ, Borton J, Said G, Le TK, Monz B, Rosilio M, Avoinet S. Estimated prevalence of peripheral neuropathy and associated pain in adults with diabetes in France. Curr Med Res Opin. 2007;23:2035–2042. doi: 10.1185/030079907X210516. [DOI] [PubMed] [Google Scholar]
- Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology. 2001;41:714–723. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Magnesium suppresses neuropathic pain responses in rats via a spinal site of action. Brain Res. 1994;666:168–172. doi: 10.1016/0006-8993(94)90768-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


